Summary
In nearly every multifactorial human disease, there are three periods that characterize our understanding and definition. First, there is a period in which there is rapid accumulation of descriptive data. Second, there is a longer and slower period as information is obtained that redefines and expands basic and clinical knowledge that lacks the final and important area of understanding aetiology and therapeutic intervention. Third, which is much less common for most diseases, is the vigorous definition of pathobiology and treatment. These phases are well illustrated by our current understanding of primary biliary cirrhosis (PBC). The term PBC was first used nearly 60 years ago and for the first 40 or so years, the primary research efforts were directed at clinical definitions and pathology. Subsequently, with the advent of molecular biology, there began a rigorous dissection of the immune response and, in particular, a better understanding of anti-mitochondrial antibodies. These efforts have greatly helped in our understanding of not only the effector mechanisms of disease, but also the uniqueness of the primary target tissue, biliary epithelium. However, this research has still not led to successful translation for specific therapy.
Introduction
The history of our knowledge on primary biliary cirrhosis (PBC) focused on four major areas of research, i.e. diagnosis and clinical management, treatment and natural history, genetics, and immunopathology. However, these areas have been progressing at very different paces since the first use of the term PBC almost 60 years ago (1) and the subsequent description of the first patient series (2). Similar to all complex diseases recognizing multiple pathogenetic factors, research in PBC begun over 30 years ago with descriptive data. Further, evidence in each of the four major fields in PBC research has then witnessed rapid and slow progression rates during the past decades. In this review article, we will critically review the available data by highlighting the first two phases to ultimately encourage vigorous efforts aimed at completing the remaining unfinished business in PBC research and cure.
The diagnosis of PBC
The history and developments in the diagnosis of PBC well resemble the proposed two phases of experimental data (Table 1). Indeed, the first rapid phase is represented by the earlier major discoveries, i.e. the description of AMA specificity (3) and the establishment of solid diagnostic criteria. Nevertheless, further studies in the field have led to the progressive reduction of the AMA-negative subgroup, yet it remains to be determined whether this fraction of PBC cases represents a technical limitation of current methods or is indeed a distinct subpopulation with different serology.
Table 1.
The history of developments observed in PBC diagnosis are well representative of the different discovery paces with the early major discoveries in PBC diagnosis and the slow progression of newer techniques for AMA testing. Putative developments for the expected upcoming phases are suggested.
Most of the epidemiologic data used to determine the incidence and prevalence rates of PBC are descriptive (4). PBC is considered to be most prevalent in England, Scandinavia and in Northern areas of the United States, although a factitious prevalence due to more exhaustive epidemiological studies from these countries cannot be excluded. Indeed, prevalence rates for PBC appear to vary widely in different geographical areas and have been reported to be as high as 402/million in the Northern US (5), thus making PBC a rare disease according to the 2002 Rare Disease Act. PBC epidemiology is often based on different case-finding methods and data are thus difficult to compare, yet the concept of a PBC geoepidemiological pattern has been supported also by anedoctal reports of disease clustering (6, 7). Hence, there is the need of rigorous population-based studies in different geographical areas that do not imply selection bias, as in the case of blood donors (8), or solid case-finding methods.
Similar to the majority of autoimmune diseases, PBC manifests a striking predominance in the female sex. In fact, women with PBC are found to outnumber men by as much as 9:1, although such gender ratio varies widely in different epidemiological studies (4) with the largest non population-based study thus far reporting a 8:1 ratio (9).
Currently, the diagnosis of PBC is based on the presence of two out of three internationally accepted criteria, i.e. detectable serum AMA (titer ≥1:40), increased enzymes indicating cholestasis (i.e. alkaline phosphatase) for longer than 6 months, and a compatible or diagnostic liver histology (10). The classification proposes that a ‘definite’ diagnosis occurs only when all three criteria are encountered. We believe this is too narrow as patients lacking serum AMA appear to follow a similar natural history when compared to their AMA-positive counterparts (11). Although the need for a liver biopsy remains critical for disease staging, the issue of whether a liver biopsy is needed upon presentation is still debated. Currently, a liver biopsy should be performed when only one of the other two diagnostic criteria is met while its need should be carefully evaluated in the presence of serum AMA positivity and a biochemical cholestatic pattern for over 6 months. However, it remains to be determined whether liver histology should be obtained in the presence of serum AMA without elevated alkaline phosphatase.
The recent development in our laboratory of a sensitive bead assay able to detect AMA in 20% of otherwise AMA-negative sera (12) strengthens the hypothesis that AMA-negative PBC cases are secondary to limits in the current testing methods and do not represent an independent clinical entity. On the other hand, specific patterns of anti-nuclear antibodies (ANA) have proven to be PBC-specific (13) and are helpful tools in the management of patients with PBC, particularly when lacking AMA. From this point of view, however, prospective data on their diagnostic significance and their prevalence in AMA-negative cases are awaited. Another issue in the diagnosis of PBC includes the consistent finding of elevated serum IgM levels that do not correlate with AMA titers or disease stage yet appear to decrease when UDCA is used (14).
These observations on the PBC diagnostic process leads to the important question of what type of patients should be suspected of having PBC. This is not of secondary importance since an early diagnosis appears to be crucial to the efficacy of available medical treatment, i.e. ursodeoxyhcolic acid (UDCA), which is virtually absent in advanced stages. The diagnosis of PBC is commonly made in asymptomatic subjects in which routine tests have demonstrated elevated alkaline phosphatase levels and further analyses are consistent with earlier stages. Our series of over 400 patients followed at the San Paolo School of Medicine of the University of Milan over the past 30 years reflects this observation. The proportion of new PBC cases presenting to our attention with jaundice decreased dramatically from 50% in the 1973-1979 period to less than 5% in the 1992-1997 years and is currently an exceptional event in our practice with over 80% being diagnosed in the absence of symptoms. Indeed, classically described symptoms for PBC include fatigue and pruritus (10). The former complaint is, in our experience, poorly specific and we are convinced that rigorous prevalence studies taking into account the pre-diagnosis status and the use of well selected controls should be encouraged to minimize spurious findings. Indeed, somehow conflicting data have been reported on fatigue (15, 16) or quality of life impairment (17, 18) but their comparisons remain difficult due to social differences and the use of various questionnaires.
What should come next? The awaited third phase is represented, in our opinion, by the overcoming of current histological and serological limitations, possibly through the use of non-invasive diagnostic tools and the use of 100% sensitive AMA tests. Lastly, the diagnostic role of PBC-specific ANA has not been fully developed (13) and may hold the key to autoantibody markers to better define patients with PBC.
The natural history and treatment options of PBC
Several open questions remain in the treatment and natural history of PBC and represent the awaited third phase in this research area (Table 2). Indeed, the disease progression may vary widely and it is not uncommon in clinical practice to observe patients remaining asymptomatic for numerous years and decades and others progressing rapidly to liver failure and liver transplantation. Earlier evidence of UDCA efficacy and the use of the Mayo score first led to believe that the solution to PBC prognosis and treatment was at hand, yet the following studies have progressed slowly, as well represented by the weak results obtained with UDCA-based combination treatments. What causes PBC severity and progression of the disease remains unknown, although data seem to indicate that genetic factors other than those inducing the disease might play a role (19). Among non genetic factors, our group and others have recently proposed, based on cross-sectional (20) and longitudinal (21, 22) studies, that PBC-specific ANA are associated with a more aggressive disease. This was of particular interest in the comparison with established prognostic scores such as the Mayo score (23). This score is the only validated and widely utilized (24) based on clinical and biochemical variables as represented by cholestasis and liver function, thus possibly being less helpful in early stages when no hyperbilirubinemia is observed (22). When symptomless at diagnosis, PBC is associated with 10-year survival rates similar to age- and sex- matched healthy individuals while 67% of pre-cirrhotic patients will develop liver cirrhosis over a 7-year observation period while 70% of asymptomatic patients will develop symptoms. Based on these data, it has been hypothesized that survival rates of asymtpomatic patients with PBC is shorter than the general population if symptoms develop during follow-up (25) and this may be secondary to non liver related deaths (26).
Table 2.
PBC natural history and treatment remain an unfinished business. The rapid pace of earlier discoveries well represent phase 1 and was followed by slower developments with combination treatments and the prognostic significance of ANA. Future developments in the third phase are hypothesized.
UDCA mechanism of action in PBC remains incompletely understood but it has been hypothesized that it is based on several factors, including stimulation of impaired secretion of the bile-acid pool, reduction in proinflammatory cytokines, effects on apoptosis and on vasoactive mediators (27-29). Doses ranging from 13 to 15 mg/kg of UDCA are currently recommended and lead to optimum bile enrichment while the use of doses as high as 30-40 mg/kg/day as recommended in primary sclerosing cholangitis is currently not supported in PBC. A meta-analysis demonstrated that an increased survival is obtained only when a dose >13 mg/kg is prescribed (30) despite the fact that a complete biochemical response to UDCA is achieved in approximately 40% of treated patients. We note, however, that the definition of complete UDCA response should be a priority for future research. Indeed, a complete biochemical response to UDCA as normalization of alkaline phosphatase levels is suggested as a major determinant of UDCA effectiveness on disease progression (31). Immunosuppressive and antifibrotic drugs have been also used in PBC with poor efficacy (32). The use of corticosteroids is encouraged in combination with UDCA when an overlap with autoimmune hepatitis is present. New monoclonal antibodies and hematopoietic stem cell transplantation may be promising in PBC. Liver transplantation is the optimal treatment for end-stage PBC (33) despite common recurrence (34) influenced by immunosuppressive regimens and the recipient sex (35).
The etiology of PBC: genetics and environment
The current hypothesis on PBC onset states that the disease results from an environmental insult intervening on a genetically susceptible background. It is also clear that genetic components of PBC development cannot be related to a single gene mutation as the disease does not manifest a complete concordance in monozygotic twin sets (36). Indeed, data on the genetics of PBC are so far derived only from case-control studies (37) and this approach is clearly limited by control matching criteria and sample size and selection. Based on these data, PBC familial occurrence and twin concordance well represent the first (descriptive) phase of research efforts while the plethora or following association studies represent a slower progression rate (Table 3).
Table 3.
PBC genetic and environmental bases recognize three bases with an earlier rapid development of descriptive data on familial PBC and twin concordance subsequently followed by a slower phase with unconclusive association studies while recapitulation of the role of sex chromosome, xenobiotics, and bacteria is awaited. Epigenetic studies may constitute the key to a awaited third phase.
| Reference | |||
|---|---|---|---|
| Phase 1 | Earlier breakthrough | Familial PBC | (4) |
| Twin concordance | (98) | ||
| Risk factors | (9) | ||
| Phase 2 | Later developments | HLA/non-HLA associations | (99) |
| X chromosome defects | (100) | ||
| Bacterial candidates | (101) | ||
| Xenobiotic reactivity | (56) | ||
| Phase 3 | What is next? | Genome-wide association studies | |
| Spontaneous animal models | |||
| Epigenetic studies | |||
| Recapitulation of bacteria and chemicals |
The concordance rates in monozygotic twins for late-onset autoimmune diseases range are on average well below 50%. We reported that concordance rates for PBC are 63% in 8 monozygotic sets and null in dizygotic twins (36). It is of interest to note that in some cases the natural history of PBC as well as the comorbidities vary significantly between concordant twins. The phenotypical discordance could be caused by epigenetic factors, differences in exposure to environmental factors such as infections or xenobiotics, as illustrated below, as well as serendipity. Consistent with twin data, we note the occurrence of multiple cases among family member (i.e. ‘familial PBC’) with different prevalence rates (38) and the resulting risk for first-degree relatives of PBC cases to develop the disease (9) or to manifest serum AMA positivity (39).
Significant associations with specific major histocompatibility complex (MHC) alleles have been reported in many autoimmune diseases (40) while data in PBC have been conflicting. The example of the MHC class II DRB8 positive association is paradigmatic in the fact that this has been repeatedly suggested without achieving universal agreement. In our Milan case series, this association was not confirmed when smaller patient and control series were used (41) while the study of the largest population of patients and controls demonstrated a significant association with the DR8 allele (42, 43). Other association studies have investigated the importance of class III MHC and other candidate genes in determining PBC onset but, once again, associations have proven to be weak or failed independent confirmation (44).
A susceptible genetic background is necessary, yet not sufficient, to induce PBC. Among environmental candidates, evidence has been obtained for bacteria and xenobiotics. Of the bacterial strains suggested to lead to PBC through molecular mimicry (45), most evidence has been reported for Escherichia coli, based on the reports of an increased prevalence of urinary tract infections in patients with PBC (9). Contrasting evidence has been collected on the role of Chlamydia pneumoniae (46, 47) and Helicobacter pylori (48-50) in the pathogenesis of PBC while data on Lactobacillus delbruekii are promising and await further confirmation (51). We have recently provided serological data suggesting that a ubiquitous xenobiotic-metabolizing Gram-negative bacterium, Novosphingobium aromaticivorans, is the best candidate yet for the induction of PBC, as it elicits a specific antibody-reaction (estimated to be 100 to 1,000-fold higher than against E. coli) and its 16S rRNA specific sequences were detected in human fecal samples (52). Most recently, our group has contributed to determine that the infection by N. aromaticivorans induces PBC-like autoantibodies, autoreactive T cells, and liver lesions (53).
Xenobiotics are foreign compounds that may either alter or complex to defined self or non-self proteins, inducing a change in the molecular structure of the native protein sufficient to induce an immune response leading to chronic autoimmunity. Specific organic structures attached to the mitochondrial antigens are recognized by PBC sera with a higher affinity than the native forms of such antigens (54-56). Further, an halogenated compound is capable to induce AMA production (57) and PBC-like liver lesions (58) in animal models.
One current hypothesis may conjugate the evidence obtained for N. aromaticivorans and xenobiotics and, if proven, may prompt a new research impulse. The microorganism enters the human system through the digestive mucosa and bacterial mimics containing lipoic acid residues at this point might be modified by xenobiotics to form immunoreactive adducts which activate local dendritic cells. Antigen presenting cells may in turn activate autoreactive T and B cells that are directed to the liver through the portal system. T cells participate directly in the autoimmune injury and/or further recruit autoreactive lymphocytes. B cells, on the other hand, secrete AMA, particularly of the IgA type. AMA IgA are then transported to the vascular side of the bile duct cell where they react with the PDC-E2-like molecules located on the luminal surface cell membrane and induce apoptosis. The immune complexes of post-apoptotic PDC-E2 and IgG-AMA and the direct cytopathic effects of autoreactive T cells (and possibly AMA) contribute to the tissue injury observed in PBC.
The immunobiology of PBC
Since the determination of PBC autoantigens in 1987 (3) there has been an enormous number of experimental studies on the major components of PBC autoimmunity. With the exception of AMA and autoreactive T cells, data on other cell populations are of uncertain significance in PBC pathogenesis (Table 4). First, it is well established that AMA are directed against members of the 2-oxoacid dehydrogenase complexes (2-OADC), among which the major epitopes are within the lipoylated domains of the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2) (59). The pathogenic role of AMA remains debated since no clinical correlation can be found and animal models developing serum AMA do not consistently manifest PBC-like liver lesions. Second, autoreactive CD4+ and CD8+ T cells can be detected in PBC peripheral blood, regardless of the AMA status, and the infiltration of autoreactive T cells in the liver and periductular spaces is one of the most prominent immune features (60). Autoreactive T cells of both subtypes recognize PDC-E2 sequences overlapping with the AMA epitopes (61). An increase in cytotoxic T cell precursors in the blood in the early stages of the disease compared to the advanced ones and a 10-fold increase of specific liver CD8+ T cells compared to peripheral blood have been demonstrated (62). Third, additional data on the immunobiology components of PBC autoimmunity has been recently obtained in CD4+CD25 high natural regulatory T cells which appear to be numerically reduced in PBC (63). What drives the immune mediated response to a ubiquitous antigen to damage a single cell type with such specificity remains unknown. The identification of specific characteristics of BEC (64, 65) such as the differential expression of cell adhesion molecules, the response to cytokines and growth factors and their potential ability to act as antigen presenting cells, may explain the strict organ specificity of the immune mediated injury in PBC (66). PBC bile duct cells manifest unique features during apoptosis while co-culture experiments do not support a direct role for these cells in determining their immune –mediated injury (67). Indeed, Odin and colleagues first reported that glutathionylation of the lysine lipoic acid moiety of PDC-E2 is dramatically reduced via Bcl-2 by serum AMA (68). The enhanced glutathionylation, linked to the evidence that PDC-E2 is released from apoptotic BEC without caspase cleavage, appears fundamental in the recognition of the mitochondrial epitope by the immune system upon cell death. A possible unifying hypothesis has been proposed by Allina and colleagues who recently demonstrated that apoptotic cells are phagocytosed by BECs and consequently are an exogenous source of autoantigens in cholangiocytes (69), possibly through anti-CD16 (70). As a result, the impact of putative changes in apoptosis (71) and autophagy (72) specific to BEC remains to be fully determined in PBC. Fifth, the innate immune compartment has been recently investigated in PBC with promising results. PBC monocytes manifest an increased response to pathogen associated stimuli, as indicated by higher levels of pro-inflammatory cytokines (73). Further, the hyper-IgM associated with PBC is secondary to an aberrant innate immune response, potentially induced by stimulation of toll like receptor 9 by bacterial CpG-B (74). Similarly, Chuang and colleagues reported a marked increase in the frequency and absolute number of blood and liver NK cells in PBC cases (75).
Table 4.
PBC immunobiology was characterized by earlier discoveries leading to the definition of AMA autoantigens and autoreactive T cells while a second phase is well represented by the study of T regulatory cells, apoptosis in cholangiocytes, and innate immune changes. The integration of these lines of evidence is the major awaited development in the third phase.
| Reference | |||
|---|---|---|---|
| Phase 1 | Earlier breakthrough | Cloning of AMA autoantigens | (3) |
| Definition of autoreactive T cells | (61) | ||
| Phase 2 | Later developments | Definition of Treg defects | (102) |
| Bile duct cell apoptosis uniqueness | (68) | ||
| Innate immune changes in PBC | (73, 74) | ||
| Phase 3 | What is next? | Proof of apoptosis involvement in autoantigen recognition | |
| Definition of lipoic acid critical role | |||
| Recapitulation of all of the above in an animal model |
As summarized in Table 4, we currently have access to a significant amount of descriptive and partially mechanistic information on the immune alterations associated with PBC. One hypothesis for the selective destruction of cholangiocytes is based on the aberrant expression of PDC-E2 on the cell surface, possibly secondary to cell-specific overexpression or to abnormal intracellular turnover. Although it cannot be ruled out that observed reactivities are in fact due to immune complexes, it remains possible that the molecules expressed on the ductular surface may not be PDC-E2 itself, but PDC-E2 mimics. Interestingly, IgA from patients with PBC colocalize with PDC-E2 (or PDC-E2 mimic) on the apical membrane of PBC cholangiocytes (76) and these data are consistent with the epithelium-specific damage.
Of mice and women: new players may provide new research impetus
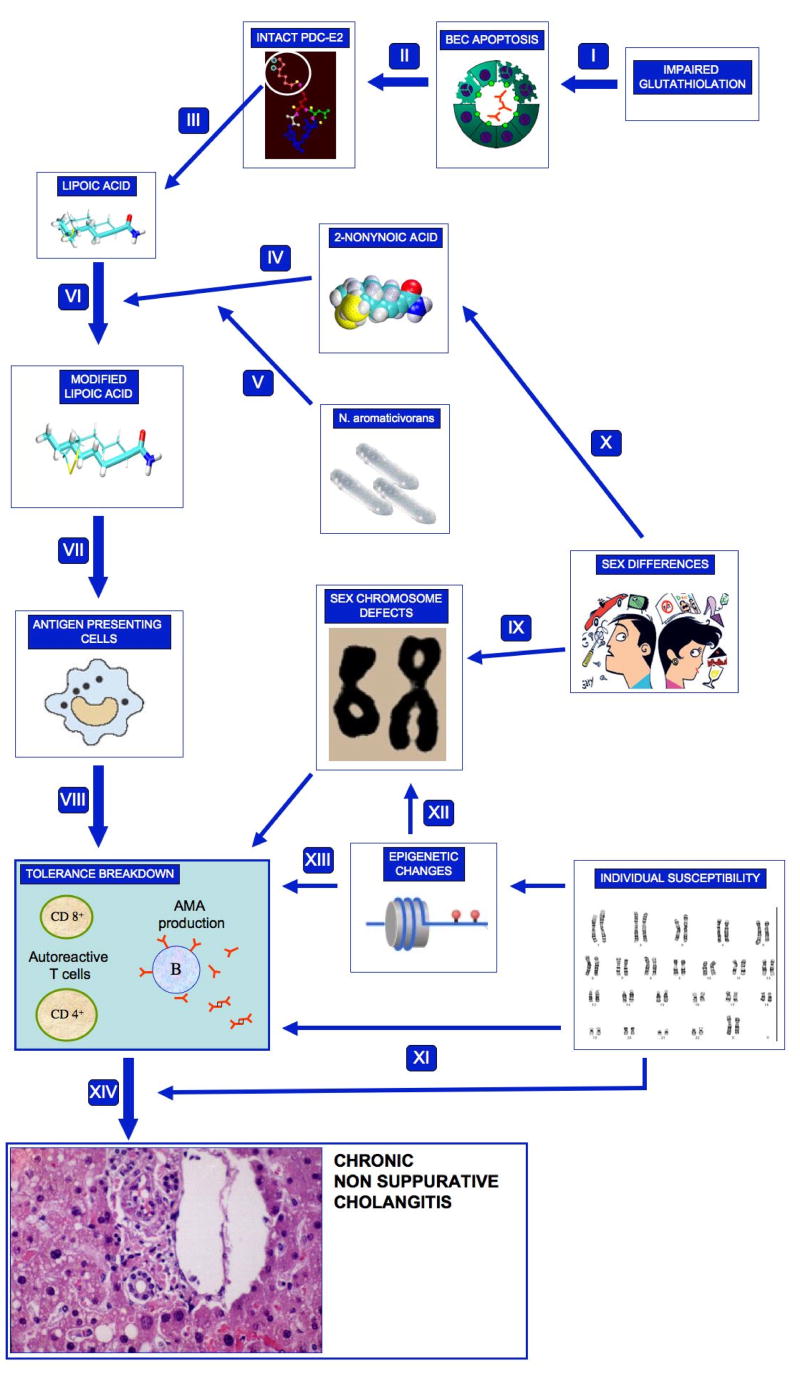
During the past 4 years, new intriguing scenarios have been proposed with important implications for PBC and the resulting working hypothesis on PBC etiology and pathogenesis is summarized in Figure 1. These include new evidence on sex chromosomes and novel comprehensive animal models.
Figure 1.
The current working hypothesis of PBC development is illustrated. The primum movens of the proposed mechanisms is based on the peculiar apoptotic features of biliary epithelial cells (BEC). Secondary to a reduced glutathiolation (I), apoptotic BEC release an intact PDC-E2 (II) which also includes lipoic acid (III). Lipoic acid in turn becomes the ideal target for xenobiotic-induced modifications with 2-octynoic acid as the best candidate (IV) and with N. aromaticivorans possibly influencing this pathway (V). The modified or substituted lipoic acid residue is then uptaken by antigen presenting cells (VII) and is capable of breaking immune tolerance (VIII) in the presence of a permissive genetic background. Female predominance of PBC may be explained by major sex chromosome defects such as X monosomy (IX) or by the increased exposure to specific xenobiotics (X). Indeed, individual susceptibility may be based on on a direct putative impact of a specific set of genes on disease development or progression (XI), as suggested by association studies, or by epigenetic changes of sex (XII) or somatic (XIII) chromosomes that remain to be investigated. The ultimate result of a permissive genetic background and an undertermined environmental exposure is the development and perpetuation of the PBC liver injury (XIV).
First, the female preponderance may hold an important key to PBC aetiology. The hypotheses based on sex hormone influence on lymphocytes at various stages in life (77-79) or fetal microchimerism (80) are inconclusive. X-linked genes determine gender-related characteristics at different levels while also regulating the immune function, particulalry to maintain tolerance. Major X chromosome defects such as those leading to Turner's syndrome (81) or premature ovarian failure (82) are commonly characterized by autoimmune comorbidities (particularly thyroid disease) and, less frequently, cholestasis. Our group first determined a significantly higher frequency of monosomy of the X chromosome in peripheral leukocytes (particularly those of the adaptive immune response, i.e. T and B cells) in women PBC (36) compared to age-matched control women. Monosomy frequency correlated with age in all three groups, as expected (83) but monosomic cells were not microchimeric cells (84). Importantly, women with two other female-predominant and late-onset autoimmune diseases with different organ specificity, i.e. systemic sclerosis and autoimmune thyroiditis, also manifested the same difference (85). This was not recapitulated in women with systemic lupus erithematosus which, however, were significantly younger (86). We further demonstrated that the X loss in PBC affected was not random but affected more frequently one parentally-inherited chromosome (87). We submit that the sex chromosome hypothesis is indeed fascinating (88) and encourage independent researchers to recapitulate our data on other patient series.
The second important development during the past few years has been the appearance of three animal models that may contribute to elucidating the undoubtedly multi-factorial causation and progression of PBC (89). First, a genomic variant of the non obese diabetic (NOD) mouse (NOD.c3c4) has been observed to manifest autoimmune cholestasis with AMA and ANA positivities in 50%–60% and 80%–90%, respectively. Liver histology demonstrated portal lymphocyte infiltration with chronic non-suppurative cholangitis and PBC-like granulomas (90). Second, a dominant negative form of trasforming growth factor β (TGFβ) receptor II (dnTGFβRII) mouse develop serum AMA in 100% of mice. The TGFβ receptor II regulates lymphocyte activation and the appearance of PBC in this model suggests a specific condition of T cells with impaired TGFβ signaling in the presence or absence of B cells is involved (91). Third, the knockout of interleukin 2 receptor α leads to a murine phenotype with 100% serum AMA positivity, 80% serum ANA positivity, and portal lymphocyte infiltration and vanishing bile ducts (92). This model is of particular interest based on the report of autoimmune cholangitis in a pediatric case of IL2Rα deficiency (93).
There appears to be a new excitement caused by these most recent lines of evidence among researchers involved in PBC aetiopathogenesis. Indeed, we are also convinced that the appearance of novel laboratory tools such as genome-wide multiplex analysis methods capable of providing high-throughput analyses of genetic polymorphisms or microRNA will prompt research efforts to finally determine the genetic bases of PBC. Nevertheless, only a multicenter study to collect a large number of PBC cases and well-matched controls and to recapitulate available evidence will provide enough power for the next needed phase in PBC.
Acknowledgments
Financial support provided by National Institutes of Health grants, DK074768, DK39588 and DK067003.
List of Abbreviations
- AIH
autoimmune hepatitis
- AMA
antimitochondrial antibody
- APC
antigen presenting cell
- BEC
biliary epithelial cell
- BSA
bovine serum albumin
- CFA
complete Freund's adjuvant
- HLA
human leukocyte antigens
- MHC
major histocompatibility complex
- OADC
2-oxo-acid dehydrogenase complex
- PBC
primary biliary cirrhosis
- PBMC
peripheral blood mononuclear cell
- PDC-E2
E2 subunit of the pyruvate dehydrogenase complex
- PSC
primary sclerosing cholangitis
- TGF
transforming growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahrens EH, Jr, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Medicine (Baltimore) Vol. 29. 1950. Primary biliary cirrhosis; pp. 299–364. [DOI] [PubMed] [Google Scholar]
- 2.Sherlock S. Primary billiary cirrhosis (chronic intrahepatic obstructive jaundice) Gastroenterology. 1959;37:574–586. [PubMed] [Google Scholar]
- 3.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 4.Selmi C, Invernizzi P, Zuin M, Podda M, Gershwin ME. Genetics and geoepidemiology of primary biliary cirrhosis: following the footprints to disease etiology. Semin Liver Dis. 2005;25:265–280. doi: 10.1055/s-2005-916319. [DOI] [PubMed] [Google Scholar]
- 5.Kim WR, Lindor KD, Locke GR, 3rd, Therneau TM, Homburger HA, Batts KP, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631–1636. doi: 10.1053/gast.2000.20197. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Mouch S, Selmi C, Benson GD, Kenny TP, Invernizzi P, Zuin M, et al. Geographic clusters of primary biliary cirrhosis. Clin Dev Immunol. 2003;10:127–131. doi: 10.1080/10446670310001626526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, et al. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43:525–531. doi: 10.1002/hep.21076. [DOI] [PubMed] [Google Scholar]
- 8.Mattalia A, Quaranta S, Leung PS, Bauducci M, Van de Water J, Calvo PL, et al. Characterization of antimitochondrial antibodies in health adults. Hepatology. 1998;27:656–661. doi: 10.1002/hep.510270303. [DOI] [PubMed] [Google Scholar]
- 9.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 11.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–1095. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 12.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, et al. Hepatology. Vol. 45. 2007. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis; pp. 659–665. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298–310. doi: 10.1055/s-2005-916321. [DOI] [PubMed] [Google Scholar]
- 14.Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza A, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32:561–566. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 15.Bjornsson E, Simren M, Olsson R, Chapman RW. Fatigue is not a specific symptom in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:351–357. doi: 10.1097/00042737-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Jones DE, Bhala N, Burt J, Goldblatt J, Prince M, Newton JL. Four year follow up of fatigue in a geographically defined primary biliary cirrhosis patient cohort. Gut. 2006;55:536–541. doi: 10.1136/gut.2005.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poupon RE, Chretien Y, Chazouilleres O, Poupon R, Chwalow J. Quality of life in patients with primary biliary cirrhosis. Hepatology. 2004;40:489–494. doi: 10.1002/hep.20276. [DOI] [PubMed] [Google Scholar]
- 18.Selmi C, Gershwin ME, Lindor KD, Worman HJ, Gold EB, Watnik M, et al. Quality of life and everyday activities in patients with primary biliary cirrhosis. Hepatology. 2007;46:1836–1843. doi: 10.1002/hep.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donaldson P, Agarwal K, Craggs A, Craig W, James O, Jones D. HLA and interleukin 1 gene polymorphisms in primary biliary cirrhosis: associations with disease progression and disease susceptibility. Gut. 2001;48:397–402. doi: 10.1136/gut.48.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34:366–372. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Shimizu-Yoshida Y, Takii Y, Komori A, Yokoyama T, Ueki T, et al. Antibody titer to gp210-C terminal peptide as a clinical parameter for monitoring primary biliary cirrhosis. J Hepatol. 2005;42:386–392. doi: 10.1016/j.jhep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Wesierska-Gadek J, Penner E, Battezzati PM, Selmi C, Zuin M, Hitchman E, et al. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology. 2006;43:1135–1144. doi: 10.1002/hep.21172. [DOI] [PubMed] [Google Scholar]
- 23.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 24.Grambsch PM, Dickson ER, Kaplan M, LeSage G, Fleming TR, Langworthy AL. Extramural cross-validation of the Mayo primary biliary cirrhosis survival model establishes its generalizability. Hepatology. 1989;10:846–850. doi: 10.1002/hep.1840100516. [DOI] [PubMed] [Google Scholar]
- 25.Springer J, Cauch-Dudek K, O'Rourke K, Wanless IR, Heathcote EJ. Asymptomatic primary biliary cirrhosis: a study of its natural history and prognosis. Am J Gastroenterol. 1999;94:47–53. doi: 10.1111/j.1572-0241.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 26.Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865–870. doi: 10.1136/gut.2003.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol. 2001;35:134–146. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 28.Paumgartner G, Beuers U. Mechanisms of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis. 2004;8:67–81. doi: 10.1016/S1089-3261(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 29.Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 30.Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD000551. CD000551. [DOI] [PubMed] [Google Scholar]
- 31.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, et al. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology. 2005;42:1184–1193. doi: 10.1002/hep.20897. [DOI] [PubMed] [Google Scholar]
- 33.Williams R, Gershwin ME. How, why, and when does primary biliary cirrhosis recur after liver transplantation? Liver Transpl. 2007;13:1214–1216. doi: 10.1002/lt.21143. [DOI] [PubMed] [Google Scholar]
- 34.Batts KP, Wang X. Recurrence of primary biliary cirrhosis, autoimmune cholangitis and primary sclerosing cholangitis after liver transplantation. Clin Liver Dis. 1998;2:421–435. doi: 10.1016/s1089-3261(05)70016-7. [DOI] [PubMed] [Google Scholar]
- 35.Charatcharoenwitthaya P, Pimentel S, Talwalkar JA, Enders FT, Lindor KD, Krom RA, et al. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2007;13:1236–1245. doi: 10.1002/lt.21124. [DOI] [PubMed] [Google Scholar]
- 36.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 37.Cardon LR, Bell JI. Association study designs for complex diseases. Nat Rev Genet. 2001;2:91–99. doi: 10.1038/35052543. [DOI] [PubMed] [Google Scholar]
- 38.Brind AM, Bray GP, Portmann BC, Williams R. Prevalence and pattern of familial disease in primary biliary cirrhosis. Gut. 1995;36:615–617. doi: 10.1136/gut.36.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazaridis KN, Juran BD, Boe GM, Slusser JP, de Andrade M, Homburger HA, et al. Increased prevalence of antimitochondrial antibodies in first-degree relatives of patients with primary biliary cirrhosis. Hepatology. 2007;46:785–792. doi: 10.1002/hep.21749. [DOI] [PubMed] [Google Scholar]
- 40.McCluskey J, Peh CA. The human leucocyte antigens and clinical medicine: an overview. Rev Immunogenet. 1999;1:3–20. [PubMed] [Google Scholar]
- 41.Invernizzi P, Battezzati PM, Crosignani A, Perego F, Poli F, Morabito A, et al. Peculiar HLA polymorphisms in Italian patients with primary biliary cirrhosis. J Hepatol. 2003;38:401–406. doi: 10.1016/s0168-8278(02)00440-3. [DOI] [PubMed] [Google Scholar]
- 42.Donaldson PT, Baragiotta A, Heneghan MA, Floreani A, Venturi C, Underhill JA, et al. HLA class II alleles, genotypes, haplotypes, and amino acids in primary biliary cirrhosis: a large-scale study. Hepatology. 2006;44:667–674. doi: 10.1002/hep.21316. [DOI] [PubMed] [Google Scholar]
- 43.Invernizzi P, Selmi C, Poli F, Bianchi I, Rosina F, Floreani A, et al. HLA-DRB1 polymorphisms in 676 Italian patients with primary biliary cirrhosis and 2028 matched healthy controls. A nationwide population-based case-control study. Hepatology. 2005;42:64964. [Google Scholar]
- 44.Donaldson PT. TNF gene polymorphisms in primary biliary cirrhosis: a critical appraisal. J Hepatol. 1999;31:366–368. doi: 10.1016/s0168-8278(99)80238-4. [DOI] [PubMed] [Google Scholar]
- 45.Selmi C, Gershwin ME. Bacteria and human autoimmunity: the case of primary biliary cirrhosis. Curr Opin Rheumatol. 2004;16:406–410. doi: 10.1097/01.bor.0000130538.76808.c2. [DOI] [PubMed] [Google Scholar]
- 46.Abdulkarim AS, Petrovic LM, Kim WR, Angulo P, Lloyd RV, Lindor KD. Primary biliary cirrhosis: an infectious disease caused by Chlamydia pneumoniae? J Hepatol. 2004;40:380–384. doi: 10.1016/j.jhep.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 47.Leung PS, Park O, Matsumura S, Ansari AA, Coppel RL, Gershwin ME. Is there a relation between Chlamydia infection and primary biliary cirrhosis? Clin Dev Immunol. 2003;10:227–233. doi: 10.1080/10446670310001642429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadstrom T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38:1072–1076. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilsson I, Kornilovs'ka I, Lindgren S, Ljungh A, Wadstrom T. Increased prevalence of seropositivity for non-gastric Helicobacter species in patients with autoimmune liver disease. J Med Microbiol. 2003;52:949–953. doi: 10.1099/jmm.0.05344-0. [DOI] [PubMed] [Google Scholar]
- 50.Durazzo M, Rosina F, Premoli A, Morello E, Fagoonee S, Innarella R, et al. Lack of association between seroprevalence of Helicobacter pylori infection and primary biliary cirrhosis. World J Gastroenterol. 2004;10:3179–3181. doi: 10.3748/wjg.v10.i21.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogdanos DP, Baum H, Okamoto M, Montalto P, Sharma UC, Rigopoulou EI, et al. Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology. 2005;42:458–465. doi: 10.1002/hep.20788. [DOI] [PubMed] [Google Scholar]
- 52.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 53.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, et al. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956–2963. doi: 10.4049/jimmunol.167.5.2956. [DOI] [PubMed] [Google Scholar]
- 55.Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, et al. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–5332. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 58.Leung PS, Park O, Tsuneyama K, Kurth MJ, Lam KS, Ansari AA, et al. Induction of primary biliary cirrhosis in guinea pigs following chemical xenobiotic immunization. J Immunol. 2007;179:2651–2657. doi: 10.4049/jimmunol.179.4.2651. [DOI] [PubMed] [Google Scholar]
- 59.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, et al. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 60.Ishibashi H, Nakamura M, Shimoda S, Gershwin ME. T cell immunity and primary biliary cirrhosis. Autoimmun Rev. 2003;2:19–24. doi: 10.1016/s1568-9972(02)00122-2. [DOI] [PubMed] [Google Scholar]
- 61.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–1845. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- 64.Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- 65.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 66.Selmi C, Mackay IR, Gershwin ME. The immunological milieu of the liver. Semin Liver Dis. 2007;27:129–139. doi: 10.1055/s-2007-979466. [DOI] [PubMed] [Google Scholar]
- 67.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, et al. Biliary epithelial cells and primary biliary cirrhosis: The role of liver-infiltrating mononuclear cells. Hepatology. 2008;47:958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 68.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, et al. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232–241. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allina J, Stanca CM, Garber J, Hu B, Sautes-Fridman C, Bach N, et al. Anti-CD16 autoantibodies and delayed phagocytosis of apoptotic cells in primary biliary cirrhosis. J Autoimmun. 2008;30:238–245. doi: 10.1016/j.jaut.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lleo A, Selmi C, Invernizzi P, Podda M, Gershwin ME. The consequences of apoptosis in autoimmunity. J Autoimmun. 2008 doi: 10.1016/j.jaut.2008.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lleo A, Invernizzi P, Selmi C, Coppel RL, Alpini G, Podda M, et al. Autophagy: highlighting a novel player in the autoimmunity scenario. J Autoimmun. 2007;29:61–68. doi: 10.1016/j.jaut.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–808. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 74.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, et al. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Malmborg AC, Shultz DB, Luton F, Mostov KE, Richly E, Leung PS, et al. Penetration and co-localization in MDCK cell mitochondria of IgA derived from patients with primary biliary cirrhosis. J Autoimmun. 1998;11:573–580. doi: 10.1006/jaut.1998.0220. [DOI] [PubMed] [Google Scholar]
- 77.Rubel LR, Rabin L, Seeff LB, Licht H, Cuccherini BA. Does primary biliary cirrhosis in men differ from primary biliary cirrhosis in women? Hepatology. 1984;4:671–677. doi: 10.1002/hep.1840040418. [DOI] [PubMed] [Google Scholar]
- 78.Grossman CJ. Regulation of the immune system by sex steroids. Endocr Rev. 1984;5:435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- 79.Parikh-Patel A, Gold E, Utts J, Gershwin ME. The association between gravidity and primary biliary cirrhosis. Ann Epidemiol. 2002;12:264–272. doi: 10.1016/s1047-2797(01)00277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Invernizzi P, De Andreis C, Sirchia SM, Battezzati PM, Zuin M, Rossella F, et al. Blood fetal microchimerism in primary biliary cirrhosis. Clin Exp Immunol. 2000;122:418–422. doi: 10.1046/j.1365-2249.2000.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ranke MB, Saenger P. Turner's syndrome. Lancet. 2001;358:309–314. doi: 10.1016/S0140-6736(01)05487-3. [DOI] [PubMed] [Google Scholar]
- 82.Davis CJ, Davison RM, Payne NN, Rodeck CH, Conway GS. Female sex preponderance for idiopathic familial premature ovarian failure suggests an X chromosome defect: opinion. Hum Reprod. 2000;15:2418–2422. doi: 10.1093/humrep/15.11.2418. [DOI] [PubMed] [Google Scholar]
- 83.Guttenbach M, Koschorz B, Bernthaler U, Grimm T, Schmid M. Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet. 1995;57:1143–1150. [PMC free article] [PubMed] [Google Scholar]
- 84.Kaplan MM, Bianchi DW. Primary biliary cirrhosis: for want of an X chromosome? Lancet. 2004;363:505–506. doi: 10.1016/S0140-6736(04)15576-1. [DOI] [PubMed] [Google Scholar]
- 85.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. J Immunol. 2005;175:575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 86.Invernizzi P, Miozzo M, Oertelt-Prigione S, Meroni PL, Persani L, Selmi C, et al. X monosomy in female systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1110:84–91. doi: 10.1196/annals.1423.010. [DOI] [PubMed] [Google Scholar]
- 87.Miozzo M, Selmi C, Gentilin B, Grati FR, Sirchia S, Oertelt S, et al. Preferential X chromosome loss but random inactivation characterize primary biliary cirrhosis. Hepatology. 2007;46:456–462. doi: 10.1002/hep.21696. [DOI] [PubMed] [Google Scholar]
- 88.Invernizzi P. The X chromosome in female-predominant autoimmune diseases. Ann N Y Acad Sci. 2007;1110:57–64. doi: 10.1196/annals.1423.007. [DOI] [PubMed] [Google Scholar]
- 89.Oertelt S, Ridgway WM, Ansari AA, Coppel RL, Gershwin ME. Murine models of primary biliary cirrhosis: Comparisons and contrasts. Hepatol Res. 2007;37:S365–S369. doi: 10.1111/j.1872-034X.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 90.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 92.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, et al. IL-2 receptor alpha(-/-) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 93.Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, et al. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun. 2006;27:50–53. doi: 10.1016/j.jaut.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Walker JG, Doniach D, Roitt IM, Sherlock S. Serological Tests in Diagnosis of Primary Biliary Cirrhosis. Lancet. 1965;1:827–831. doi: 10.1016/s0140-6736(65)91372-3. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan MM. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–1580. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 96.Miyakawa H, Tanaka A, Kikuchi K, Matsushita M, Kitazawa E, Kawaguchi N, et al. Hepatology. Vol. 34. 2001. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens; pp. 243–248. [DOI] [PubMed] [Google Scholar]
- 97.Heathcote J. Update on primary biliary cirrhosis. Can J Gastroenterol. 2000;14:43–48. doi: 10.1155/2000/989486. [DOI] [PubMed] [Google Scholar]
- 98.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–492. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 99.Selmi C, Invernizzi P, Zuin M, Podda M, Seldin MF, Gershwin ME. Genes and (auto)immunity in primary biliary cirrhosis. Genes Immun. 2005;6:543–556. doi: 10.1038/sj.gene.6364248. [DOI] [PubMed] [Google Scholar]
- 100.Selmi C, Invernizzi P, Miozzo M, Podda M, Gershwin ME. Primary biliary cirrhosis: does X mark the spot? Autoimmun Rev. 2004;3:493–499. doi: 10.1016/j.autrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Selmi C, Gershwin ME. Bacteria and human autoimmunity: the case of primary biliary cirrhosis. Curr Opin Rheumatol. 2004;16:406–410. doi: 10.1097/01.bor.0000130538.76808.c2. [DOI] [PubMed] [Google Scholar]
- 102.Lan RY, Mackay IR, Eric Gershwin M. Regulatory T cells in the prevention of mucosal inflammatory diseases: Patrolling the border. J Autoimmun. 2007;29:272–280. doi: 10.1016/j.jaut.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]