Abstract
Fire accident victims, who sustain both thermal injury to skin and smoke inhalation, have gross evidence of systemic and pulmonary oxidant damage and acute lung injury. We hypothesized that gamma-Tocopherol (gT), a reactive O2 and N2scavenger, when delivered into the airway will attenuate lung injury induced by burn and smoke inhalation. Acute lung injury was induced in chronically prepared, anesthetized sheep by 40% total burn surface area, 3rd degree skin burn and smoke insufflation (48 breaths of cotton smoke, <40°C). Study groups: 1) Sham (not injured, flax (FO)-nebulized, n=6); 2) SA-neb (injured, saline-nebulized, n=6); 3) FO-neb (injured, FO-nebulized, n=6); 4) gT+FO-neb (injured, FO+gT-nebulized, n=6). Nebulization was started 1 h post-injury and 24 ml of FO with or without gT (51 mg/ml) was delivered into airways over 47 h using our newly developed lipid aerosolization device (droplet size-2.5–5 μm). The burn and smoke inhalation-induced pathological changes seen in the saline group were attenuated by FO nebulization; gT addition further improved pulmonary function. Pulmonary gT delivery along with a FO source may be a novel effective treatment strategy in management of patients with acute lung injury.
Keywords: Gamma, Tocopherol, Smoke inhalation injury, Burn, Acute lung injury, Ovine model, Free radicals
Introduction
Burn injury is very traumatic, especially when the thermal injury is associated with smoke inhalation. This combination greatly increases morbidity and mortality [1, 2]. The degree of oxidant stress in lung and systemic organs after smoke exposure appears to reflect the degree of organ injury and subsequent mortality [3, 4] and iNOS-generated NO has an important role in the changes in both systemic and pulmonary microvascular permeability that follow combined cutaneous burn and smoke inhalation injury [5, 6]. Combined burn and smoke inhalation injury is typically associated with a systemic inflammatory response and increased levels of reactive nitrogen (RNS) and oxygen (ROS) species in the lung [7]. Following the combined burn and smoke inhalation injury, cells capable of generating both superoxide anion and nitric oxide, especially neutrophils when activated, can thus produce peroxynitrite [8, 9]. Peroxynitrite is a powerful oxidant, nitrosating and nitrating agent [10]. It can readily trigger DNA single strand breakage and induce poly (ADP-ribose) polymerase (PARP) activation [11]. However, the overactivation of poly (ADP-ribose) polymerase leads to a depletion in ATP and NAD+, resulting in cellular dysfunction and ultimately to necrotic cell death [12, 13]. Activation of PARP also upregulates nuclear factors and the inducible form of nitric oxide synthase (iNOS or NOS-2) and interleukin-8 (IL-8) [14–17].
Various antioxidants prevent damage from oxygen-free radical reactions. For example, vitamins C and E and coenzyme Q are known as in vivo antioxidants [18]. Vitamin E is the major chain-breaking antioxidant within biological membranes and includes tocopherols and tocotrienols [19]. Four major forms of the tocopherols are alpha, beta, gamma, and delta-tocopherol, which differ only in number and location of methyl substitutents on the aromatic chromanol ring [20]. Of the tocopherols, alpha- and gamma-tocopherols (aT and gT) are principal forms found in human and animal diets and comprise most of the vitamin E content of tissues [21]. Cooney et al. reported that gT might be a more effective cellular protectant against nitrogen dioxide (NO2) and demonstrated that gT suppressed NO2-induced neoplastic transformation more effectively than aT in vitro [22]. However despite the advantages of gT and its prevalence in the diet, its concentration in the body is low as it is rapidly excreted. aT is the major tocopherol in mammals because the liver expresses the aT transfer protein that maintains plasma aT concentrations. In contrast, the liver actively metabolizes gT preventing plasma and tissue gT accumulation [23].
Our previous studies in sheep with combined burn and smoke injury demonstrated that most of the oxidative damage took place in the lung and this bronchopulmonary damage preceded systemic injury [6]. Consequently we hypothesized that by delivering gT into the lung via the airway, gT would act as a scavenger of both reactive oxygen and nitrogen species and thus minimize injury [24, 25].
Previously, we have shown that aerosolizing an emulsion of aT into the airway reduced the acute lung injury resulting from combined burn and smoke inhalation injury in our ovine model [26]. However a commercially available gT emulsion does not exist. gT is a thick oily material that is difficult to aerosolize using an ultrasonic nebulizer. Therefore, we developed a device that nebulizes lipids to droplet sizes of 2.5–5.0 μm. Flax oil (FO) containing high alpha-linolenic acid (omega-3 PUFA) was used as a diluent. The FO has the added benefit that omega-3 PUFAs interfere with early inflammatory signal transduction processes and thus is capable of blunting hyperinflammatory processes [27]. We utilized this new lipid delivery system to test our hypothesis that gT delivered to the lungs of injured sheep would ameliorate the extent of the oxidative stress and injury processes.
Materials and methods
Animals
Twenty-four adult female sheep were cared for in the Investigative Intensive Care Unit at our institution. The experimental procedure was approved by the Animal Care and Use Committee of the University of Texas Medical Branch. The National Institutes of Health and American Physiological Society guidelines for animal care were strictly followed. The Investigative Intensive care unit is accredited by The Association for the Assessment and Accreditation of Laboratory Animal Care International.
Animal model
Sheep (30–40 kg) were surgically prepared, as described in detail previously [28]. A Swan-Ganz thermal dilution catheter was inserted through the right external jugular vein (model 93A-1317-F, Edwards Critical Care Division, Irvine, CA) for the measurement of the core body temperature to evaluate blood gas and the fluid resuscitations. An arterial catheter was inserted into the right femoral artery (16 gauge, 24 in., Intracath, Becton Dickinson, Sandy, UT) for the measurement of arterial blood gas. To evaluate changes in lung lymph flow, an efferent lymph vessel from the caudal mediastinal lymph node was cannulated (Silastic catheter 0.025-in ID, 0.047-in OD; Dow Corning, Midland, MI) according to a modification of the technique described by Staub and colleagues [29, 30]. After a 7-day recovery period, the sheep were deeply anesthetized with halothane and were given a burn (40% total body surface area [TBSA], third degree) and inhalation injury (48 breaths of cotton smoke, <40°C). After burn/smoke injury, all sheep were placed on a ventilator with positive end-expiratory pressure set to 5 cm H2O and tidal volume maintained at 15 mL/kg. The latter tidal volume is equal to about 10 ml/kg in humans due to the large dead space of sheep [31]. All animals were given fluid resuscitation with Lactated Ringer’s solution strictly according to the Parkland formula (4 mL/kg/% TBSA burned/24 h). Experiment was conducted in awake condition for 48 h.
Experimental Design
The sheep were randomly assigned to one of the following four groups: 1) Sham (not injured, FO-nebulized, n=6); 2) SA-neb (injured, saline-nebulized, n=6); 3) FO-neb (injured, FO-nebulized, n=6); 4) gT+FO-neb (injured, gT+FO-nebulized, n=6). Sham animals received no injury but were surgically prepared similarly to injured animals, placed on a ventilator, and given fluid resuscitation and nebulized with 24 ml FO over 47h. SA-neb animals were nebulized with 24 ml of 0.9 % NaCl over 47 h after injury. FO-neb animals were nebulized 24 ml FO over 47 h after injury. gT+FO-neb animals were nebulized 24 ml gT+FO mixture solution (gT:1220 mg; 51 mg/ml) over 47h after injury.
Aerosol Delivery & Material
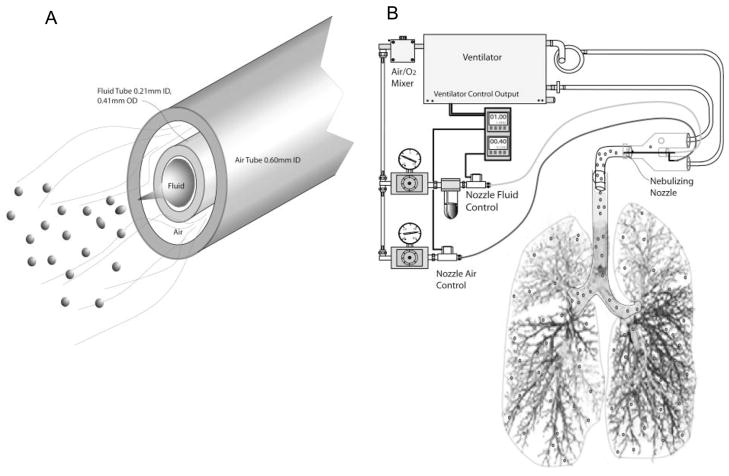
We developed a novel viscous lipid formulation nebulization nozzle and control system in our laboratory and adapted this system to a Siemans® 900c servo ventilator (Siemans-Elema AB, Sweden) as shown in Fig. 1. Briefly, the nebulizing nozzle (Fig. 1A) is fabricated from hypodermic needle stock material with a center fluid delivery tube and an outer air delivery tube. Calibrated blood counting slides were waved through the ventilator inspiratory airflow containing the nebulized flax oil formulations allowing droplets to impact onto the slide. The slides were then observed under light microscopy (200×) and sized visually and counted. The vast majority (100:1) of observed impacted deformed and flattened droplets larger than 2 μm and smaller than 10 μm in diameter were conservatively considered to be in the 2–5 μm spherical range. The FO and gT+FO were nebulized using a 0.203mm fluid channel in the nebulizing nozzle. Saline was nebulized using a 0.0152mm fluid channel. The smaller nozzle size was used for the much less viscous saline. Droplets in both instances were in the 2–5 μm range.
Fig. 1.
Novel Lipid Nebulization Device Schema. Fluid tube (0.21 mm ID, 0.41 mm OD) was inserted into air tube (0.60 mm OD) in spray nozzle (A). The spray nozzle was directly put into the center of tracheotomy tube. The nebulization system was synchronized with ventilation expiratory cycle. The delivery rate was adjusted by changing nozzle fluid control pressure (B).
The output end of the nebulizing nozzle is positioned in the center of flow within the “Y” connector of the ventilator circuit immediately adjacent to and directed toward the tracheotomy tube connector. The nozzle is fed from an air/liquid flow control system adapted to and controlled by the electronic output of the 900c ventilator. The oxygen-air mixer blending the inspired air for the ventilator is tapped to provide the nebulizing air supply providing the nebulization air at the same FiO2 as the ventilator air FiO2. The control cycle timers are programmed to provide 1.0 second of air flow and 0.4 seconds of fluid flow with each inspiration cycle. The fluid flow and subsequent nebulization is configured to occur in the first 0.6 seconds of inspiration thereby providing the nebulized droplets at the beginning of the inspired air flow into the lungs. This nebulizer configuration provides an additional inspired air flow volume of 50 mL/second/breath which was deducted from the total tidal volume.
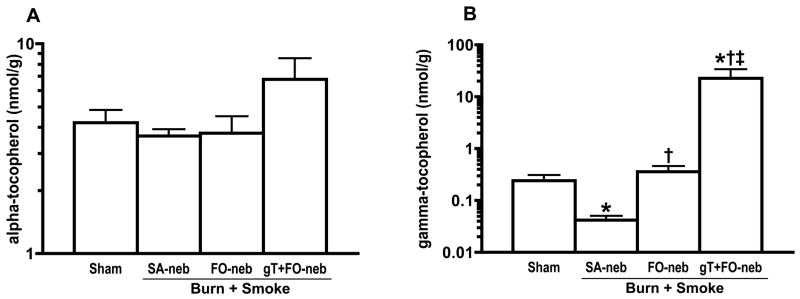
Cold pressed, filtered flax seed oil (Spectrum Organic Products LLC, Melville, N.Y.) was used alone and as a carrier for 8.3% solution w/w of mixed tocopherols (Decanox™ MTS-90G, a gift from Dr. Brent Flickinger, Archer Daniels Midland Co., Decatur, IL.) which was measured to contain gT (610 mg/g) and aT (91 mg/g). The flax oil mixtures were sterile filtered through 0.22 μm pore filter prior to use. Flax oil alone or flax oil containing 8.3% Decanox™ was administered by continuous pulse nebulization synchronized with the inspiration cycle at a rate of 0.45–0.5 ml/hour or 11–12 ml/24 hours. Lung tissue gT concentrations (Fig. 2) demonstrate that the aerosolized material was deposited into the lung.
Fig. 2.
Concentrations of alpha and gamma-tocopherol in lung tissue (A and B). Right lower lobe was collected for the measurement of lung tissue tocopherol levels. Burn and smoke inhalation injury significantly reduced gT concentration level in lung tissue. However, gT nebulization significantly increased gT concentration in lung tissue. aT concentration in lung was slightly, but not significantly, increased in gT+FO-neb. Data are expressed as mean ± SEM. Θ p < 0.05; significantly different from Sham, † p < 0.05; significantly different form SA-neb, ‡ p < 0.05; significantly different from FO-neb.
Measured variables
Arterial and mixed venous blood samples were taken at different time points for measurement of blood gases (IL GEM Premier 3000 Blood Gas Analyzer; GMI, Minnesota). PaO2/FiO2 ratio, which reflects the status of oxygenation, was calculated from the concentration of oxygen an animal is breathing (FiO2) and the PaO2 value from arterial blood gas. Pulmonary shunt fraction, which shows oxygen-transfer efficiency, was calculated using standard equations. The pulmonary microvascular fluid flux was evaluated by measuring the lung lymph flow. Sheep were sacrificed under deep ketamine anesthesia 48 h after injury. The right lung was then removed, and a 1-cm-thick section was taken from the middle of the lower lobe, injected with 10% formalin, and immersed in formalin. Four tissue samples were taken at predetermined sites for histological examination. Fixed samples were embedded in paraffin, sectioned at 4μm, and stained with hematoxylin and eosin. A pathologist without knowledge of the group assignments evaluated the lung histology. Levels of airway obstruction were obtained with a standardized protocol. Fifteen bronchi were investigated, and the percentage of area obstructed by the cast was estimated (0%–100%) [32]. The remaining lower one-half of the right lower lobe was used for the determination of bloodless wet-to-dry weight ratio [33].
Alpha-Tocopherol and Gamma-Tocopherol Measurement
A modification of the method by Podda and colleagues [34] was used for aT and gT analyses, as described previously [26]. Briefly, tissue (~50 mg) or plasma (100 μL) was saponified with alcoholic KOH, extracted with hexane, the extract dried under nitrogen, the residue resuspended in 1:1 ethanol-methanol, then injected into an HPLC system. Tocopherols were detected using an electrochemical detector, and quantitated by comparison to authentic standards.
Myeloperoxidase Measurement
The activity of myeloperoxidase (MPO), an indicator of neutrophil accumulation, is determined directly in whole lung homogenates. MPO concentrations were evaluated on homogenized right lung with a commercially available assay. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance at 450 nm during a 10 minute incubation. Myeloperoxidase activity was adjusted by lung tissue bloodless wet-dry ratio and reported as U/g dry tissue.
Malondialdehyde Measurement
Malondialdehyde (MDA) concentrations were utilized to estimate the lipid peroxidation in the lung and were measured as thiobarbituric acid reactive material. Lung tissue MDA levels were quantified with a commercially available assay (Northwest Life Science Specialties, Vancouver, WA). The level of lipid peroxides is expressed as MDA per milligram protein.
3-Nitotyrosine, IL-6, IL-8 protein Measurement
Lung tissue was homogenized in phosphate buffered saline containing 1 mM phenylmethylsulfonylfluoride (PMSF) and protease inhibitor cocktail (Sigma, St. Louis. MO). The lysate was centrifuged at 14,000g for 5 minutes to remove cellular debris. Protein concentration in the supernatant was determined by Bradford assay (Biorad, Hercules,CA). Seventy-five micrograms of protein was resuspended in sample loading buffer, boiled and resolved in 4–20% SDS-PAGE (Biorad). Protein was electrophoretically transferred to a polyvinyl difluoride membrane in Tris-glycine-methanol buffer at 55 volt for 2 hour. After transfer, the membrane was rinsed in water and then blocked in 5% nonfat milk in TBS buffer (50mM Tris.Hcl, pH 7.4, 150mM Nacl, 0.05% Tween 20). The blot was then probed with Anti-Nitrotyrosine antibody (Upstate)1:1000, Anti-IL-6 antibody (Chemicon) 1: 500, Anti-IL-8 antibody (Santa Cruz) 1:1000 in 5% Bovine serum albumin, 0.1% TBS-Tween 20 buffer overnight. The blot was washed 5 times in TBS-Tween 20 buffer and then incubated with Horseradish peroxidase conjugated anti-rabbit IgG for an hour. After washing, immunoreactive bands were detected by using the enhanced chemiluminiscence kit (Amersham, Piscataway, NJ) and exposure to an X-ray film. Relative density of bands was then scanned and quantitated using Image J software.
Immunohistochemistry of Poly (ADP-ribose) polymerase activity
For the immunohistochemical detection of poly ADP-ribose (PAR), monoclonal anti-PAR antibody (Calbiochem, San Diego, CA, USA) (1:1000, overnight, 4°C) was used after antigen retrieval. Secondary labeling was achieved by using biotinylated horse anti-mouse antibody (Vector Laboratories, Burlingame, CA, USA) (30min room temperature). Horseradish peroxidase-conjugated avidin (30 min, room temperature) and brown colored diaminobenzidine (6min, room temperature) was used to visualize the labeling (Vector Laboratories, Burlingame, CA, USA). The sections were counterstained with hematoxylin (blue color).
The intensity of PAR staining of individual sections was determined by a blinded experimenter according to a semiquantitative PAR-positivity score from 1–10. (1: no staining, 2: light cytoplasmic staining, 3: few positive nuclei, 4: light nuclear staining in approximately 10% of cells, 5: light nuclear staining in approximately 25% of cells, 6: light nuclear staining in approximately 50% of cells, 7: strong nuclear staining in approximately 50% of cells, 8: approximately 75% of the nuclei are positive, 9: approximately 90% of the nuclei are positive, 10: few negative cells) [35, 36]
IL-6 and IL-8 mRNA Measurement
Lung tissue was excised at the time of sacrifice and immersed in liquid N2. Total RNA is obtained using a commercially available total RNA purification kit, Purescript TM (Gentra Systems, Inc., Minneapolis, MN). Briefly 100 mg of the freshly frozen lung was lysed and homogenized using a mortar and pestle with 3 ml of lysis buffer containing EDTA, citric acid, and SDS according to the manufacturer’s protocol, except that the homogenized tissue was incubated overnight at room temperature in the lysis buffer. Precipitation buffer was added and incubated 10 min on ice to precipitate protein and DNA and centrifuged at 3,000 g. The supernatant was placed in 3 ml of isopropanol and centrifuged at 3,000 g for 5 min. The pellet was washed with 3 ml of 70% ethanol, centrifuged again and air-dried for 10 min. The pellet was resuspended in DEPC-treated water. Total RNA was quantitated spectrophotometrically at 260 nm. Quality of the isolated RNA was controlled by measuring the ratio of 28s/18s rRNA. Messenger RNA was isolated from the total RNA by the Straight A’s TM mRNA Isolation System (Novagen, Madison, WI) purification procedure in which mRNA was first hybridized to oligo dT coupled magnetic beads, washed, and then eluted to obtain polyadenylated mRNA according to the manufacturer’s protocol. First strand cDNA was synthesized by reverse transcription of the mRNA samples using MMLV-derived reverse transcriptase (Perkin Elmer, Branchburg, NJ) and random hexamers for priming according to standard techniques [37]. The cDNA was then used as a template for real-time PCR. Primers and probes were designed using a commercial online primer design program (Biosearch Technologies, Inc., Novato, CA) and purchased from the same company. QPCR was performed with a RotorGene 3000 (Corbett Research, San Francisco, CA). The reaction mixtures consisted of dilutions of cDNA from 256 ng of RNA, primer and probe concentrations, and subjected to amplification using a final, optimized concentration of MgCl2, 0.375 U of Taq polymerase (AmpliTaq, Perkin-Elmer) and 0.2 mM dTP’s in a reaction volume of 15 μl. The mixtures were amplified for 40 cycles at a melting temperature of 95° C for 10 min, an annealing temperature of 55° C for 10s, and extension at 60° C for 45s.The threshold amplifications (Ct) for each dilution, and reaction efficiencies were determined for each analyte using RotorGene software (Corbett Research). The copy numbers were normalized between samples using GAPDH copy numbers obtained by determination of GAPDH copy number using an external standard constructed from the v-erb gene. All results were expressed as copy numbers per μg of total RNA.
Statistical analysis
Summary statistics of data are expressed as means ± standard error of the mean. Statistical significance was determined using a two-factor analysis of variance with repeated measures. The two factors were treatment and time. Fisher’s least significant difference procedure with Bonferoni’s adjustment for number of comparisons is used for the multiple comparisons (or post-hoc analysis). Effects and interactions were assessed at the p<0.05 level of significance.
Results
All animals survived the 48 h experimental period after the combined injury with 40% TBSA burn and smoke inhalation. Since vitamin E may decrease platelet adhesion [38], we evaluated the clotting time. The nebulization did not result in a bleeding tendency in any of the groups. The activated clotting time was 144 ± 14 s at baseline, 163 ± 3 s at 24h, and 160 ± 10 s at 48 h in the gT+FO group and 158 ± 3 s at baseline, 177 ± 10 s at 24h, and 183 ± 12 s at 48 h in the nebulized saline group. There was no significant difference in each group.
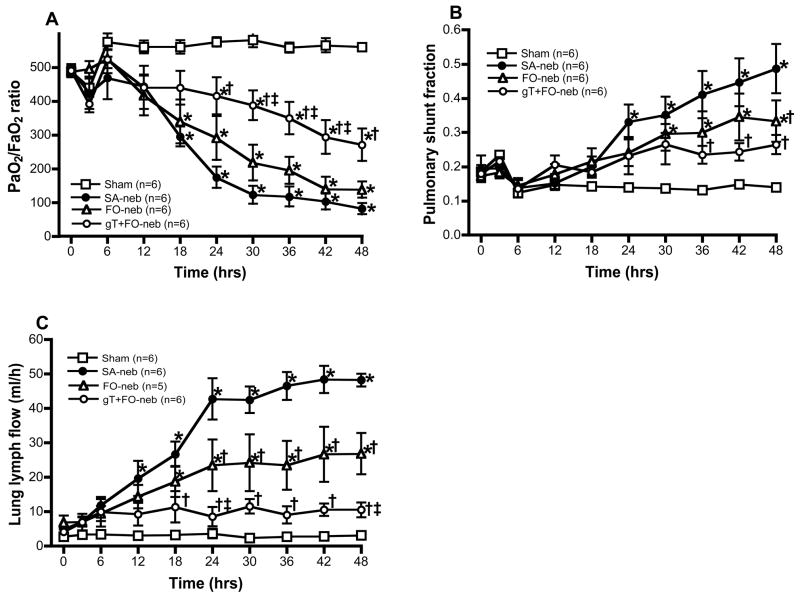
Burn and smoke inhalation injury significantly reduced the gT levels in lung tissue. However, gT concentrations were significantly increased in the gT+FO nebulization group compared with the other groups. The lung aT concentrations were somewhat increased but there was no significant difference in aT concentration levels (Fig. 2). No increases were found in plasma gT (data not shown) documenting that the gT administration is confined to the lung. PaO2/FiO2 ratio (Fig. 3A) was markedly decreased in animals that were nebulized with saline (injured) as compared with sham animals (uninjured). Nebulization of gT+FO attenuated the PaO2/FiO2 ratio decrease. The PaO2/FiO2 levels fell after injury to levels below 200, the threshold for diagnosis of the acute respiratory distress syndrome. Treatment with gT prevented this fall in oxygenation. Mean PaO2/FiO2 ratio was 270 in gT group, but only one animal decreased to 158 at 48h; all the other animals’ PaO2/FiO2 ratios were above 200. Statistically significant differences were observed at 24, 30, 36, 42 and 48 h compared with the SA-neb group and at 30, 36, and 42 h compared with FO-neb group. An increase in pulmonary shunt fraction (Fig. 3B) seen in the SA-neb group was significantly attenuated by FO nebulization at 48h and gT+FO nebulization at 36, 42 and 48 h after the combined injury.
Fig. 3.
Effect of gT nebulization on physiological changes. PaO2/FiO2 ratio is an index of pulmonary gas exchange, which reflects the status of oxygenation. Burn and smoke inhalation injury decreased PaO2/FiO2 ratio in FO-neb and SA-neb groups. However, gT nebulization significantly attenuated this change from 24 h (A). Pulmonary shunt fraction shows oxygen-transfer efficiency. Burn and smoke inhalation injury deteriorated the pulmonary shunt fraction. However, gT nebulization significantly attenuated this change from 36h (B). Lung lymph flow shows pulmonary transvascular fluid flux. There were significant increases in FO-neb and SA-neb groups. gT nebulization significantly attenuated this change from 18 h (C). Data are expressed as mean±SEM. Θ p < 0.05; significantly different from Sham, † p < 0.05; significantly different form SA-neb, ‡ p < 0.05; significantly different from FO-neb.
Lung lymph flow, a characteristic of pulmonary transvascular fluid flux, was markedly increased in injured, saline nebulized animals compared with the sham group (Fig. 3C). The lymph flow began to increase 12 h after the insult and a peak was observed at 42 h. However, gT+FO nebulization reversed this increase in pulmonary transvascular fluid flux and significant differences were observed between gT+FO-neb and SA-neb groups at 18, 24, 30, 36, 42 and 48 h, and gT+FO-neb and FO-neb groups at 24 and 48 h after the combined injury.
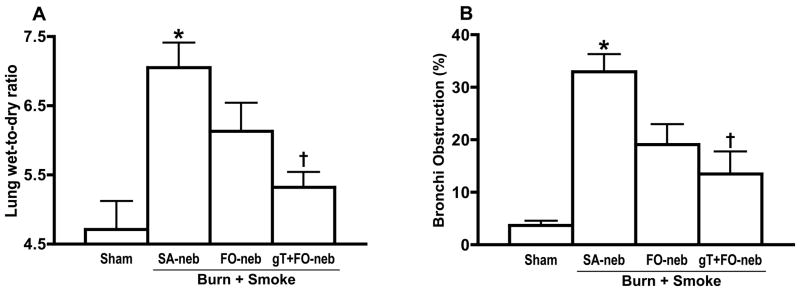
Lung bloodless wet-to-dry weight ratio, a measure of lung water content, was significantly increased at 48 h after insult in the SA-neb group as compared with the sham group (Fig. 4A). However, gT+FO nebulization significantly reduced the edema. The airway obstruction score revealed a significant increase in mean obstruction of bronchi (Fig. 4B) in the saline group as compared with the sham group. Treatment with gT+FO nebulization significantly reduced the obstruction score.
Fig. 4.
Lung wet-to dry ratio shows water content in lung tissue. There was significant increase in SA-neb group. gT nebulization significantly attenuated the change (A). The lung histology was evaluated by pathologist to determine the percentage of airway obstruction. There was significant increase in SA-neb group. gT nebulization significantly attenuated the change. Data are expressed as mean±SEM. Θ p < 0.05; significantly different from Sham, † p < 0.05; significantly different form SA-neb.
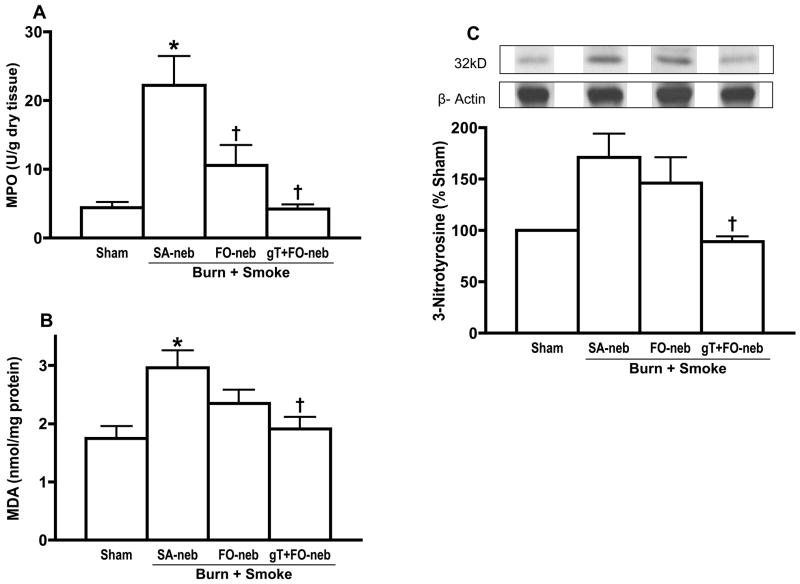
Lung myeloperoxidase activity is an indicator of neutrophil accumulation. Burn and smoke inhalation injury significantly increased myeloperoxidase activity in lung. However, both FO and gT+FO nebulization abrogated changes in myeloperoxidase (Fig. 5A). Fig. 5B shows the effect of gT+FO nebulization on malondialdehyde concentration which is an index of lipid peroxidation (ROS) in lung tissue. Injury increased malondialdehyde concentrations (saline group as compared with the sham group), while gT+FO nebulization prevented the increase. 3-Nitrotyrosine is a marker of nitrosative stress, resulting from reactive nitrogen species (RNS) such as peroxynitrite. Burn and smoke injury caused a marked increase in lung 3-nitrotyrosine 48 h after the insult. gT+FO nebulization significantly prevented the increase in 3-nitrotyrosine (Fig. 5C).
Fig. 5.
The activity of myeloperoxidase (MPO), an indicator of neutrophil accumulation, was remarkably increased in SA-neb group. gT and FO nebulization significantly attenuated the change (A). Malondialdehyde (MDA) shows lipid peroxidation in lung tissue. There was significant increase in saline group. gT nebulization significantly attenuated the change (B). 3-Nitrotyrosine is one of index of peroxynitrite. There was significant increase in saline group. gT nebulization significantly attenuated the change (C). Data are expressed as mean±SEM. Θ p < 0.05; significantly different from Sham, † p < 0.05; significantly different form SA-neb.
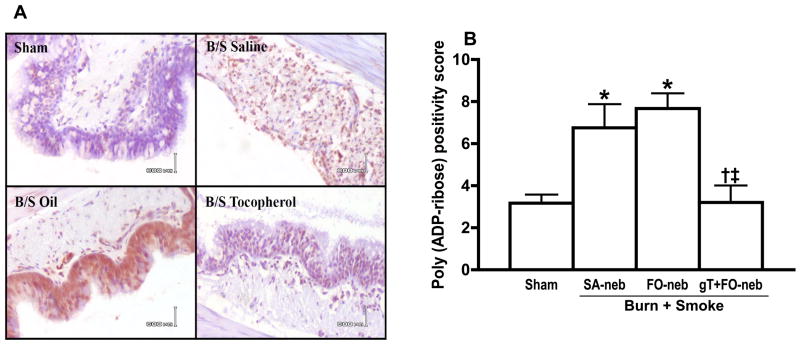
After burn and smoke injury, there was a marked increase in poly (ADP-ribose) polymerase activity in the SA-neb and FO-neb groups. Treatment with gT+FO nebulization prevented this increase in activity (Fig. 6A). Fig. 6B shows the poly (ADP-ribose) positivity score graph which quantified the degree of poly (ADP-ribose) histochemical stain. Burn and smoke injury caused a significant increase in lung poly (ADP-ribose) polymerase activity. However, gT+FO nebulization significantly prevented the increase in lung poly (ADP-ribose) polymerase activity.
Fig. 6.
Effect of gT nebulization on Poly (ADP-ribose) Polymerase Activity in lung tissue. Poly (ADP-ribose) was stained by monoclonal antibody (A). Immunohistochemistry stain slide was semi-quantified and scored. There were significant increases in FO-neb and SA-neb group. gT nebulization significantly attenuated the change (B). Data are expressed as mean±SEM. Θ p < 0.05; significantly different from Sham, † p < 0.05; significantly different form SA-neb, ‡ p < 0.05; significantly different from FO-neb.
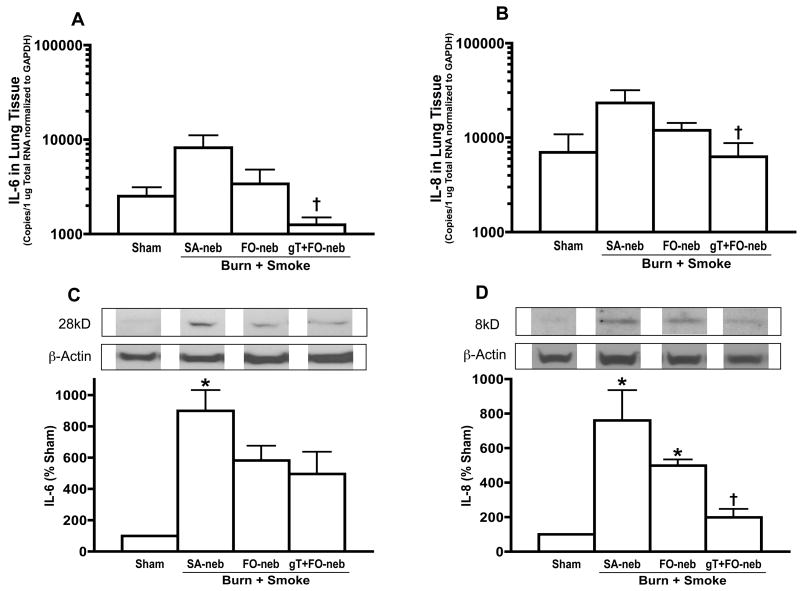
To determine the pro-inflammatory chemokines, IL-6 and IL-8 mRNA and protein were measured in lung tissue. Burn and smoke injury caused a significant increase in lung IL-6 and IL-8 protein at 48 h after the insult. gT+FO nebulization prevented the increase in both IL-6 and IL-8 mRNA and protein, respectively (Fig. 7).
Fig. 7.
Effect of gT nebulization on IL-6, IL-8 mRNA and protein in lung tissue. mRNA was normalized to GAPDH. Burn and smoke inhalation injury increased IL-6 and IL-8 protein levels in SA-neb group. gT nebulization significantly attenuated the elevation of IL-6 and IL-8 in both of mRNA and protein. Data are expressed as mean±SEM. Θp < 0.05; significantly different from Sham, † p < 0.05; significantly different form SA-neb.
Discussion
In the present study, the sheep that had burn and smoke injuries and were treated with gT+FO nebulization or with FO nebulization alone, demonstrated more effective pulmonary gas exchange (PaO2/FiO2 and pulmonary shunt fraction) and less pulmonary microvascular leakage (lung lymph flow and lung water content). We hypothesized that delivery of gT into the airway would place gT at the site of injury so that it would act as an antioxidant, scavenge RNS and attenuate acute lung injury following combined burn and smoke inhalation injury.
In our present study, the plasma aT and gT levels did not change dramatically. We have previously published the sheep plasma aT levels and their response to injury [39]. In our present study, the plasma aT and gT levels did not change dramatically. We have previously published the sheep plasma aT levels and their response to injury [39]. Sheep have markedly lower circulating lipid concentrations than do humans, so extrapolation to humans relative to plasma concentrations is not particularly useful. That being the case, our group has also reported that plasma levels in burned patients can fall dramatically in some cases to concentrations almost at the levels of detection [18]. The levels of lung tissue were 21 nmol/g and 25 nmol/g in two cadaver patents [40], 20 nmol/g in swine [41]. There is a report to show aT levels in bronchoalveolar lavage (BAL), the aT levels in BAL was 0.3 μM [42]. However, the lung aT concentration (5.8±0.4 nmol/g) in sheep was lower than human and swine lung [39], and as we showed previously, burn and smoke inhalation injury further reduced aT concentration in lung tissue [43]. In our present study, there were no significant differences in aT concentration in lung tissue; however, burn and smoke inhalation injury significantly reduced the gT concentration in lung tissue (Fig 2).
In our previous study, a commercially available emulsion of aT (Vital E, Schering Plough) was aerosolized with an ultrasonic nebulizer (DeVilbiss Pro-UltraNeb Large Volume Nebulizer-099HD) [26]. Decanox™ (ADM) is a commercially available source of mixed tocopherols that is a more viscous lipid material than Vital E and is more difficult to aerosolize using a commercially available nebulizer. Although we had some success with this device, the nebulizer was frequently damaged, consequently we designed a new nebulizer to aerosolize gT. Our novel lipid nebulization device has a suitable size spray nozzle to make 2.5–5.0 μm droplets and is synchronized with the ventilator to deliver material only during the inspiratory cycle. With this device, we can aerosolize viscous lipid materials effectively. Here we demonstrate that the gT concentration in lung tissue was significantly increased in gT+FO nebulization group (Fig. 2). We selected FO that has a high alpha-linolenic acid concentration as lipid carrier. Mineral oil and saturated oil like paraffin or Vaseline easily cause lipoid pneumonia but vegetable oil is less harmful to alveolar tissue [44, 45]. FO has paracellular permeability and has previously been used as absorption enhancer in pulmonary drug delivery [46, 47]. In the uninjured sham group, nebulization of FO did not produce any pulmonary injury (Fig. 3,4). Remarkably, FO administration prevented some of the injury in the animals given the double insult of smoke and burn (Fig. 3), possibly due to the high n-3 polyunsaturated fatty acid content that could be used by the tissue for synthesis of anti-inflammatory compounds, such as resolvins [48–50].
In the past we have reported evidence that activated polymorphonuclear cells (PMNs), especially neutrophils, release ROS and proteases that damage the parenchyma after insult of smoke and burn [51, 52]. Several studies on burn and smoke inhalation injury have demonstrated that myeloperoxidase, an indicator of neutrophil accumulation, appear in lung parenchyma [53]. The level of myeloperoxidase was significantly less in the sheep treated with FO and gT+FO nebulization (Fig. 5A) suggesting that gT, as well as FO, reduced the neutrophil accumulation and decrease their degree of activation. Superoxide also played a crucial role in mediating the lung injury associated with smoke inhalation [4]. Lipid peroxides appear in the systemic circulation within minutes after injury [3, 18]. The level of malondialdehyde in lung tissue at 48 h was used as a marker of lipid peroxidation of the cell membrane [54]. Malondialdehyde was significantly less in the sheep treated with gT+FO nebulization (Fig. 5B), which shows that gT scavenged ROS. We also measured lung tissue 3-nitrotyrosine as an index of formation of RNS, such as peroxynitrite [55]. Direct administration of gT, compared with the saline, significantly decreased the lung tissue of 3-nitrotyrosine (Fig. 5C). Beckman and colleagues [55] reported that the prevention of decomposition of NOx by scavenging superoxide anion using superoxide dismutase (SOD) reduced the tissue 3-nitrotyrosine content. We have previously measured the chemical content of the smoke from burning cotton [56]. The majority of the offending materials were aldehydes and acrolein. The latter has been considered to be the most important of the reactive species [57] and cutaneous burn wound itself also generates RNS [58]. In our present study, we were not able to detect the RNS and ROS time course changes, however MPO, MDA, 3-nitrotyrosine significantly increased at 48h in control group, and these parameters were significantly reduced in gT+ FO neb group. We were not also able to show reactive nitrogen species in the smoke, but these results strongly suggested that burn and smoke inhalation injury cased an amount of RNS as well as ROS in lung tissue. gT is a potent RNS scavenger, as well as ROS and several articles supported that gT and RNS interaction [59–62]. In our study, we speculate that gT delivery into airway may scavenge RNS, as well as ROS.
Peroxynitrite is a potent oxidant produced by the reaction of NOx and superoxide anion [63, 64] and is produced in burn and smoke models of injury [58, 65]. Peroxynitrite induces the development of DNA single-strand breakage with resulting poly (ADP-ribose) polymerase activation. Intracellular NAD and ATP levels are depleted as a consequence of poly (ADP-ribose) polymerase activation. Depletion of the high-energy phosphates could lead to apoptosis and/or death of the cells and thus injure various organs. PARP activation can also lead to activation of nuclear factors and thus up regulation of nitric oxide synthase and a number of cytokines [66]. Depletion of cellular NAD leads to inhibition of ATP generating pathways, which leads to cellular dysfunction. In our study, poly (ADP-ribose) polymerase activity was significantly reduced by gT suggesting that cellular dysfunction was prevented because the gT scavenged ROS, RNS (Fig. 6).
In addition, it was recently reported that poly (ADP-ribose) polymerase activates NF-kB and thereby the expression the inducible isoform of NO synthase (iNOS) as well as the production of various proinflammatory cytokines such as IL- 6, IL-8 etc and chemokine mediators [67–69]. In our study, the dual injury from burn and smoke inhalation significantly increased IL-6 and IL-8 protein levels. However, gT attenuated the increase of IL-6, IL-8 mRNA and protein (Fig. 7). Given the role of reactive nitrogen and oxygen species in triggering the response to inhalation injury, it is probable the changes in cytokines and other mediators we identify following the double injury of combined smoke and burn injuries may the result of scavenging these species. The prevention of cytokine expression, NOx elevation, 3-nitrotyrosine formation and PARP activation by gT delivery into airway supports this hypothesis.
Overall, in the present study, we report that 1) burn and smoke injury significantly reduces gT concentrations in lung tissue, as well as increases severe signs of acute lung injury as evidenced by deteriorated pulmonary gas exchange, massive airway obstruction, pulmonary edema and excessive production of RNS and ROS. 2) FO nebulization itself attenuates many of the above pathological changes. 3) gT combined with FO nebulization had a greater effect than FO alone on these pathological changes.
Pulmonary gT delivery may be a novel effective treatment strategy in management of patients with acute lung injury after burn and smoke injury.
Acknowledgments
This work was supported by National Institute for General Medical Sciences Grant GM66312, GM060688 and Grants 8954, 8450 and 8460 from the Shriners of North America.
Abbreviations
- gT
gamma-Tocopherol
- aT
alpha-Tocopherol
- FO
flaxseed oil
- SA-neb
saline nebulization
- FO-neb
flaxseed oil nebulization
- gT+FO-neb
gamma-Tocopherol and flaxseed oil nebulization
- RNS
reactive nitrogen spices
- ROS
reactive oxygen spices
- MPO
myeloperoxidase
- MDA
malondialdehyde
- PAR
poly ADP-ribose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saffle J, Davis B, Williams P The American Burn Association Registry Participant Group. Recent outcomes in the treatment of burn injury in the United States: a report from the American Burn Association Patient Registry. J Burn Care Rehabil. 16:219–232. doi: 10.1097/00004630-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Barrow R, Spies M, Barrow L, Herndon D. Influence of demographics and inhalation injury on burn mortality in children. BURNS. 30:72–77. doi: 10.1016/j.burns.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Demling R, Ikegami K, Lalonde C. Increased lipid peroxidation and decreased antioxidant activity correspond with death after smoke exposure in the rat. J Burn Care Rehabil. 16:104–110. doi: 10.1097/00004630-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen T, Cox C, Herndon D, Biondo N, Traber L, Bush P. Effects of manganese superoxide dimutase on lung fluid balance after smoke inhalation. J Appl Physiol. 78:2161–2168. doi: 10.1152/jappl.1995.78.6.2161. [DOI] [PubMed] [Google Scholar]
- 5.Soejima K, Traber L, Schmalstieg F, Hawkins H, Jodoin J, Szabo C. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 163:745–752. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 6.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips G. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 167:1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 7.Traber D, Hawkins H, Enkhbaatar P, Cox R, Schmalstieg F, Zwischenberger J, Traber L. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. pulmonary pharmacology and therapeutics. 20:163–166. doi: 10.1016/j.pupt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Ischiropoulos H, Zhu L, Beckman J. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 9.Carreras M, Pargament G, Catz S, Poderoso J, Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 341:65–68. doi: 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 10.Bose M. Immunol Lett. 48:59–64. doi: 10.1016/0165-2478(95)02444-1. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C. Potential role of the peroxinitrate-poly(ADP-ribose) synthetase pathway in a rat model of severe hemmorrhagic shock. SHOCK. 9:341–344. doi: 10.1097/00024382-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Dawson V, Dawson T, Synder S. Nitric oxide Activation of poly(ADP-ribose) Synthetase in Neurotoxicity. Science. 263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 13.Szabo C. Mechanisms of cell necrosis. Crit Care Med. 2005;33:S530–S534. doi: 10.1097/01.ccm.0000187002.88999.cf. [DOI] [PubMed] [Google Scholar]
- 14.Boulares A, Zoltoski A, Sherif Z, Jolly P, Massaro D, Smulson M. Gene konckout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am J Respir Cell Mol Biol. 28:322–329. doi: 10.1165/rcmb.2001-0015OC. [DOI] [PubMed] [Google Scholar]
- 15.Chiarugi A, Moskowitz A. Poly(ADP-ribose) polymerase-1 activity promotes NF-kappa-B-dreven transcription and microglial activation: implication for neurodegenerative disorders. J Neurochem. 85:306–317. doi: 10.1046/j.1471-4159.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- 16.Hauschildt S, Scheipers P, Bessler W. Inhibitor of poly (ADP-ribose) polymerase suppress lipopolysaccharide-induced nitrite formation in macrophages. Biochem Biophys Res Commun. 179:865–871. doi: 10.1016/0006-291x(91)91898-m. [DOI] [PubMed] [Google Scholar]
- 17.Ibuki Y, Mizuno S, Goto R. gamma-irradiation-induced DNA damage enhances NO production via NF-kappaB activation in RAW264.7 cells. Biochm Biophys Acta. 1593:159–167. doi: 10.1016/s0167-4889(02)00385-3. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen T, Cox C, Traber D, Gasser H, Redl H, Schlag G, Herndon D. Free radical activity and loss of plasma antioxidants, vitamin E, and sulfhydryl groups in patients with burns: the 1993 Moyer Award. J Burn Care Rehabil. 14:602–609. doi: 10.1097/00004630-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kagan V. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. Ann N Y Acad Sci. 570:121–135. doi: 10.1111/j.1749-6632.1989.tb14913.x. [DOI] [PubMed] [Google Scholar]
- 20.Podda M, Weber C, Traber M, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 37:893–901. [PubMed] [Google Scholar]
- 21.Machlin L. Vitamin E. New York: Marcel Dekker; 1991. [Google Scholar]
- 22.Cooney R, Franke A, Harwood P, Hatch–Pigott V, Custer L, Mordan L. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc Natl Acad Sci USA. 90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 24.Christen S, Woodall A, Shigenaga M, Southwell-Keely P, Duncan M, Ames B. γ-Tocopherol traps mutagenic electrophiles such as NOx and complements α-tocopherol: Physiological implications. Proc Natl Acad Sci USA. 94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoglen N, Waller S, Sipes I, Liebler D. Reactions of Peroxynitrite with γ-Tocopherol. Chem Res Toxicol. 10:401–407. doi: 10.1021/tx960200h. [DOI] [PubMed] [Google Scholar]
- 26.Morita N, Traber M, Enkhbaatar P, Westphal M, Murakami K, Leonard S, Cox R, Hawkins H, Herndon D, Traber L, Traber D. Aerosolized alpha-tocopherol ameriorates acute lung injury followin combined burn and smoke inhalation injury in sheep. SHOCK. 25:277–282. doi: 10.1097/01.shk.0000208805.23182.a7. [DOI] [PubMed] [Google Scholar]
- 27.Heller A, Theilen H, Koch T. Fish or Chips? News Physiol Sci. 18:50–54. doi: 10.1152/nips.01419.2002. [DOI] [PubMed] [Google Scholar]
- 28.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, McGuire R, Schmalstieg F, Cox R, Hawkins H, Jodoin J, Lee S. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole attenuates acute lung injury in an ovine model. Am J Physiol Regul Inter Comp Physiol. 2003;285:R366–R372. doi: 10.1152/ajpregu.00148.2003. [DOI] [PubMed] [Google Scholar]
- 29.Staub N, Bland R, Brigham K, Demling R, Erdmann A, Woolverton W. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 19:315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.Traber D, Adams T, Henriksen NJ, Traber D, Thomson P. Reproducibility of cardiopulmonary effects of different endotoxins in the same sheep. J Appl Physiol. 54:1167–1171. doi: 10.1152/jappl.1983.54.4.1167. [DOI] [PubMed] [Google Scholar]
- 31.Vidal Melo M, Haris R, Layfield D, Musch G, Venegas J. Changes in regional ventilagtion after autologous blood clot pulmonary embolism. Anesthesiology. 97:671–681. doi: 10.1097/00000542-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Cox RABA, Soejima K, Murakami K, Katahira J, Traber LD, Herndon DN, Schmalstieg FC, Traber DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 29:295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 33.Pearce MLYJ, Beazell J. Measurement of pulmonary edema. Circ Res. 16:484–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- 34.Podda M, Weber C, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 37:893–901. [PubMed] [Google Scholar]
- 35.Beller CJRT, Kosse J, Gero D, Szabo C, Szabo G. Activation of the peroxynitrite-poly (adenosine diphosphate-ribose) polymerase pathway during neointima proliferation: A new target to prevent restenosis after endarterectomy. Journal of Vascular Surgery. 43:824–830. doi: 10.1016/j.jvs.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Toth-Zsamboki E, Horvath E, Vargova K, Pankotai E, Murthy K, Zsengeller Z, Barany T, Pek T, Fekete K, Kiss RG, Preda I, Lacza Z, Gero D, Szabo C. Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes. Molecular medicine (Cambridge, Mass. 12:221–228. doi: 10.2119/2006-00055.Toth-Zsamboki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Freedman JE, Farhat JH, Loscalzo J, Keaney JF., Jr alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation. 94:2434–2440. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 39.Traber MG, Shimoda K, Murakami K, Leonard SW, Enkhbaatar P, Traber LD, Traber DL. Burn and smoke inhalation injury in sheep depletes vitamin E: kinetic studies using deuterated tocopherols. Free radical biology & medicine. 42:1421–1429. doi: 10.1016/j.freeradbiomed.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. The American journal of clinical nutrition. 67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 41.Lauridsen C, Engel H, Jensen SK, Craig AM, Traber MG. Lactating sows and suckling piglets preferentially incorporate RRR- over all-rac-alpha-tocopherol into milk, plasma and tissues. J Nutr. 132:1258–1264. doi: 10.1093/jn/132.6.1258. [DOI] [PubMed] [Google Scholar]
- 42.van der Vliet A, O’Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. The American journal of physiology. 1999;276:L289–296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- 43.Morita NSK, Traber M, Westphal M, Enkhbaatar P, Murakami K, Leonard S, Traber L, Traber D. Vitamin E attenuates acute lung injury in sheep with burn and smoke inhalation injury. Redox Report. 11:61–70. doi: 10.1179/135100006X101020. [DOI] [PubMed] [Google Scholar]
- 44.Spickardlll A, Hirschmann J. Exogenous Lipoid Pneumonia. Arch Intern Med. 154:686–692. [PubMed] [Google Scholar]
- 45.Pinkerton H. The reaction to oils and fats in the lung. Archives of Pathology. 1927:380–401. [Google Scholar]
- 46.Wang L, Ma J, Pan W, Velasquez D, Malanga C, Rojonasakul Y. Alveolar Permiability Enhancement by Oleic Acid and Related Fatty Acids: Evidence for a Calciumn-Dependent Mechanism. Pharmaceuticla Research. 11:513–517. doi: 10.1023/a:1018906330308. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi S, Kondo S, Juni K. Study on Pulmonary Delivery of Salmon Calcitonin in Rates: Effects of Protease Inhibitors and Absorption Enhancers. Pharmaceuticla Research. 11:1239–1243. doi: 10.1023/a:1018926007902. [DOI] [PubMed] [Google Scholar]
- 48.Paschos GK, Rallidis LS, Liakos GK, Panagiotakos D, Anastasiadis G, Votteas V, Zampelas A. Background diet influences the anti-inflammatory effect of alpha-linolenic acid in dyslipidaemic subjects. The British journal of nutrition. 92:649–655. doi: 10.1079/bjn20041230. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American journal of clinical nutrition. 2006;83:1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 50.Das UN. Biological significance of essential fatty acids. The Journal of the Association of Physicians of India. 54:309–319. [PubMed] [Google Scholar]
- 51.Sakurai H, Schmalstieg FC, Traber LD, Hawkins HK, Traber DL. Role of L-selectin in physiological manifestations after burn and smoke inhalation injury in sheep. J Appl Physiol. 86:1151–1159. doi: 10.1152/jappl.1999.86.4.1151. [DOI] [PubMed] [Google Scholar]
- 52.Traber D, Herndon D, Enkhbaatar P, Maybauer MO, Maybauer DM. The pathophysiology of inhalation injury. London: W. S. SAUNDERS; 2007. [Google Scholar]
- 53.Shimoda K, Murakami K, Enkhbaatar P, Traber LD, Cox RA, Hawkins HK, Schmalstieg FC, Komjati K, Mabley JG, Szabo C, Salzman AL, Traber DL. Effect of poly(ADP ribose) synthetase inhibition on burn and smoke inhalation injury in sheep. American journal of physiology. 2003;285:L240–249. doi: 10.1152/ajplung.00319.2002. [DOI] [PubMed] [Google Scholar]
- 54.De Zwart L, Meerman J, Commandeur J, Vermeulen N. Biomarkers of Free Radical Damage Applications in Experimental Animals and in Humans. Free Rad Biol Med. 26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 55.Beckman J, Beckman T, Chen J, Marshall P, Freeman B. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura R, Traber LD, Herndon DN, Linares HA, Lubbesmeyer HJ, Traber DL. Increasing duration of smoke exposure induces more severe lung injury in sheep. J Appl Physiol. 64:1107–1113. doi: 10.1152/jappl.1988.64.3.1107. [DOI] [PubMed] [Google Scholar]
- 57.Hales CA, Barkin PW, Jung W, Trautman E, Lamborghini D, Herrig N, Burke J. Synthetic smoke with acrolein but not HCl produces pulmonary edema. J Appl Physiol. 64:1121–1133. doi: 10.1152/jappl.1988.64.3.1121. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira GV, Shimoda K, Enkhbaatar P, Jodoin J, Burke AS, Chinkes DL, Hawkins HK, Herndon DN, Traber L, Traber D, Murakami K. Skin nitric oxide and its metabolites are increased in nonburned skin after thermal injuries. Shock (Augusta, Ga. 22:278–282. doi: 10.1097/01.shk.0000135259.90311.33. [DOI] [PubMed] [Google Scholar]
- 59.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr. 17:296–304. doi: 10.1053/j.jrn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Papas KA, Sontag MK, Pardee C, Sokol RJ, Sagel SD, Accurso FJ, Wagener JS. A pilot study on the safety and efficacy of a novel antioxidant rich formulation in patients with cystic fibrosis. J Cyst Fibros. 7:60–67. doi: 10.1016/j.jcf.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Wu JH, Ward NC, Indrawan AP, Almeida CA, Hodgson JM, Proudfoot JM, Puddey IB, Croft KD. Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin Chem. 53:511–519. doi: 10.1373/clinchem.2006.076992. [DOI] [PubMed] [Google Scholar]
- 62.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 63.Brovkovych V, Patton S, Borvkovych S, Kiechle F, Huk I. In situ measurment of nitric oxide, superoxide and peroxinitrite during endotoxemia. J Physiol Pharmaco. 48:633–644. [PubMed] [Google Scholar]
- 64.Xia Y, Zweier J. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA. 94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soejima K, McGuire R, Snyder N, Uchida T, Szabo C, Salzman A, Traber L, Traber D. The effect of inducible nitric oxide synthase (iNOS) inhibition on smoke inhalation injury in sheep. SHOCk. 13:261–266. doi: 10.1097/00024382-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Espinoza LA, Smulson ME, Chen Z. Prolonged poly(ADP-ribose) polymerase-1 activity regulates JP-8-induced sustained cytokine expression in alveolar macrophages. Free radical biology & medicine. 42:1430–1440. doi: 10.1016/j.freeradbiomed.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 67.Szabo E, Virag L, Bakondi E, Gyure L, Hasko G, Bai P, Hunyadi J, Gergely P, Szabo C. Peroxinitrite Production, DNA Breakage, and Poly (ADP-ribose) Polymerase Activation in a Mouse Model of Oxazolone-Induced Contact Hypersensitivity. J Invest Dermatol. 117:74–80. doi: 10.1046/j.0022-202x.2001.01388.x. [DOI] [PubMed] [Google Scholar]
- 68.Hadda H, Rhinn H, Bloquel C, Coqueran B, Szabo C, Plotkine M, Scherman D, Margaill I. Anti-inflammatory effects of PJ34, a poly (ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. British Journal of Pharmacology. 149:23–30. doi: 10.1038/sj.bjp.0706837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliver F, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, Rubia G, Murica G. Resistance to endotoxic shock as a consequence of defective NF-kB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]