Abstract
Divalent metal ions play a critical role in the removal of N-terminal methionine from nascent proteins by methionine aminopeptidase (MetAP). Being an essential enzyme for bacteria, MetAP is an appealing target for the development of novel antibacterial drugs. Although purified enzyme can be activated by several divalent metal ions, the exact metal ion used by MetAP in cells is unknown. Many MetAP inhibitors are highly potent on purified enzyme, but they fail to show significant inhibition of bacterial growth. One possibility for the failure is a disparity of the metal used in activation of purified MetAP and the metal actually used by MetAP inside bacterial cells. Therefore, the challenge is to elucidate the physiologically relevant metal for MetAP and discover MetAP inhibitors that can effectively inhibit cellular MetAP. We have recently discovered MetAP inhibitors with selectivity toward different metalloforms of Escherichia coli MetAP, and with these unique inhibitors, we characterized their inhibition of MetAP enzyme activity in a cellular environment. We observed that only inhibitors that are selective for the Fe(II)-form of MetAP were potent in this assay. Further, we found that only these Fe(II)-form selective inhibitors showed significant inhibition of growth of five E. coli strains and two Bacillus strains. We confirmed their cellular target as MetAP by analysis of N-terminal processed and unprocessed recombinant glutathione S-transferase proteins. Therefore, we conclude that Fe(II) is the likely metal used by MetAP in E. coli and other bacterial cells.
Methionine aminopeptidase (MetAP)2 plays a critical role in maturation of proteins by catalyzing removal of the N-terminal initiator methionine from nascent proteins (1). Consistent with this role is the ubiquitous presence of the enzyme in prokaryotes and eukaryotes, and N-terminal methionine excision affects between 55 and 70% of proteins (2). MetAP is coded by a single gene in bacteria (type I or type II), while eukaryotic cells have both type I and type II MetAP enzymes. The lethal effect of MetAP gene deletion has been reported for Escherichia coli (3), Salmonella typhimurium (4), and Saccharomyces cerevisiae (5). Therefore, MetAP is an appealing target for the development of antibacterial and antifungal drugs with novel mechanisms of action (6). In addition, some anticancer compounds, such as fumagillin and bengamides, are potent inhibitors of mammalian MetAPs, possibly due to the involvement of MetAPs in angiogenesis and cell proliferation (7–10).
Divalent metal ions play an important role in the MetAP-catalyzed hydrolysis of proteins and peptides. Purified MetAP apoenzyme can be reproducibly activated by a number of divalent metal ions, such as Co(II), Mn(II), Ni(II), and Fe(II) (11, 12). Among them, Co(II) is one of the best activators of the enzyme, and many MetAP inhibitors were discovered by screening on purified MetAP with high Co(II) concentrations in the activity assay (13). Several x-ray structures of MetAP with or without an inhibitor bound showed that the catalytic site is a shallow pocket with two Co(II) ions situated at the bottom for a dinuclear arrangement (14). A protein or peptide substrate is believed to bind to the catalytic site with its N-terminal methionine and coordinate with the Co(II) ions around its scissile bond. A water molecule, often seen bridging the two Co(II) ions, attacks the scissile bond during the hydrolysis (15, 16). A modified mechanism based on a single metal ion at the active site is also proposed (17, 18). Although there is no question for a central role of divalent metal ions in catalysis, possibility exists that a divalent metal ion other than Co(II) may function as the activating metal for MetAP in a physiologically relevant environment. Walker and Bradshaw were the first who questioned the metal identity with observation of activation of S. cerevisiae MetAP in the presence of a physiological concentration of reduced glutathione and suggested the possibility of Zn(II) as the cofactor for yeast MetAP (19). Recent theoretical calculation also favors Zn(II) as the cofactor (20). Based on whole cell metal analyses and activity measurements of purified enzyme under anaerobic condition, D'Souza and Holz (11) suggested that E. coli MetAP functions as a Fe(II) enzyme. Wang et al. (21) analyzed inhibition of MetAP by two MetAP inhibitors, one non-selective for different metalloforms and the other lack of inhibition on the Mn(II)-form, and concluded that human type II MetAP is a Mn(II) enzyme.
Potent MetAP inhibitors have been identified, but none of the inhibitors have shown significant antibacterial activity (13, 22, 23). Although most of the current MetAP inhibitors inhibit the Co(II)-form of MetAP effectively, their potency on other metalloforms often has not been characterized. We reported that potent inhibitors of the Co(II)-form may or may not inhibit other MetAP metalloforms with a metal ion other than Co(II) at the catalytic site (12, 24). Therefore, it is plausible that lack of antibacterial activity by MetAP inhibitors is due to their inability to inhibit MetAP inside bacterial cells. It becomes critical to elucidate the native metal used by MetAP inside cells and identify MetAP inhibitors that can effectively inhibit the cellular MetAP.
By using high throughput screening of large chemical libraries, we have previously identified several small molecule MetAP inhibitors with high potency and superb selectivity toward either the Co(II)-form or the Mn(II)-form of E. coli MetAP (24). Recently, we discovered additional inhibitors with selectivity for the Fe(II)-form of E. coli MetAP.3 With these unique MetAP inhibitors available as research tools, we established a novel MetAP enzyme activity assay using live E. coli cells and characterized these metalloform-selective inhibitors on this cellular MetAP activity assay. We demonstrated that the Fe(II)-form selective inhibitors inhibit not only the cellular MetAP activity but also growth of bacterial cells. These findings shed important lights on the development of MetAP inhibitors as useful therapeutics.
EXPERIMENTAL PROCEDURES
Materials—Fluorogenic substrate, Met-AMC, is a methionine derivatized with 7-amino-4-methylcoumarin (AMC), which was purchased from Bachem Bioscience (King of Prussia, PA). Resazurin dye was purchased from Acros Organics (Morris Plains, NJ). Mueller Hinton broth and agar were purchased from Remel Products (Lenexa, KS). The pBACE vector with the cGSTA1 insert was generously provided by Prof. Ming F. Tam at Institute of Molecular Biology, Academia Sinica, Taiwan. The recombinant E. coli MetAP was expressed in E. coli BL21(DE3) cells and purified as an apoenzyme (12).
Bacterial Strains—Bacillus megaterium, Bacillus subtilis, and E. coli ATCC 25922 were acquired from Fisher Scientific. E. coli strains D22 (26), RL25 (27), and RL436 (27) were obtained from the E. coli Genetic Stock Center at Yale. E. coli AS19 strain was obtained as a gift from Prof. Liam Good at Karolinska Institute. Strain AS19 has a severely depleted lipopolysaccharides (LPS) layer (28), but its exact mutation is unknown.
MetAP Activity and Inhibition Assay on Purified Enzyme—Enzyme activity was monitored by fluorescence on a Spectra-Max Gemini XPS plate reader (Molecular Devices, Sunnyvale, CA), following hydrolysis of the fluorogenic substrate Met-AMC (λex 360 nm, λem 460) at room temperature (12, 24, 29). All kinetic experiments were carried out on 384-well plates with an 80-μl assay volume. The IC50 values were calculated from non-linear regression curve fitting of percent inhibitions as a function of inhibitor concentrations.
Cellular MetAP Activity and Inhibition Assay—BL21(DE3) cells overexpressing the recombinant E. coli MetAP were let to grow to exponential phase, harvested, and washed twice with water. The final cell pellet was resuspended in 10 mm CaCl2 in 100 mm Tris, pH 7.5, and then an equal volume of glycerol was added. The cell suspension was aliquoted and kept at –80 °C for storage. For the cellular MetAP activity assay, the cell suspension was diluted 400-fold with 10 mm CaCl2 in 100 mm Tris (pH 7.5). The cells, substrate Met-AMC, and inhibitor at 12 serial concentrations were combined in wells of a 384-well plate. The final assay volume was 80 μl with 150 μm Met-AMC, 5 mm CaCl2, and 50 mm Tris, pH 7.5. Production of AMC was monitored via fluorescence (λex 360 nm, λem 460) at room temperature every 2 min for 6–8 h. The IC50 values were calculated from the rate of substrate hydrolysis within the first 4 h.
Inhibition of Bacterial Growth—The assay was carried out on 384-well plates containing 12 serial concentrations for each inhibitor (the highest final concentration in the assay was 1 mm). A suspension of bacterial cells grown to exponential phase in Mueller Hinton medium was adjusted to 0.5 McFarland optical density (30) and then further diluted by 1000-fold in the same medium containing 100 mm Tris, pH 7.5. The cell suspension was dispensed into the microplate by Multidrop Combi reagent dispenser (Thermo Scientific, Waltham, MA). In the case of E. coli AS19, 40 μl of cell culture was dispensed into 40 μl of inhibitor, and cell growth was monitored by absorbance at 600 nm using a SpectraMax 340PC384 plate reader (Molecular Devices). Because of excessive variation of absorbance at 600 nm for other bacterial strains, analysis of these strains was carried out by monitoring fluorescence by including resazurin with the cells (31). Inhibitor (20 μl), cells (40 μl), and resazurin dye (450 μm, 20 μl) were mixed. The conversion from resazurin to resofurin was monitored kinetically by fluorescence (λex 530 nm and λem 590 nm) using a SpectraMax Gemini XPS plate reader. Both absorbance and fluorescence kinetic experiments were carried out for 10 h at 37 °C, with readings taken every 5 min. Signal intensities at time points along the exponential phase of the growth curve corresponding to 50–85% of total intensity of an uninhibited sample were averaged and converted to percent inhibitions to calculate IC50 values by non-linear regression curve fitting. MIC values were calculated as the concentration of compound resulting in 90% inhibition of cell growth.
Expression and Purification of Recombinant Glutathione S-Transferase—E. coli AS19 cells were transformed with the pBACE expression vector encoding for chicken liver glutathione S-transferase A1–1 with a phenylalanine to alanine substitution at position 111 (32). When cells reached exponential phase in LB medium containing 50 μg/ml ampicillin, the cells were diluted by 10,000-fold into an induction medium described by Craig et al. (33). Cells were allowed to grow at 25 °C for an additional 50 h in the presence or absence of an inhibitor. Harvested cells were resuspended in PBS buffer containing 5 mm EDTA and lysed by French press. The glutathione S-transferase proteins were purified by affinity chromatography using a GSTrap HP column (GE Healthcare, Piscataway, NJ). After washing impurities out of the column with PBS buffer, the recombinant proteins were eluted with 50 mm Tris containing 20 mm glutathione, pH 7.8.
Characterization of N-terminal Processed and Unprocessed Glutathione S-Transferases by Mass Spectrometry and N-terminal Sequencing—Purified proteins were incubated with 5 mm dithiothreitol to remove protein-bound glutathione, after which the sample was dialyzed into deionized water. The proteins dissolved in a solution containing 50% methanol and 3% acetic acid were injected into an Agilent G1946B mass spectrometer interfaced with an electrospray ionization source in the positive mode. Deconvoluted mass spectrum was obtained with the software MagTran (34). The first six amino acids at the N terminus of glutathione S-transferase purified from AS19 cells grown in the presence of inhibitor 6 were identified by Edman sequencing as a service by Alphalyse (Palo Alto, CA).
Physicochemical Calculation of the Compounds—The program ALOGPs was used to compute the calculated logP of the compounds (35). Physicochemical parameters describing Lipinski's rule of five (such as molecular weight, hydrogen bond donors, and acceptors) were evaluated by Molinspiration Property Calculation Service.
RESULTS
Development of a Cell-based MetAP Enzyme Activity Assay—To evaluate the ability of a compound to inhibit MetAP inside cells, we employed a strategy to make live E. coli cells permeable to small molecules, including substrates and inhibitors, and to assess inhibition by the compound when MetAP carries out hydrolysis of its substrate in a cellular environment. Ca(II) cation has been widely used to make bacterial cells permeable for molecules as big and charged as DNA molecules during bacterial transformation (36, 37). Tris also enhances outer-membrane permeability, making bacteria more sensitive to antibiotics (38). We overexpressed E. coli MetAP protein in E. coli BL21(DE3) cells and used the recombinant MetAP still inside live bacterial cells as the enzyme reagent. Ca(II) is not a MetAP activator and does not inhibit purified MetAP enzyme. With the cells suspended in 50 mm Tris buffer, we investigated the effect of Ca(II) on cell permeability by monitoring fluorescence from hydrolysis of the fluorogenic substrate Met-AMC (Fig. 1). Clearly, no significant amount of fluorescence was detectable when Ca(II) was below 10 μm, possibly due to inability of the substrate Met-AMC to pass though intact cell membranes. As CaCl2 concentration increases, rate of hydrolysis of Met-AMC accelerates as reflected by change of fluorescence with increasing amounts of CaCl2. The rate reached maximum at around 200 μm of CaCl2 and maintained at that level to 10 mm of CaCl2, suggesting easy penetration of Met-AMC into the cells at this concentration range. We chose a condition of 5 mm CaCl2 and 50 mm Tris in our following cellular enzyme activity assay.
FIGURE 1.
Effect of Ca(II) on the cellular MetAP activity. E. coli cells were suspended in 50 mm Tris, and increasing amounts of CaCl2 were added. MetAP enzyme activity was measured as hydrolysis of Met-AMC by fluorescence.
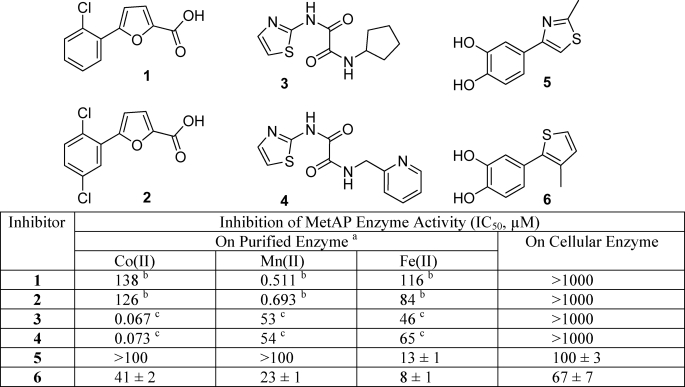
Inhibition of the Enzymatic Activity of MetAP in the Cell-based Assay—We have previously observed MetAP inhibitors that are either selective for the Mn(II)-form of E. coli MetAP or the Co(II)-form (24). However, no compounds in either of the two classes have significant inhibitory activity for the Fe(II)-form. Therefore, we carried out a high throughput screening campaign recently with a Fe(II)-activated E. coli MetAP and identified another unique class of MetAP inhibitors with both high potency and selectivity for the Fe(II)-form.3 We selected two compounds from each of the three metalloform-selective inhibitor classes (1 and 2 selective for the Mn(II)-form, 3 and 4 for the Co(II)-form, and 5 and 6 selective for the Fe(II)-form) and characterized them on the newly established cellular MetAP enzyme activity assay in comparison with their inhibitory activity on the purified enzymes activated by Co(II), Mn(II), or Fe(II) (Table 1). No inhibitory activity was detectable at the highest concentration (1 mm) for the Co(II)-form and the Mn(II)-form selective inhibitors (1–4) in this cellular MetAP assay. In contrast, the Fe(II)-form selective inhibitors (5 and 6) displayed significant inhibition, indicating that Fe(II) ion is likely the metal used by MetAP in the cellular environment. X-ray structures of E. coli MetAP in complex with these inhibitors (39)3 confirm that they directly interact with the enzyme at the active site.
TABLE 1.
Inhibition of the enzymatic activity of MetAP in purified form and in cellular environment by metalloform-selective MetAP inhibitors 1–6
Inhibition of Growth of E. coli and Bacillus Strains—Because of the importance of post-translational modification by MetAP, it is conceivable that MetAP inhibition will lead to inhibition of bacterial cell growth. Five E. coli strains and two Bacillus strains were selected for such testing. Among the E. coli strains, ATCC25922 is a wild-type strain, while AS19, D22, RL25, and RL436 have different mutations that make their membrane more permeable to small molecules (26–28). Two wild-type Bacillus strains (B. subtilis and B. megaterium) were also included because they are Gram-positive with a membrane structure different from that of Gram-negative E. coli. Inhibition of growth was determined in a quantitative way by calculating IC50 values for bacteria that grow in wells of a 384-well microplate according to continuous absorbance readings (600 nm, for AS19) or continuous fluorescence readings (λex 530 nm, λem 590, for the other 6 strains). Consistent with their ability to inhibit MetAP in the cellular assay, the Fe(II)-form selective inhibitors 5 and 6 inhibited all of the seven strains tested (Table 2). Generally, the IC50 values are lower for the Gram-positive Bacillus couple, compared with the Gram-negative E. coli strains. Although all E. coli strains were inhibited, strain D22 with membrane mutation was the most susceptible to compounds 5 and 6. It is important to note that none of the Co(II)-form and Mn(II)-form selective inhibitors 1–4 showed any detectable inhibitory activity under the same condition. This growth inhibition was tested in the presence of 50 mm Tris to increase cell permeability to small molecules (38). Compounds 5 and 6 were also tested in the absence of Tris on E. coli ATCC 25922, and they both showed reduced but still significant antibacterial activity on the wild-type E. coli cells (281 μm and 179 μm, respectively).
TABLE 2.
Inhibition of growth of some Gram-positive and Gram-negative bacterial strains by metalloform-selective MetAP inhibitors 1–6
|
Bacterial strain
|
Inhibition of bacterial cell growth (IC50,
μm)a,b
|
|
|---|---|---|
| 5 | 6 | |
| E. coli AS19 | 117 (91) | 15 (7) |
| E. coli D22 | 6 (4) | 9 (3) |
| E. coli RL25 | 107 (54) | 90 (41) |
| E. coli RL436 | 113 (33) | 52 (15) |
| E. coli ATCC 25922 | 110 (65) | 58 (30) |
| B. subtilis | 67 (41) | 22 (8) |
| B. megaterium | 70 (73) | 15 (13) |
Inhibitors 1-4 showed no inhibition for all seven strains tested at the highest concentration of 1 mm.
Values in parentheses are MIC in mg/liter.
Confirmation of the Cellular Target as MetAP Enzyme by Analysis of Recombinant GST—To make a connection between observed inhibition of bacterial cell growth to inhibition of MetAP enzyme activity inside bacterial cells, we used a construct that expresses recombinant GST and measured the ratio of N-terminal processed and unprocessed GSTs as an indication of MetAP inhibition in cells. N-terminal methionine residue of chicken liver GST was efficiently removed when the protein was expressed in E. coli (32), and analysis of the extent of N-terminal processing of the recombinant GST will provide the important information of MetAP inhibition under physiological conditions. E. coli cells harboring the GST-expressing plasmid were cultured at sublethal concentrations of either inhibitor 5 or inhibitor 6, and bacterial cell growth was retarded with the concomitant accumulation of unprocessed glutathione S-transferase protein. Both processed and unprocessed GST proteins were purified from cell extracts by affinity chromatography, and processed and unprocessed GST proteins were identified by mass spectrometry (Fig. 2). The deconvoluted spectra shows two peaks with masses 26114, corresponding to the processed GST, and 26245, corresponding to the unprocessed GST, and the mass difference of 131 is a shift due to a methionine residue. In the absence of 5 or 6, almost all of the GST was processed, and only the protein missing the first methionine was detected. In contrast, significant amount of unprocessed GST was accumulating in the sample derived from cells cultured with 5 or 6, consistent with the inhibition of the N-terminal processing by MetAP in the presence of the Fe(II)-form selective inhibitors. The same sample collected in the presence of 6 was analyzed by Edman degradation for the first six amino acids at the N terminus, the sequence obtained was mixture of Met-Ala-Ala-Lys-Pro-Val and Ala-Ala-Lys-Pro-Val, validating presence of the processed and unprocessed proteins.
FIGURE 2.
Effect of inhibition of MetAP on N-terminal processing of recombinant GST protein. A, ESI-MS protonation multiplicity spectra of a mixture of processed and unprocessed GST with charge states ranging from +19 to +26. B, spectra were transformed to a mass scale. The mass difference between both peaks corresponds to a methionine residue. Positions for the processed and unprocessed GST proteins are indicated by arrows. In both A and B, the spectrum using the sample in the absence of 6 is shown on the top, and the spectrum using the sample in the presence of 6 is shown on the bottom.
Physicochemical Properties of the Inhibitors—A key question is the ability of the compounds tested to cross cell membranes in order to reach MetAP target that resides in the cytoplasm. LogP values are considered the principal parameter to estimate lipophilicity that correlates largely with access of compounds into cells. Calculated logP values for inhibitors 1–6 by ALOGPs program are 3.50, 4.10, 1.35, 1.44, 2.03, and 2.53, respectively. According to a thorough analysis of the Comprehensive Medicinal Chemistry (CMC) data base by Ghose (40), the range of calculated logP that meets the criteria for drug-like properties lies between –0.4 and 5.6, and therefore, all six compounds used in the current work comply with that criterion. 1 and 2 have only 1 hydrogen donor, while the rest have 2. The number of hydrogen acceptors is 3, 3, 5, 6, 3, and 2 for compounds 1–6, respectively. The molecular mass of the six compounds is within the narrow range from 206 to 262 Da. Thus, all six compounds have drug-like physiochemical properties (41) and their penetration into the cells is likely under our experimental conditions.
DISCUSSION
Undoubtedly, Co(II) is an excellent activator of purified MetAP apoenzyme, and the Co(II)-form MetAP is responsible for the discovery of most of the current MetAP inhibitors (22, 23, 42–44) and has provided structural and mechanistic insight of the catalysis and inhibition of this therapeutically important family of enzymes (14). However, most Co(II) proteins are vitamin B12-dependent enzymes, and MetAP is among the limited number of enzymes that have Co(II) in a non-B12 form (45). Co(II) is certainly an activator for these enzymes but has not been firmly established as the native cofactor in some cases. MetAP has never been isolated with sufficient amount of cobalt present with the protein sample (12). Considering the cobalt concentration in E. coli cells is low with total cobalt at a submicromolar concentration (46), Co(II) is probably not the native metal for bacterial MetAPs. Activation of yeast MetAP by Zn(II) was reported (19), and E. coli MetAP can be activated only weakly (11, 12). Calculation showed Zn(II) could be an efficient cofactor for the catalysis (20). However, the activation of E. coli MetAP by Zn(II) is not always reproducible (47), partially due to the narrow range of concentration for the activation and the strong inhibition often displayed by Zn(II) at higher concentrations. The inactivity of Zn(II)-loaded MetAP was explained by unfavorable coordination geometry and Lewis acidity (47). Ni(II) is a weak but reproducible activator of E. coli MetAP (12, 24); nevertheless, Ni(II) is not a common cofactor with only seven nickel enzymes known (48). Mn(II) enzymes are numerous (49), and activation of E. coli and Pyrococcus furiosus MetAPs was extensively characterized (47). Both Mn(II) and Fe(II) were detectable in E. coli with or without MetAP overexpression, while Co(II) was not detectable under the same conditions (11). Therefore, Co(II), Mn(II), and Fe(II) are among the best activators of purified MetAPs (11, 12), and Mn(II) and Fe(II) are the top candidates for the native metal ion of bacterial MetAPs.
Metal analysis of isolated MetAP holoenzymes has not offered convincing evidence as to the native metal, because the metal ions are not tightly bound. There are no significant differences in affinity among the common divalent ions either. The affinity (Kd) of the two Co(II) ions to the dinuclear site of E. coli MetAP was 0.3 μm (tight position) and 2.5 mm (loose position), respectively, while the affinity of Fe(II) ion to the tight position was 0.2 μm (50). Similarly, Mn(II) binds to the same position at 6 μm (47). In this difficult situation, our unique MetAP inhibitors, selective for Co(II)-, Mn(II)-, and Fe(II)-forms, respectively (24, 29),3 provide powerful tools to resolve the ambiguity and define the native metal ion of MetAP, which is critical in the development of effective MetAP inhibitors for therapeutic applications.
To assess the inhibition by these metalloform-selective inhibitors, an enzyme activity assay using live E. coli cells where MetAP is in its native environment was established. The cells were made permeable to small molecule substrates and inhibitors by Ca(II) treatment, and hydrolysis of the fluorogenic substrate Met-AMC was easily detected by fluorescence. Although there are possibilities that this hydrolysis could result from action of other peptidases in the cells, the fact that our MetAP inhibitors 5 and 6 could inhibit this activity completely indicates that MetAP contributes at least in large part to the hydrolysis. PepN, not a metalloenzyme (51), was suggested to be responsible for the majority of aminopeptidase activity in E. coli extracts, and Met was a much poorer residue than Arg and Ala in a panel of aminopeptidase substrates tested (52). Further support for MetAP playing a significant role in the hydrolysis is the complete inhibition by metal chelators phenanthrolin and EDTA (IC50 12 mm and 1.9 mm, respectively), and dependence of the hydrolysis on metal ion is consistent with the requirement of metal for MetAP activity.
In the presence of Ca(II) at concentrations below 10 μm, no hydrolysis of Met-AMC was detectable in the cellular MetAP assay, indicating that all MetAP existed inside cells and there was no leakage. The hydrolysis was maximal at the Ca(II) concentrations ranging from 200 μm to 10 mm, suggesting that under this condition, Met-AMC could freely access MetAP inside the cells. At the testing condition of 5 mm of Ca(II), it is likely that the inhibitors could penetrate cell membrane and reach the cellular MetAP for inhibition. Although there are differences in their structures, these inhibitors (1–6) all have physicochemical properties that obey the empirical rule for drug leads (41) and have no special structural features that would prevent them from entering cells under this condition. Therefore, the observation that only Fe(II)-form selective inhibitors 5 and 6 showed significant inhibition of the cellular MetAP activity provides a strong evidence that Fe(II) is the likely native metal for MetAP.
MetAP is an essential enzyme in bacteria (3, 4). The significant inhibition of growth of five E. coli strains (Gram-negative) and two Bacillus strains (Gram-positive) by the Fe(II)-selective inhibitors further support the role of Fe(II) as the native metal. Most bacteria have only one MetAP gene, but as an exception, Bacillus was reported to have two homologous MetAP genes, one essential and the other non-essential (53). It is reasonable that the MetAP inhibitors 1–6 that we discovered and characterized on E. coli MetAP also inhibit the Bacillus MetAP enzymes and MetAP enzymes from other bacteria because of their sequence homology with E. coli MetAP. Nevertheless, although the antibacterial activity of 5 and 6 is likely the result of MetAP inhibition, possibility still exists that this observed activity is due to action on other cellular targets when the inhibitors were at micromolar concentrations.
The use of a recombinant GST protein as a marker proved to be a valuable tool to confirm inhibition of MetAP under physiological conditions. At sublethal concentrations of an inhibitor, cells grew at a slower rate than in the absence of the inhibitor. The amount of unprocessed GST increased, and the unprocessed GST was unequivocally identified as retaining the initiator methionine by mass spectrometry and Edman sequencing of the first six amino acid residues at the N terminus. Only those compounds selective toward the Fe(II) enzyme (5 and 6) retarded microbial growth, possibly as a direct result of MetAP inhibition.
It is intriguing that Mn(II) was suggested to be the native metal for human type II MetAP (21). Although further studies are needed, this is not without precedence. Prolidase and aminopeptidase share the same five metal ligands at the active site and share the same “pita-bread” overall structural fold with MetAP (54). E. coli prolidase was reported as a Co(II) enzyme (55), while human prolidase is believed to be a Mn(II) enzyme (56). Although E. coli aminopeptidase P (57, 58) and human cytosolic homolog (59) were characterized as Mn(II) enzymes, both human and porcine membrane-bound aminopeptidase P were described as Zn(II)-containing enzymes (60, 61). Several bacterial nitrile hydratases have been characterized, and they contain either cobalt or ferric ions, although they have similar sequences and share the same metal ligand residues (45, 62). Apparently, isozymes with homologous sequences and same metal ligand residues can accommodate different metal ions at the same site. The similar sizes of several divalent metal ions make this isomorphous replacement possible (63).
For many enzymes that require metal ions for activity, assignment of native metal has been difficult, and change in the assignment is not uncommon. For example, E. coli peptide deformylase was initially characterized as a Zn(II) enzyme (64) and later changed to a Fe(II) enzyme (65, 66). Increasing evidence shows that metal ions could dictate the catalysis and inhibition. In addition to what we have reported for MetAP (12, 24), Lactococcus lactis prolidase could be activated by either Mn(II) and Zn(II), and the two metalloforms preferred different substrates (25). Our approach of using metalloform-selective inhibitors demonstrates a successful example for another strategy for the assignment in the often confusing world of metalloenzymes.
Acknowledgments
We thank Dr. Liam Good and Dr. Ming F. Tam for their kind gifts of E. coli AS19 strain and the plasmid expressing a chicken liver GST protein, respectively.
This work was supported, in whole or in part by National Institutes of Health Grant AI065898 (to Q.-Z. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: MetAP, methionine aminopeptidase; GST, glutathione S-transferase.
Wang, W.-L., Chai, S. C., Huang, M., He, H. Z., and Ye, Q.-Z. (2008) J. Med. Chem. in press.
References
- 1.Bradshaw, R. A., Brickey, W. W., and Walker, K. W. (1998) Trends Biochem. Sci. 23 263–267 [DOI] [PubMed] [Google Scholar]
- 2.Giglione, C., Boularot, A., and Meinnel, T. (2004) Cell Mol. Life Sci. 61 1455–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, S. Y., McGary, E. C., and Chang, S. (1989) J. Bacteriol. 171 4071–4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller, C. G., Kukral, A. M., Miller, J. L., and Movva, N. R. (1989) J. Bacteriol. 171 5215–5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, X., and Chang, Y. H. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 12357–12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan, M. D., Sampson, P. B., and Honek, J. F. (2002) Curr. Med. Chem. 9 385–409 [DOI] [PubMed] [Google Scholar]
- 7.Griffith, E. C., Su, Z., Turk, B. E., Chen, S., Chang, Y. H., Wu, Z., Biemann, K., and Liu, J. O. (1997) Chem. Biol. 4 461–471 [DOI] [PubMed] [Google Scholar]
- 8.Griffith, E. C., Su, Z., Niwayama, S., Ramsay, C. A., Chang, Y. H., and Liu, J. O. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 15183–15188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, S., Widom, J., Kemp, C. W., Crews, C. M., and Clardy, J. (1998) Science 282 1324–1327 [DOI] [PubMed] [Google Scholar]
- 10.Towbin, H., Bair, K. W., DeCaprio, J. A., Eck, M. J., Kim, S., Kinder, F. R., Morollo, A., Mueller, D. R., Schindler, P., Song, H. K., van Oostrum, J., Versace, R. W., Voshol, H., Wood, J., Zabludoff, S., and Phillips, P. E. (2003) J. Biol. Chem. 278 52964–52971 [DOI] [PubMed] [Google Scholar]
- 11.D'Souza, V. M., and Holz, R. C. (1999) Biochemistry 38 11079–11085 [DOI] [PubMed] [Google Scholar]
- 12.Li, J. Y., Chen, L. L., Cui, Y. M., Luo, Q. L., Li, J., Nan, F. J., and Ye, Q. Z. (2003) Biochem. Biophys. Res. Commun. 307 172–179 [DOI] [PubMed] [Google Scholar]
- 13.Schiffmann, R., Heine, A., Klebe, G., and Klein, C. D. (2005) Angew Chem. Int. Ed. Engl. 44 3620–3623 [DOI] [PubMed] [Google Scholar]
- 14.Lowther, W. T., and Matthews, B. W. (2000) Biochim. Biophys. Acta 1477 157–167 [DOI] [PubMed] [Google Scholar]
- 15.Lowther, W. T., Orville, A. M., Madden, D. T., Lim, S., Rich, D. H., and Matthews, B. W. (1999) Biochemistry 38 7678–7688 [DOI] [PubMed] [Google Scholar]
- 16.Lowther, W. T., Zhang, Y., Sampson, P. B., Honek, J. F., and Matthews, B. W. (1999) Biochemistry 38 14810–14819 [DOI] [PubMed] [Google Scholar]
- 17.Copik, A. J., Swierczek, S. I., Lowther, W. T., D'Souza V, M., Matthews, B. W., and Holz, R. C. (2003) Biochemistry 42 6283–6292 [DOI] [PubMed] [Google Scholar]
- 18.Ye, Q. Z., Xie, S. X., Ma, Z. Q., Huang, M., and Hanzlik, R. P. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9470–9475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker, K. W., and Bradshaw, R. A. (1998) Protein Sci. 7 2684–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leopoldini, M., Russo, N., and Toscano, M. (2007) J. Am Chem. Soc. 129 7776–7784 [DOI] [PubMed] [Google Scholar]
- 21.Wang, J., Sheppard, G. S., Lou, P., Kawai, M., Park, C., Egan, D. A., Schneider, A., Bouska, J., Lesniewski, R., and Henkin, J. (2003) Biochemistry 42 5035–5042 [DOI] [PubMed] [Google Scholar]
- 22.Oefner, C., Douangamath, A., D'Arcy, A., Hafeli, S., Mareque, D., Mac Sweeney, A., Padilla, J., Pierau, S., Schulz, H., Thormann, M., Wadman, S., and Dale, G. E. (2003) J. Mol. Biol. 332 13–21 [DOI] [PubMed] [Google Scholar]
- 23.Luo, Q. L., Li, J. Y., Liu, Z. Y., Chen, L. L., Li, J., Qian, Z., Shen, Q., Li, Y., Lushington, G. H., Ye, Q. Z., and Nan, F. J. (2003) J. Med. Chem. 46 2631–2640 [DOI] [PubMed] [Google Scholar]
- 24.Ye, Q. Z., Xie, S. X., Huang, M., Huang, W. J., Lu, J. P., and Ma, Z. Q. (2004) J. Am Chem. Soc. 126 13940–13941 [DOI] [PubMed] [Google Scholar]
- 25.Yang, S. I., and Tanaka, T. (2008) FEBS J. 275 271–280 [DOI] [PubMed] [Google Scholar]
- 26.Normark, S., Boman, H. G., and Matsson, E. (1969) J. Bacteriol. 97 1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lathe, R., Buc, H., Lecocq, J. P., and Bautz, E. K. (1980) Proc. Natl. Acad. Sci. U. S. A. 77 3548–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zorzopulos, J., de Long, S., Chapman, V., and Kozloff, L. M. (1989) FEMS Microbiol. Lett. 52 23–26 [DOI] [PubMed] [Google Scholar]
- 29.Huang, Q. Q., Huang, M., Nan, F. J., and Ye, Q. Z. (2005) Bioorg. Med. Chem. Lett. 15 5386–5391 [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. (2000) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard M7-A4, National Committee for Clinical Laboratory Standards, Wayne, PA
- 31.Sarker, S. D., Nahar, L., and Kumarasamy, Y. (2007) Methods 42 321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, L. F., Liaw, Y. C., and Tam, M. F. (1997) Biochem. J. 327 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig, S. P., 3rd, Yuan, L., Kuntz, D. A., McKerrow, J. H., and Wang, C. C. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 2500–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, Z., and Marshall, A. G. (1998) J. Am Soc. Mass Spectrom. 9 225–233 [DOI] [PubMed] [Google Scholar]
- 35.Tetko, I. V., Gasteiger, J., Todeschini, R., Mauri, A., Livingstone, D., Ertl, P., Palyulin, V. A., Radchenko, E. V., Zefirov, N. S., Makarenko, A. S., Tanchuk, V. Y., and Prokopenko, V. V. (2005) J. Comput. Aided Mol. Des. 19 453–463 [DOI] [PubMed] [Google Scholar]
- 36.Dagert, M., and Ehrlich, S. D. (1979) Gene (Amst.) 6 23–28 [DOI] [PubMed] [Google Scholar]
- 37.Brass, J. M., Boos, W., and Hengge, R. (1981) J. Bacteriol. 146 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irvin, R. T., MacAlister, T. J., and Costerton, J. W. (1981) J. Bacteriol. 145 1397–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie, S. X., Huang, W. J., Ma, Z. Q., Huang, M., Hanzlik, R. P., and Ye, Q. Z. (2006) Acta Crystallogr. D. Biol. Crystallogr. 62 425–432 [DOI] [PubMed] [Google Scholar]
- 40.Ghose, A. K., Viswanadhan, V. N., and Wendoloski, J. J. (1999) J. Comb. Chem. 1 55–68 [DOI] [PubMed] [Google Scholar]
- 41.Lipinski, C. A., Lombardo, F., Dominy, B. W., and Feeney, P. J. (2001) Adv. Drug Deliv. Rev. 46 3–26 [DOI] [PubMed] [Google Scholar]
- 42.Douangamath, A., Dale, G. E., D'Arcy, A., Almstetter, M., Eckl, R., Frutos-Hoener, A., Henkel, B., Illgen, K., Nerdinger, S., Schulz, H., Mac Sweeney, A., Thormann, M., Treml, A., Pierau, S., Wadman, S., and Oefner, C. (2004) J. Med. Chem. 47 1325–1328 [DOI] [PubMed] [Google Scholar]
- 43.Huang, M., Xie, S. X., Ma, Z. Q., Hanzlik, R. P., and Ye, Q. Z. (2006) Biochem. Biophys. Res. Commun. 339 506–513 [DOI] [PubMed] [Google Scholar]
- 44.Huang, M., Xie, S. X., Ma, Z. Q., Huang, Q. Q., Nan, F. J., and Ye, Q. Z. (2007) J. Med. Chem. 50 5735–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi, M., and Shimizu, S. (1999) Eur. J. Biochem. 261 1–9 [DOI] [PubMed] [Google Scholar]
- 46.Outten, C. E., and O'Halloran, T. V. (2001) Science 292 2488–2492 [DOI] [PubMed] [Google Scholar]
- 47.D'Souza, V. M., Swierczek, S. I., Cosper, N. J., Meng, L., Ruebush, S., Copik, A. J., Scott, R. A., and Holz, R. C. (2002) Biochemistry 41 13096–13105 [DOI] [PubMed] [Google Scholar]
- 48.Wattt, R. K., and Ludden, P. W. (1999) Cell Mol. Life Sci. 56 604–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yocum, C. F., and Pecoraro, V. L. (1999) Curr. Opin. Chem. Biol. 3 182–187 [DOI] [PubMed] [Google Scholar]
- 50.D'Souza, V. M., Bennett, B., Copik, A. J., and Holz, R. C. (2000) Biochemistry 39 3817–3826 [DOI] [PubMed] [Google Scholar]
- 51.Golich, F. C., Han, M., and Crowder, M. W. (2006) Protein Expr. Purif 47 634–639 [DOI] [PubMed] [Google Scholar]
- 52.Chandu, D., and Nandi, D. (2003) Microbiology 149 3437–3447 [DOI] [PubMed] [Google Scholar]
- 53.You, C., Lu, H., Sekowska, A., Fang, G., Wang, Y., Gilles, A. M., and Danchin, A. (2005) BMC Microbiol. 5 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowther, W. T., and Matthews, B. W. (2002) Chem. Rev. 102 4581–4608 [DOI] [PubMed] [Google Scholar]
- 55.Maher, M. J., Ghosh, M., Grunden, A. M., Menon, A. L., Adams, M. W., Freeman, H. C., and Guss, J. M. (2004) Biochemistry 43 2771–2783 [DOI] [PubMed] [Google Scholar]
- 56.Lupi, A., Della Torre, S., Campari, E., Tenni, R., Cetta, G., Rossi, A., and Forlino, A. (2006) FEBS J. 273 5466–5478 [DOI] [PubMed] [Google Scholar]
- 57.Wilce, M. C., Bond, C. S., Dixon, N. E., Freeman, H. C., Guss, J. M., Lilley, P. E., and Wilce, J. A. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham, S. C., Bond, C. S., Freeman, H. C., and Guss, J. M. (2005) Biochemistry 44 13820–13836 [DOI] [PubMed] [Google Scholar]
- 59.Cottrell, G. S., Hooper, N. M., and Turner, A. J. (2000) Biochemistry 39 15121–15128 [DOI] [PubMed] [Google Scholar]
- 60.Molinaro, G., Carmona, A. K., Juliano, M. A., Juliano, L., Malitskaya, E., Yessine, M. A., Chagnon, M., Lepage, Y., Simmons, W. H., Boileau, G., and Adam, A. (2005) Biochem. J. 385 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooper, N. M., Hryszko, J., Oppong, S. Y., and Turner, A. J. (1992) Hypertension 19 281–285 [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi, M., and Shimizu, S. (1998) Nat. Biotechnol. 16 733–736 [DOI] [PubMed] [Google Scholar]
- 63.Hanzlik, R. P. (1976) Inorganic Aspects of Biological and Organic Chemistry, Academic Press, New York
- 64.Meinnel, T., Lazennec, C., and Blanquet, S. (1995) J. Mol. Biol. 254 175–183 [DOI] [PubMed] [Google Scholar]
- 65.Rajagopalan, P. T., Datta, A., and Pei, D. (1997) Biochemistry 36 13910–13918 [DOI] [PubMed] [Google Scholar]
- 66.Giglione, C., Pierre, M., and Meinnel, T. (2000) Mol. Microbiol. 36 1197–1205 [DOI] [PubMed] [Google Scholar]