Abstract
Histamine, a potent inflammatory mediator, has multiple effects on the pathogenesis of atherosclerosis. This study investigates the effect of histamine on the expression of early growth response factor 1 (Egr-1), a master transcription factor that regulates the expression of an array of atherogenic genes in atherosclerotic lesions. Histamine markedly and rapidly induces Egr-1 mRNA and protein expression in primary human aortic endothelial cells (HAECs). Histamine-induced Egr-1 expression is dependent on the activation of the H1 receptor. Histamine also rapidly and transiently activates protein kinase C-δ (PKCδ), extracellular signal-regulated kinase (ERK)1/2, p38 kinase, and c-Jun N-terminal kinase (JNK) prior to Egr-1 induction. Using specific pharmacological inhibitors and small interfering RNA technology, we determined that PKCδ and ERK, but not p38 and JNK, mediate histamine-induced Egr-1 expression. Our data provide the first evidence that histamine regulates expression of Egr-1 in mammalian cells and demonstrate a novel role of PKCδ in up-regulation of Egr-1 expression. The present study reveals the following regulatory mechanism: histamine up-regulates Egr-1 expression in primary HAECs via the H1 receptor and the PKCδ-dependent ERK activation pathway. Our data also imply that CREB, a downstream component of the ERK pathway, regulates Egr-1 expression in HAECs. Importantly, these results suggest a central role of Egr-1 in histamine-induced gene expression and in histamine-induced vascular disease.
Histamine, a low molecular weight amine, is produced by histidine decarboxylase (HDC)2 in mast cells and macrophages in atherosclerotic lesions (1). The expression of the histamine-producing enzyme HDC is increased during the development of atherosclerosis in human aortas and is localized in macrophage-derived foam cells and mononuclear cells (2). The concentrations of histamine detected in both pig restinotic neointima (30–140 μm) (3) and human atherosclerotic intima (16 μm) are higher than those in human tunica media (2.2 μm) (4). Histamine receptors, through which histamine exerts its functions, are expressed in intimal atherosclerotic lesions (5). Histamine induces endothelial cells to produce proinflammatory cytokines such as interleukin 6 (IL6) and interleukin 8 (IL8) (6–8); adherent molecules such as p-selectin (9), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) (10), and tissue factor (11), a prominent initiator of blood coagulation. Histamine also induces tissue factor expression in smooth muscle cells (11), and smooth muscle cell proliferation (12, 13). Most importantly, the antagonists of histamine receptor 1 (H1) reduce thickened intimas in mice (13), and recently, HDC knock-out mice showed reduced neointimal thickening (14). All of this accumulating evidence supports the notion that histamine promotes the development and progression of atherosclerosis.
Early growth response factor 1 (Egr-1) has emerged as a key regulator in the development of atherosclerosis. A zinc finger nuclear protein, Egr-1 regulates a set of genes implicated in the pathogenesis of atherosclerosis, with subsequent thrombosis and restenosis, by acting as a master transcription factor (15, 16). The products of this set of genes include pro-inflammatory cytokines, chemokines, adhesion molecules, growth factors, coagulation factors, and matricellular modulators.
To the best of our knowledge, whether histamine has an influence on Egr-1 expression in any mammalian cell type is unknown. Therefore, in this study, we aimed to understand the relationship between histamine and the key transcription factor Egr-1 in primary human aortic endothelial cells (HAECs), one type of vascular wall cells involved in the development of atherosclerosis. Our data reveal a novel effect of histamine on Egr-1 expression. Furthermore, the results from this study determined for the first time the molecular mechanism by which histamine regulates Egr-1 expression, as well as reveal a novel function of protein kinase C-δ (PKCδ) in up-regulation of Egr-1 expression. Most significantly, our data point to a central role of Egr-1 in histamine-triggered inflammation and atherosclerosis.
EXPERIMENTAL PROCEDURES
Reagents—Histamine, mepyramine, chlorpheniramine, and cimetidine were obtained from Sigma; non-silencing control siRNA, PKCδ siRNA, and PKCα siRNA from Qiagen (Valencia, CA); siRNA transfection reagent, RNAi MAX from Invitrogen (Carlsbad, CA); dCTP-32P from MP Biochemicals (Solon, OH); DNA labeling kit from GE Health Care (Piscataway, NJ); pertussis toxin, PD98059, SB203580, U0126, SP600125, rottlerin, GF109203X, Go6976, and Go6983 from Biomol International (Plymouth Meeting, PA); antibodies against p42/44 mitogen-activated protein kinase (MAPK), p38 MAPK, c-Jun N-terminal kinase (JNK) MAPK, phospho-extracellular signal-regulated kinase (ERK), phospho-p38, phospho-JNK, PKCδ, phospho-PKCα/β (Thr-638/641), phospho-PKCδ (Thr-505), phospho-PKCθ (Thr-538), phospho-PKCζ/λ (Thr-410/403) and phospho-CREB (Ser-133) from Cell Signaling Technology (Beverly, MA); antibodies against phospho-PKCε (Ser-729) and Egr-1 (C-19) from Santa Cruz Biotechnology (Santa Cruz, CA); antibody against phospho-PKCη (Ser-674) from BIOSOURCE International (Camarillo, CA); and antibody against PKCα from BD Biosciences (Franklin Lakes, NJ).
Cell Culture—HAECs supplied by Cascade Biologics (Portland, OR) were cultured in Medium 200 with special LSGS supplements (Cascade Biologics). Cells were starved for 16 h before stimulation with histamine.
Small Interfering RNA (siRNA) Transfection—Non-silencing siRNA (20 nm), PKCδ siRNA (20 nm), and PKCα siRNA (20 nm) were transfected into HAECs using the RNAi MAX transfection reagent according to the manufacturer's instructions (Invitrogen). The non-silencing siRNA was used as a negative control. 48 h after transfection, the cells were starved for 16 h followed by treatment either with or without histamine.
Northern Blot Analysis—Total RNA of the cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions and subjected to electrophoresis in formaldehyde/agarose gels. RNA was transferred onto Nylon membranes (Amersham Biosciences, Piscataway, NJ) and hybridized with radiolabeled cDNA probes as described previously (17). A 752-bp fragment of human Egr-1 cDNA was amplified by PCR and used as a probe to detect human Egr-1 mRNA. The primers used were: forward, 5′-CTA CGA GCA CCT GAC CGC AG-3′ and reverse, 5′-GAT CAC AGG ACT CCA CTG GGC-3′.
Western Blot Analysis—HAECs were rinsed with cold phosphate-buffered saline and lysed in Western blot lysis buffer (50 mm Tris-HCl, pH 6.8, 8 m urea, 5%-mercaptoethanol, 2% SDS, and protease inhibitors) with sonication for 20 s on ice. Cellular proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and were transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore). The membranes were then probed with the specific antibodies, and the specific protein bands were visualized by ECL-Plus (GE Healthcare, Piscataway, NJ) as described previously (18).
Data Analysis—All data are representative of a minimum of three experiments. Results are expressed as means ± S.E. Comparisons between multiple groups were performed using one-way analysis of variance with post-hoc t tests. A single comparison analysis was made using two-tailed, unpaired Student's t tests. A p value of < 0.05 was considered to be statistically significant.
RESULTS
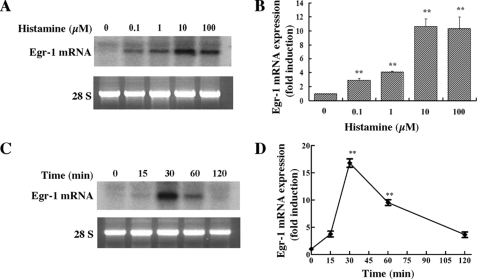
Histamine Markedly Induces Egr-1 mRNA Expression in HAECs—To determine whether histamine has any effect on Egr-1 expression, cultured HAECs were serum-starved for 16 h and then treated with various concentrations of histamine (0.1–100 μm) for 30 min. Total RNA was extracted from cells, and Egr-1 mRNA was detected by Northern blot analysis. As shown in Fig. 1, A and B, histamine markedly induced Egr-1 mRNA expression in a dose-dependent manner. The maximum increase in the level of Egr-1 mRNA was produced by a histamine concentration of 10 μm, a concentration that falls in the range detected in human atherosclerotic lesions (4). We also noticed that histamine rapidly and transiently induced Egr-1 mRNA accumulation, which peaked at 30 min and declined to the basal level 2 h after commencing stimulation (Fig. 1, C and D).
FIGURE 1.
Histamine induction of Egr-1 mRNA expression in HAECs. A, concentration dependence of histamine-stimulated Egr-1 mRNA expression. Cultured cells were starved for 16 h prior to various concentrations of histamine stimulation for 30 min. Total RNA was isolated using TRIzol reagent and subjected to Northern blot analysis. Visualized bands of 28S were used to assess RNA loading. B, quantitative analysis of Egr-1 mRNA expression (concentration dependence). Averaged data quantified by densitometry of Northern blots are shown. **, p < 0.01 versus control (unstimulated). C, time course of histamine induction of Egr-1 mRNA expression. Cells were stimulated with 10μm histamine for the indicated time periods. D, quantitative analysis of Egr-1 mRNA expression (time course). Averaged data quantified by densitometry of Northern blots are shown. **, p < 0.01 versus control (unstimulated).
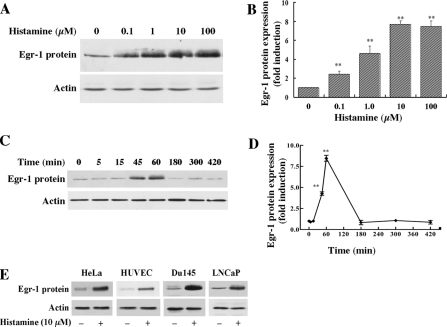
Histamine Increases Egr-1 Protein Expression in HAECs and Other Mammalian Cell Types—To determine whether histamine-induced Egr-1 mRNA expression is associated with increased levels of Egr-1 protein, we evaluated the effect of histamine on Egr-1 protein expression by Western blot analysis. As shown in Fig. 2, A and B, histamine dose-dependently induced Egr-1 protein expression and reached its maximal induction level at 10 μm. Histamine rapidly and transiently increased the production of Egr-1 protein (Fig. 2, C and D); maximal expression was reached at ∼1 h and declined to basal levels by 3 h. These data indicate that histamine-induced levels of Egr-1 mRNA contributed to the increased levels of Egr-1 protein in HAECs.
FIGURE 2.
Histamine stimulation of Egr-1 protein expression in HAECs and other types of mammalian cells. A, concentration dependence of histamine-stimulated Egr-1 protein expression in HAECs. Cultured cells were starved for 16 h prior to various concentrations of histamine stimulation for 1 h. Cell lysates were subjected to Western blot analysis. The same membrane was re-probed with an actin antibody to assess protein loading. B, quantitative analysis of Egr-1 protein expression (concentration dependence) in HAECs. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 versus control (unstimulated). C, time course of histamine induction of Egr-1 protein expression in HAECs. Cells were stimulated with 10 μm histamine for the indicated time periods. Cell lysates were subjected to Western blot analysis. D, quantitative analysis of Egr-1 protein expression (time course) in HAECs. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the increase of Egr-1 protein expression versus control (unstimulated). E, histamine stimulation of Egr-1 protein expression in other types of mammalian cells. Cultured HeLa cells, HUVECs, Du145 cells, and LNCaP cells were starved 16 h prior to 10 μm histamine stimulation for 1 h. Cell lysates were examined by Western blot analysis.
In testing whether histamine induces Egr-1 expression in mammalian cell types other than HAECs, we found that histamine induces Egr-1 expression in epithelial-like HeLa cells, human umbilical vein endothelial cells (HUVEC), and the prostate adenocarcinoma cells DU145 and LNCaP (Fig. 2E). These data indicate that histamine induces Egr-1 expression in various types of mammalian cells.
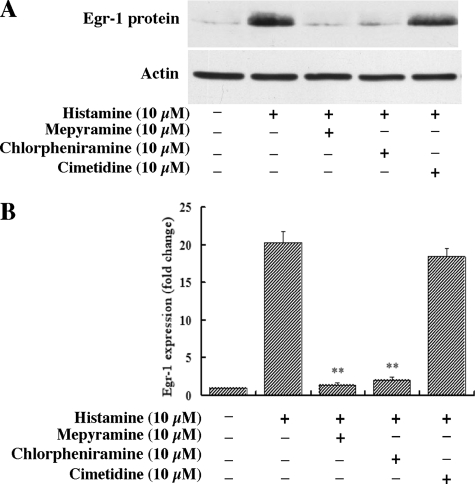
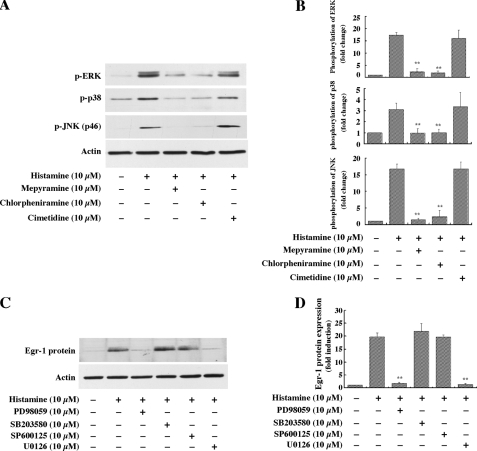
Histamine Induction of Egr-1 Expression Is Dependent on the H1 Receptor but Not the H2 Receptor—Histamine exerts its function through four types of G-protein-coupled receptors (1). An early study indicates that H1 receptor is expressed in human endothelial cells (5), and a recent report suggests that histamine receptor 2 (H2) is also expressed in human endothelial cells (19). To determine which of the receptors indeed mediates histamine-induced Egr-1 expression in HAECs, we pretreated HAECs with one of the H1 receptor-specific antagonists, either mepyramine or chlorpheniramine, or with the H2 receptor-specific antagonist cimetidine for 40 min. The cells were then stimulated with histamine (10 μm) for 1 h, and the expression of Egr-1 protein was detected by Western blot analysis. Pretreatment with one of the specific H1 antagonists, either mepyramine (10 μm) or chlorpheniramine (10 μm), completely blocked the histamine-induced expression of Egr-1 protein; however, pretreatment with the H2 receptor-specific antagonist cimetidine (10 μm) had no effect on histamine-induced Egr-1 expression (Fig. 3, A and B). These results indicate that histamine induction of Egr-1 expression depends on the activation of the H1 receptor, but not the H2 receptor.
FIGURE 3.
Role of histamine receptors in histamine-stimulated Egr-1 expression. HAECs were pretreated with the specific histamine receptor antagonists mepyramine (10 μm), chlorpheniramine (10 μm), or cimetidine (10 μm) for 40 min, and then were treated with histamine (10 μm) for 1 h. A, Western blot analysis. Protein loading was confirmed by re-probing the membrane with an actin antibody. B, quantitative analysis. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the increase of Egr-1 protein expression in the presence of inhibitors versus in the absence of inhibitors.
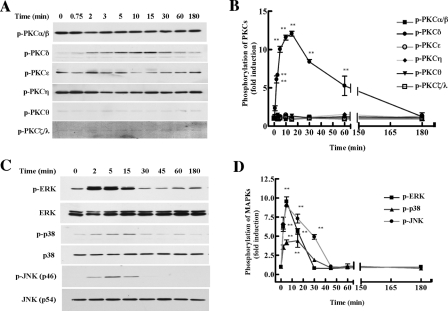
Histamine Activates PKCδ, and ERK, p38, and JNK MAPKs in HAECs—To investigate the intracellular signaling pathways involved in histamine-induced Egr-1 expression, we first assessed histamine activation of PKC. PKC consists of three subgroups: conventional PKC (cPKC) (α, β, and γ), novel PKC (nPKC) (ε, δ, η, and θ), and atypical PKC (aPKC) (ζ and λ). Although PKCα, β, ε, δ, η, θ, and ζ have been reported to be expressed in endothelial cells (20, 21), we found that histamine induced phosphorylation of only PKCδ, but not the other sub-types of PKC (Fig. 4, A and B). Histamine induction of PKCδ phosphorylation was detectable after a 45-s stimulation, reaching a peak at 2–15 min and declining to basal level after 60 min (Fig. 4, A and B). We also evaluated the effect of histamine on the activation of MAPK pathways. MAPKs include ERK, p38 and JNK. As shown in Fig. 4, C and D, starved HAECs were treated with histamine (10 μm) for various time periods, and we found that histamine activated all three MAPKs in HAECs. The maximum effect occurred around 2–15 min and dropped to basal levels after 30 min of stimulation. These data suggest that the activation of PKCδ and MAPKs may be involved in histamine-induced Egr-1 expression.
FIGURE 4.
Effects of histamine on the phosphorylation of various isoforms of PKC and MAPKs in HAECs. The cellular phosphorylation levels of each PKC isoform, ERK, p38 and JNK were analyzed using phosphospecific antibodies (see “Experimental Procedures”). A, time course for PKC phosphorylation by histamine. Cells were stimulated with 10 μm histamine for the indicated times. B, illustration of the dynamics of PKC phosphorylation. Averaged data quantified by densitometry of immunoblots, expressed as fold increase in phosphorylation, in which the phosphorylation observed in cells at time 0 was defined as 1.0 (control). **, p < 0.01 versus control. C, time course for the phosphorylation of ERK, p38, and JNK MAPKs by histamine. Equal loading was confirmed by reprobing the membranes with anti-total ERK, p38, and JNK antibodies. D, illustration of the dynamics of MAPK phosphorylation. Averaged data quantified by densitometry of immunoblots, expressed as fold increase in phosphorylation, in which the phosphorylation observed in cells at time 0 was defined as 1.0 (control). **, p < 0.01 versus control.
H1 Receptor Mediates the Activation of ERK, p38 and JNK, but Only the Activation of ERK Is Responsible for Histamine-induced Egr-1 Expression—Data presented in Fig. 3 clearly indicated that histamine-induced Egr-1 expression is mediated by the H1 receptor. To determine which histamine receptor is responsible for the histamine-induced phosphorylation of these MAPKs, the antagonists specific for the H1 and H2 receptors were used to treat HAECs for 40 min, prior to 5 min of stimulation with histamine. As shown in Fig. 5, A and B, the H1 receptor antagonists, either mepyramine (10 μm) or chlorpheniramine (10 μm), completely blocked the phosphorylation of these MAPKs. In contrast, the H2 receptor antagonist cimetidine had no effect on phosphorylation of the three MAPKs. These results, in combination with the data presented in Fig. 3, indicate that both histamine-induced Egr-1 expression and MAPK activation are mediated by the H1 receptor, but not the H2 receptor in HAECs. Next, we determined whether and which specific MAPK is functionally involved in histamine induction of Egr-1 expression. Pretreatment of HAECs with either of the ERK kinase inhibitors PD98059 or U0126, but not the JNK inhibitor SP600125 or p38 MAPK inhibitor SB203580, completely blocked histamine-induced Egr-1 expression (Fig. 5, C and D). These results indicate that H1 receptor-mediated ERK activation, but not H1 receptor-mediated p38 or JNK activation mediates histamine-induced Egr-1 expression.
FIGURE 5.
Role of histamine receptors in MAPK activation and role of specific MAPK in histamine-induced Egr-1 expression in HAECs. A, the effects of the specific histamine receptor antagonists on MAPK phosphorylation. Cells were pretreated with mepyramine (10 μm), chlorpheniramine (10 μm), or cimetidine (10 μm) for 40 min, and then were treated with histamine (10 μm) for 5 min. B, quantitative analysis of the effects of the specific histamine receptor antagonists on MAPK phosphorylation. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the increase of phosphorylation in the presence of antagonists versus in the absence of antagonists. C, effects of specific MAPK inhibitors on Egr-1 expression induced by histamine. Cells were pretreated with PD98059 (10 μm), SB203580 (10 μm), SP600125 (10 μm), or U0126 (10 μm) for 40 min, and then were treated with histamine (10 μm) for 1 h. D, quantitative analysis of the effect of the specific inhibitors of MAPKs on Egr-1 expression. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the increase of protein expression in the presence of inhibitors versus in the absence of inhibitors.
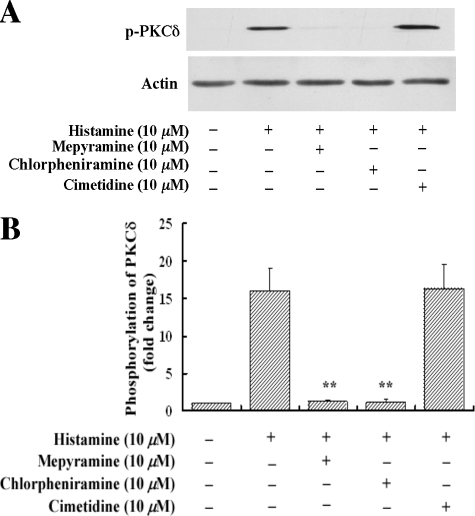
Histamine Activates PKCδ via the H1 Receptor—We next examined which histamine receptor mediates histamine-induced PKCδ activation. As shown in Fig. 6, A and B, specific histamine H1 receptor antagonists mepyramine (10 μm) and chlorpheniramine (10 μm) completely blocked histamine-induced phosphorylation of PKCδ. In contrast, the specific H2 inhibitor cimetidine (10 μm) had no effect on histamine activation of PKCδ. These results along with the results shown in Fig. 5, A and B, indicate that histamine activates PKCδ and MAPK pathways via the H1 receptor.
FIGURE 6.
Role of histamine receptors in histamine-stimulated PKCδ phosphorylation. HAECs were pretreated with mepyramine (10 μm), chlorpheniramine (10 μm) or cimetidine (10 μm) for 40 min, and then were treated with histamine (10 μm) for 5 min. A, the effects of the specific histamine receptor antagonists on PKCδ phosphorylation. The protein loading was confirmed by re-probing the membrane with an actin antibody. B, quantitative analysis of the effects of the specific histamine receptor antagonists on PKCδ phosphorylation. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the increase of PKCδ phosphorylation in the presence of inhibitors versus in the absence of inhibitors.
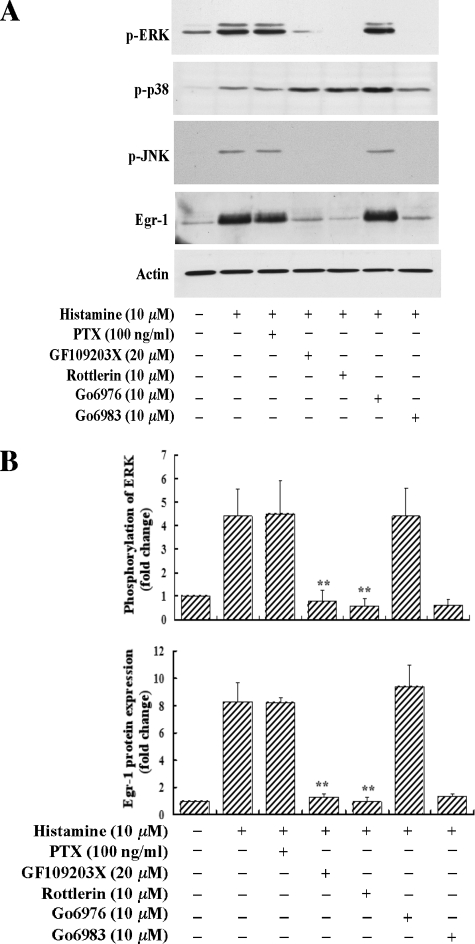
Specific PKC Inhibitors GF109203X and Go6983, and PKCδ-selective Inhibitor Rottlerin Inhibit ERK MAPK Activation and Egr-1 Expression—To determine the functional role of histamine-activated PKCδ in Egr-1 expression, we applied several specific inhibitors of PKC isotypes to evaluate their effects on histamine-induced Egr-1 expression. As shown in Fig. 7, A and B, we observed that GF109203X (20 μm), a specific inhibitor of PKCα, β, ε, δ, and ζ (22, 23), or Go6983 (10 μm), a specific inhibitor of PKCα, β, γ, δ, and ζ (24), completely blocked both histamine-induced ERK activation and Egr-1 expression. These results suggest that PKC is involved in histamine-induced Egr-1 expression. Pretreatment of cells with Go6976 (10 μm), a specific PKCα and β inhibitor (23), had no effect on histamine-induced ERK activation and Egr-1 expression, suggesting that PKCα and β are not involved in histamine induction of Egr-1 expression. These results, in combination with the fact that PKCα, β, ε, δ, η, θ, and ζ are reportedly expressed in endothelial cells (20, 21), suggest that PKCε, δ, η, θ, and ζ may be involved in the regulation of Egr-1 expression in response to histamine stimulation. However, it is noted that among these PKC isotypes, only PKCδ is rapidly and markedly phosphorylated upon histamine treatment (Fig. 4, A and B). To determine the functional role of PKCδ in histamine-induced Egr-1 expression, we examined whether rottlerin, a selective PKCδ inhibitor (25), affected Egr-1 expression. As shown in Fig. 7, A and B, pretreatment of HAECs with rottlerin (10 μm) for 40 min prior to histamine stimulation completely blocked histamine-induced ERK activation and Egr-1 expression. Taken together, these results strongly suggest that PKCδ mediates histamine induction of ERK activation and Egr-1 expression.
FIGURE 7.
Effects of the selective PKC inhibitors and pertussis toxin on histamine-stimulated phosphorylation of ERK, JNK, and p38 and expression of Egr-1. HAECs were pretreated with GF109203X (20 μm), rottlerin (10 μm), Go6976 (10 μm), or Go6983 (10 μm) for 40 min, or pretreated with pertussis toxin (PTX, 100 ng/ml) for 16 h, and then were treated with histamine (10 μm) either for 5 min (phosphorylation of ERK, JNK, and p38) or 1 h (Egr-1 protein expression). A, Western blot analyses of the phosphorylation of ERK, JNK, and p38 and expression of Egr-1. Protein loading was confirmed by re-probing the Egr-1 protein membrane with an actin antibody. B, quantitative analysis of ERK phosphorylation and Egr-1 expression. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the increase of ERK phosphorylation and Egr-1 expression in the presence of inhibitors versus in the absence of inhibitors.
We also noticed that the general PKC inhibitors GF109203X and Go6983, as well as the PKCδ inhibitor rottlerin, completely blocked histamine-induced JNK phosphorylation, but did not block p38 phosphorylation (Fig. 7A). These results suggest that PKCδ also mediates activation of JNK, but not of p38, although the histamine-induced activation of JNK and p38 does not contribute to Egr-1 expression (Fig. 5, C and D).
In addition, we observed that pretreatment with 100 ng/ml of pertussis toxin, a Gi/o inhibitor, had no effect on histamine-induced ERK phosphorylation and Egr-1 expression, indicating that the G-proteins, which contribute to histamine-induced Egr-1 expression in HAECs, are not Gi/o proteins.
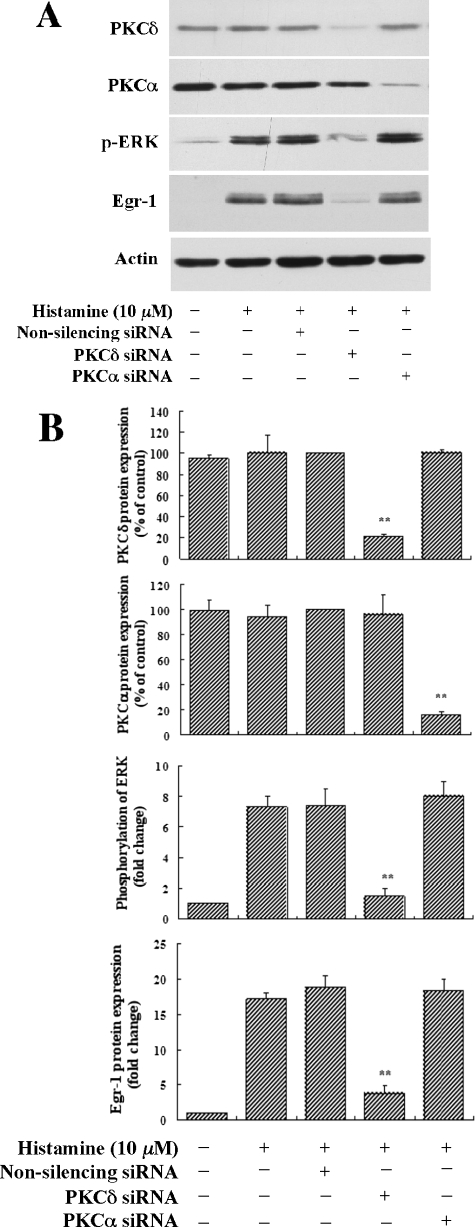
Knockdown of PKCδ Expression by Specific PKCδ siRNA Abolished Histamine Induction of Both ERK MAPK Activation and Egr-1 Expression—To confirm our finding that both histamine-induced ERK activation and Egr-1 expression are mediated by PKCδ, we employed the RNA interference approach by using specific siRNA to deplete endogenous PKCδ expression. We examined whether depletion of PKCδ affected histamine-induced ERK activation and Egr-1 expression in HAECs. PKCδ siRNA, non-silencing siRNA (negative control) and PKCα siRNA (relative kinase control) were transfected into HAECs for 48 h, and then the cells were starved for 16 h followed by treatment with 10 μm histamine. As shown in Fig. 8, A and B, we found that PKCδ-specific siRNA, which knocked down endogenous PKCδ expression by nearly 85%, reduced ERK activation and Egr-1 expression by 80 and 86%, respectively. In contrast, a related kinase control, PKCα siRNA, which knocked down PKCα expression by 80%, had no effect on either ERK activation or Egr-1 expression. The non-silencing RNA (the negative control), as expected, also had no effect on ERK activation and Egr-1 expression. Taken together, these data strongly support the conclusion that histamine induces Egr-1 expression via a PKCδ-mediated ERK pathway.
FIGURE 8.
Effects of PKCδ siRNA, PKCα siRNA and nonsilencing siRNA on histamine-induced ERK phosphorylation and Egr-1 expression. HAECs were transfected with PKCδ siRNA (20 nm), PKCα siRNA (20 nm), or non-silencing siRNA (20 nm). 48 h after transfection, cells were starved for 16 h followed by stimulation with histamine (10 μm) for 5 min (phosphorylation of ERK) or 1 h (expression of Egr-1, PKCδ and PKCα). A, Western blot analysis of cellular protein levels of PKCδ, PKCα, and Egr-1, and phosphorylation levels of ERK. B, quantitative analysis of the effects of PKCδ siRNA, PKCα siRNA, and nonsilencing siRNA on ERK phosphorylation and Egr-1 expression. Averaged data quantified by densitometry of Western blots are shown. **, p < 0.01 for the decrease of protein expression (PKCδ or PKCα) versus non-silencing control (100%), and the decrease of ERK phosphorylation and Egr-1 protein expression in the specific siRNA treatment versus in non-silencing RNA treatment.
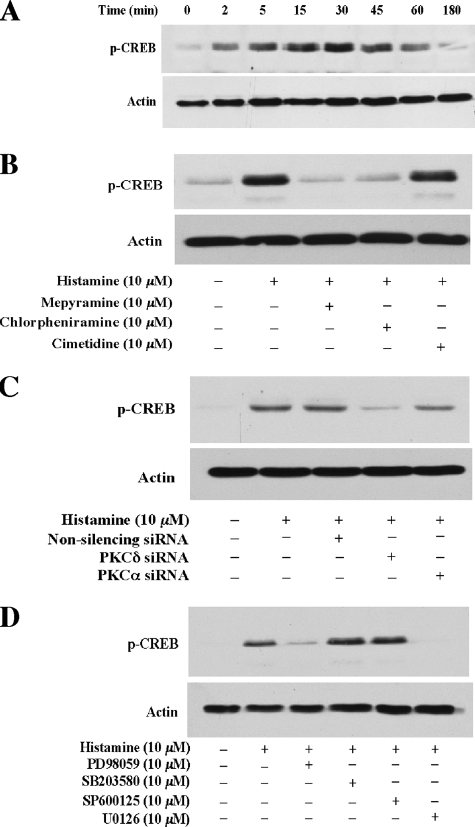
cAMP Response Element-binding Protein (CREB), a Nuclear Transcription Factor, Is a Downstream Component of the ERK Pathway and Is Activated by Histamine; CREB Is Implied in the Regulation of Histamine-induced Egr-1 Expression in HAECs—To further understand the molecular mechanism of the regulation of histamine-induced Egr-1 expression downstream from ERK in HAECs, we examined whether histamine activates CREB, which has been shown to mediate Egr-1 expression in various types of cells (17, 26). As shown in Fig. 9, the results from a series of experiments reveal (1) histamine induces rapid and profound phosphorylation of CREB, which is detectable at 2 min and peaks at 30 min; (2) specific histamine H1 receptor antagonists mepyramine (10 μm) and chlorpheniramine (10 μm) completely blocked histamine-induced phosphorylation of CREB. In contrast, the specific H2 inhibitor cimetidine (10 μm) had no effect on histamine activation of CREB, indicating that the histamine receptor H1 mediates CREB phosphorylation; (3) knockdown of PKCδ expression using siRNA of PKCδ markedly reduces histamine-induced phosphorylation of CREB; and (4) pretreatment with the ERK kinase inhibitor PD98059 or U0126 blocks histamine-induced phosphorylation of CREB. Taken together, these results are in line with our findings that histamine induces Egr-1 expression via the histamine receptor H1-mediated PKCδ-regulated ERK pathway. These results reveal that CREB is a downstream component of the histamine-triggered ERK pathway and imply that histamine-activated CREB mediates Egr-1 expression in HAECs.
FIGURE 9.
Histamine activation of CREB, and H1 receptor, PKCδ, and ERK mediation of histamine-induced CREB phosphorylation. A, time course of histamine induction of CREB phosphorylation in HAECs. Cultured HAECs were starved for 16 h prior to 10 μm histamine stimulation. Cell lysates were examined by Western blot analysis. B, effects of histamine receptor antagonists on histamine-induced CREB phosphorylation. HAECs were pretreated with the specific histamine receptor antagonists mepyramine (10 μm), chlorpheniramine (10 μm), or cimetidine (10 μm) for 40 min, and then were treated with histamine (10 μm) for 5 min. Cell lysates were examined by Western blot analysis. C, effect of PKCδ siRNA on histamine-induced phosphorylation of CREB. HAECs were transfected with PKCδ siRNA (20 nm), PKCα siRNA (20 nm), or non-silencing siRNA (20 nm). 48 h after transfection, cells were starved for 16 h followed by stimulation with histamine (10 μm) for 5 min. Cell lysates were examined by Western blot analysis. D, effects of specific MAPK inhibitors on CREB phosphorylation induced by histamine. Cells were pretreated with PD98059 (10 μm), SB203580 (10 μm), SP600125 (10 μm), or U0126 (10 μm) for 40 min, and then were treated with histamine (10 μm) for 5 min. Cell lysates were examined by Western blot analysis. Protein loading was confirmed by re-probing the membrane with an actin antibody (A–D).
DISCUSSION
The role of histamine in the development and progression of atherosclerosis has been highlighted by two independent in vivo studies. One of these studies showed that the histamine H1 receptor antagonist reduced intimal hyperplasia (13); the other study reported that histamine synthesis enzyme knock-out mice (HDC–/– mice) showed reduced neointimal thickening and a decreased intima-to-media thickness ratio (14). In regard to how histamine influences inflammation and atherosclerosis in endothelial cells, evidence has shown that histamine induces expression of genes such as p-selectin (9), ICAM1, VCAM1 (10), IL6, IL8 (8), cyclooxygenase-2 (27), and tissue factor (11). The products of these genes have been shown to promote inflammation and atherogenesis (28, 29). Importantly, Egr-1 functions as a key transcription factor, regulating the expression of these genes (15, 30–32). However, to our knowledge, there is no information regarding whether and how histamine regulates Egr-1 expression in mammalian cells. To address this question, we set out to determine the effect of histamine on the expression of Egr-1 in primary HAECs. Our results reveal, for the first time, that histamine markedly and rapidly induces transcription factor Egr-1 expression in mammalian cells.
Although both the H1 and H2 histamine receptors are reportedly expressed in endothelial cells (5, 19), our data indicate that the H1 receptor, but not the H2 receptor, mediates the histamine signal leading to Egr-1 expression. Related to our finding, previous studies have shown that histamine-induced expression of p-selectin (9), ICAM1, VCAM1 (10), IL6, IL8 (8), cyclooxygenase-2 (27), and tissue factor (11) is mediated by the H1 receptor in vascular endothelial cells. Taken together, our results and the previous observations support a notion that histamine-induced expression of the pro-inflammatory transcription factor Egr-1 and its downstream inflammatory genes is selectively mediated by the H1 receptor in endothelial cells.
Our data from the experiments investigating the intracellular signaling cascade demonstrate that ERK MAPK mediates histamine-induced Egr-1 expression. The role of MAPK in histamine induction of endothelial gene expression has not been well explored, although a previous report suggested that ERK, p38, and JNK MAPK may all contribute to histamine induction of tissue factor expression (11). Our data clearly show that activation of ERK, but not of JNK or p38, is required for histamine-induced Egr-1 expression. These results not only provide further support for the essential role of MAPK in histamine activation of vascular endothelial cells, but also reveal a specific ERK MAPK pathway leading to histamine-induced Egr-1 expression.
We systematically determined which specific PKC isoform was responsible for histamine-induced Egr-1 expression. Information available in the literature shows that PKCα, β, ε, δ, η, θ, and ζ are expressed in endothelial cells (20, 21). First, we examined which specific isoform of PKC was activated by histamine. Interestingly, we observed that PKCδ was the only one being rapidly phosphorylated upon histamine stimulation, starting after 45 s and reaching its maximum phosphorylation between 2 and 15 min (Fig. 4, A and B). Second, the fact that the H1 receptor antagonists blocked both histamine-induced Egr-1 expression (Fig. 3) and histamine-induced PKCδ activation (Fig. 6) suggests the possibility that PKCδ may be involved in histamine-induced Egr-1 expression. Third, general PKC inhibitors GF109203X and Go6983 completely blocked Egr-1 expression, indicating that PKC is indeed involved in histamine-induced Egr-1 expression (Fig. 7). Fourth, rottlerin, a selective PKCδ inhibitor, dramatically reduced histamine-induced Egr-1 expression, strongly suggesting a functional role of PKCδ in histamine-induced Egr-1 expression (Fig. 7). This speculation is further supported by the fact that Go6976, a specific inhibitor of PKCα and -β, had no effect on histamine-induced Egr-1 expression, ruling out the involvement of PKCα and β in histamine induction of Egr-1 expression (Fig. 7). Fifth, to confirm the functional role of PKCδ in histamine-induced Egr-1 expression, we employed the RNA interference approach, which has recently emerged as a powerful tool to deplete the expression of a protein of interest. We found that depletion of PKCδ, but not PKCα, nearly completely blocked histamine-induced Egr-1 expression and ERK activation (Fig. 8). Thus, our data clearly establish a functional role of PKCδ in histamine-induced Egr-1 expression in HAECs.
Our findings provide the first evidence that activated PKCδ mediates up-regulation of the expression of the key transcription factor Egr-1. In a previous study, PKCδ was constitutively activated in senescent fibroblast cells, and overactivation of PKCδ may have accounted for the loss of the ability of these cells to replicate in response to growth factors. As cells become senescent, they become unable to express growth-associated immediate-early genes, including c-fos and Egr-1 (33). Therefore, PKCδ could have divergent functions in either up-regulating or down-regulating gene expression in proliferating or non-proliferating cells. Indeed, the involvement of PKCδ in both anti-apoptosis and pro-apoptosis has been reported (34).
Our results also reveal that the histamine-triggered pathway leading to Egr-1 expression is distinct from the hypoxia-induced pathway, in which activation of PKCα or PKCβ leads to Egr-1 expression in endothelial cells and in a monocyte-like cell line (35, 36). These results indicate that these specific PKC isoforms serve as the central mediators for Egr-1 expression in various cell types and in response to different stimuli.
In further pursuit of the molecular mechanism of the regulation of Egr-1 expression downstream from ERK, we identified that CREB, a nuclear transcription factor shown to turn on Egr-1 transcription in several cell types (17, 26), is a downstream component of the histamine-induced ERK pathway. These results imply a nuclear regulatory role of CREB in histamine-induced Egr-1 expression.
Taken together, our results not only provide the first evidence that histamine induces Egr-1 expression in mammalian cells, but also reveal novel insights into the mechanism by which histamine regulates Egr-1 expression. Our data clearly establish a signaling pathway that mediates histamine induction of Egr-1, i.e. the effect of histamine is specifically mediated by the H1 receptor, the activated PKCδ and the ERK cascade. Our data also imply that CREB, a downstream component of the ERK pathway, regulates Egr-1 expression in HAECs. Because it was reported previously that histamine induces the expression of p-selectin (9), ICAM1, VCAM1 (10), IL6, IL8 (8), cyclooxygenase-2 (27), and tissue factor (11) in endothelial cells, and that Egr-1 can serve as a transcription factor for regulating these genes in response to other stimuli (15, 30–32), our result that histamine induces Egr-1 expression points out that Egr-1 may be the previously missed link between histamine and the pro-inflammatory genes. Of particular significance is that our findings imply that histamine-induced Egr-1 transcription activity could serve as a central control regulating transduction of the histamine signal and consequently governing histamine induction of multiple inflammatory genes, which in turn, trigger inflammation and atherosclerosis.
Acknowledgments
We thank Dr. Donald McGavin and Misty Bailey for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL074341 (to M.-Z. C.) and AG026640 (to X. X.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HDC, histidine decarboxylase; Egr-1, early growth response factor 1; HAEC, human aortic endothelial cell; PKC, protein kinase C; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; IL, interleukin; siRNA, small interfering RNA; CREB, cAMP response element-binding protein.
References
- 1.Tanimoto, A., Sasaguri, Y., and Ohtsu, H. (2006) Trends Cardiovasc. Med. 16 280–284 [DOI] [PubMed] [Google Scholar]
- 2.Higuchi, S., Tanimoto, A., Arima, N., Xu, H., Murata, Y., Hamada, T., Makishima, K., and Sasaguri, Y. (2001) FEBS Lett. 505 217–222 [DOI] [PubMed] [Google Scholar]
- 3.Fang, Y. I., Namiki, H., Tsunoda, E., Shioda, S., Shibata, M., Nakatani, M., Katagiri, T., Takeyama, Y., Ohata, H., Honda, K., and Momose, K. (2005) Life Sci. 77 241–251 [DOI] [PubMed] [Google Scholar]
- 4.Tanimoto, A., Wang, K. Y., Murata, Y., Kimura, S., Nomaguchi, M., Nakata, S., Tsutsui, M., and Sasaguri, Y. (2007) Arterioscler. Thromb. Vasc. Biol. 27 1556–1561 [DOI] [PubMed] [Google Scholar]
- 5.Takagishi, T., Sasaguri, Y., Nakano, R., Arima, N., Tanimoto, A., Fukui, H., and Morimatsu, M. (1995) Am. J. Pathol. 146 981–988 [PMC free article] [PubMed] [Google Scholar]
- 6.Delneste, Y., Lassalle, P., Jeannin, P., Joseph, M., Tonnel, A. B., and Gosset, P. (1994) Clin. Exp. Immunol. 98 344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeannin, P., Delneste, Y., Gosset, P., Molet, S., Lassalle, P., Hamid, Q., Tsicopoulos, A., and Tonnel, A. B. (1994) Blood 84 2229–2233 [PubMed] [Google Scholar]
- 8.Li, Y., Chi, L., Stechschulte, D. J., and Dileepan, K. N. (2001) Microvasc. Res. 61 253–262 [DOI] [PubMed] [Google Scholar]
- 9.Asako, H., Kurose, I., Wolf, R., DeFrees, S., Zheng, Z. L., Phillips, M. L., Paulson, J. C., and Granger, D. N. (1994) J. Clin. Investig. 93 1508–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura, S., Wang, K. Y., Tanimoto, A., Murata, Y., Nakashima, Y., and Sasaguri, Y. (2004) Pathol. Int. 54 465–474 [DOI] [PubMed] [Google Scholar]
- 11.Steffel, J., Akhmedov, A., Greutert, H., Luscher, T. F., and Tanner, F. C. (2005) Circulation 112 341–349 [DOI] [PubMed] [Google Scholar]
- 12.Satoh, T., Sugama, K., Matsuo, A., Kato, S., Ito, S., Hatanaka, M., and Sasaguri, Y. (1994) Atherosclerosis 110 53–61 [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa, N., Watanabe, S., Matsuda, A., Kondo, K., Hashimoto, H., Umemura, K., and Nakashima, M. (1998) Eur. J. Pharmacol. 362 53–59 [DOI] [PubMed] [Google Scholar]
- 14.Sasaguri, Y., Wang, K. Y., Tanimoto, A., Tsutsui, M., Ueno, H., Murata, Y., Kohno, Y., Yamada, S., and Ohtsu, H. (2005) Circulation Res. 96 974–981 [DOI] [PubMed] [Google Scholar]
- 15.Khachigian, L. M. (2006) Circulation Res. 98 186–191 [DOI] [PubMed] [Google Scholar]
- 16.Blaschke, F., Bruemmer, D., and Law, R. E. (2004) Rev. Endocr. Metab. Disord. 5 249–254 [DOI] [PubMed] [Google Scholar]
- 17.Cui, M. Z., Laag, E., Sun, L., Tan, M., Zhao, G., and Xu, X. (2006) Arterioscler. Thromb. Vasc. Biol. 26 1029–1035 [DOI] [PubMed] [Google Scholar]
- 18.Cui, M. Z., Zhao, G., Winokur, A. L., Laag, E., Bydash, J. R., Penn, M. S., Chisolm, G. M., and Xu, X. (2003) Arterioscler. Thromb. Vasc. Biol. 23 224–230 [DOI] [PubMed] [Google Scholar]
- 19.Li, H., Burkhardt, C., Heinrich, U. R., Brausch, I., Xia, N., and Forstermann, U. (2003) Circulation 107 2348–2354 [DOI] [PubMed] [Google Scholar]
- 20.Krizbai, I., Szabo, G., Deli, M., Maderspach, K., Lehel, C., Olah, Z., Wolff, J. R., and Joo, F. (1995) J. Neurochem. 65 459–462 [DOI] [PubMed] [Google Scholar]
- 21.Haller, H., Ziegler, W., Lindschau, C., and Luft, F. C. (1996) Arterioscler. Thromb. Vasc. Biol. 16 678–686 [DOI] [PubMed] [Google Scholar]
- 22.Toullec, D., Pianetti, P., Coste, H., Bellevergue, P., Grand-Perret, T., Ajakane, M., Baudet, V., Boissin, P., Boursier, E., Loriolle, F., Duhamel, L., Charon, D., and Kirilovsky, J. (1991) J. Biol. Chem. 266 15771–15781 [PubMed] [Google Scholar]
- 23.Martiny-Baron, G., Kazanietz, M. G., Mischak, H., Blumberg, P. M., Kochs, G., Hug, H., Marme, D., and Schachtele, C. (1993) J. Biol. Chem. 268 9194–9197 [PubMed] [Google Scholar]
- 24.Gschwendt, M., Dieterich, S., Rennecke, J., Kittstein, W., Mueller, H. J., and Johannes, F. J. (1996) FEBS Lett. 392 77–80 [DOI] [PubMed] [Google Scholar]
- 25.Gschwendt, M., Muller, H. J., Kielbassa, K., Zang, R., Kittstein, W., Rincke, G., and Marks, F. (1994) Biochem. Biophys. Res. Commun. 199 93–98 [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto, K. M., Fraser, J. K., Lee, H. J., Lehman, E., and Gasson, J. C. (1994) Mol. Cell. Biol. 14 5975–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan, X., Essengue, S., Talreja, J., Reese, J., Stechschulte, D. J., and Dileepan, K. N. (2007) J. Immunol. 179 7899–7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packard, R. R., and Libby, P. (2008) Clin. Chem. 54 24–38 [DOI] [PubMed] [Google Scholar]
- 29.Linton, M. F., and Fazio, S. (2004) Curr. Opin. Pharmacol. 4 116–123 [DOI] [PubMed] [Google Scholar]
- 30.Sayasith, K., Brown, K. A., Lussier, J. G., Dore, M., and Sirois, J. (2006) J. Mol. Endocrinol. 37 239–250 [DOI] [PubMed] [Google Scholar]
- 31.Prince, J. M., Ming, M. J., Levy, R. M., Liu, S., Pinsky, D. J., Vodovotz, Y., and Billiar, T. R. (2007) Shock (Augusta, Ga) 27 157–164 [DOI] [PubMed] [Google Scholar]
- 32.Choi, E. Y., Park, Z. Y., Choi, E. J., Oh, H. M., Lee, S., Choi, S. C., Lee, K. M., Im, S. H., Chun, J. S., and Jun, C. D. (2007) J. Cell. Biochem. 102 1442–1457 [DOI] [PubMed] [Google Scholar]
- 33.Wheaton, K., and Riabowol, K. (2004) Mol. Cell. Biol. 24 7298–7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodie, C., and Blumberg, P. M. (2003) Apoptosis 8 19–27 [DOI] [PubMed] [Google Scholar]
- 35.Lo, L. W., Cheng, J. J., Chiu, J. J., Wung, B. S., Liu, Y. C., and Wang, D. L. (2001) J. Cell. Physiol. 188 304–312 [DOI] [PubMed] [Google Scholar]
- 36.Yan, S. F., Lu, J., Zou, Y. S., Soh-Won, J., Cohen, D. M., Buttrick, P. M., Cooper, D. R., Steinberg, S. F., Mackman, N., Pinsky, D. J., and Stern, D. M. (1999) J. Biol. Chem. 274 15030–15040 [DOI] [PubMed] [Google Scholar]