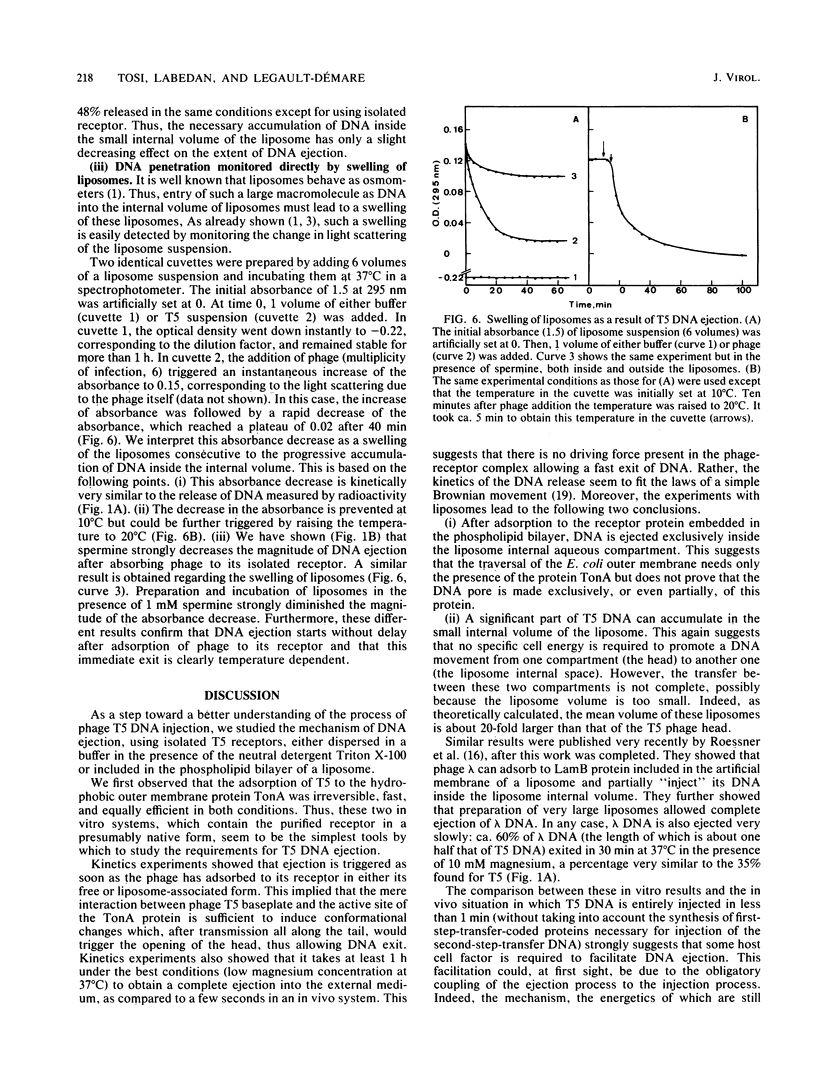
Abstract
Outer membrane protein TonA, the receptor for coliphage T5, has been partially purified and incorporated into the phospholipid bilayer of liposomes. Adsorption of the phage to its receptor in either a free or liposome-associated form is fast and sufficient to trigger the ejection of encapsidated DNA. In both in vitro systems the exit of DNA from the phage capsid is a very slow process. Ejected DNA can partially accumulate inside the liposome aqueous compartment, but the transfer from the phage head to the liposome internal space is never complete, perhaps because the liposome volume is too small. The presence of polyamines or divalent cations (magnesium) or both in the incubation medium diminished the extent of DNA ejection, possibly by stabilizing DNA inside the head. DNA movement was slowed as the temperature was decreased from 37 to 18 degrees C. Furthermore, incubation at 4 degrees C totally prevented this DNA movement, even if a large part of the DNA had already exited the capsid.
Full text
PDF

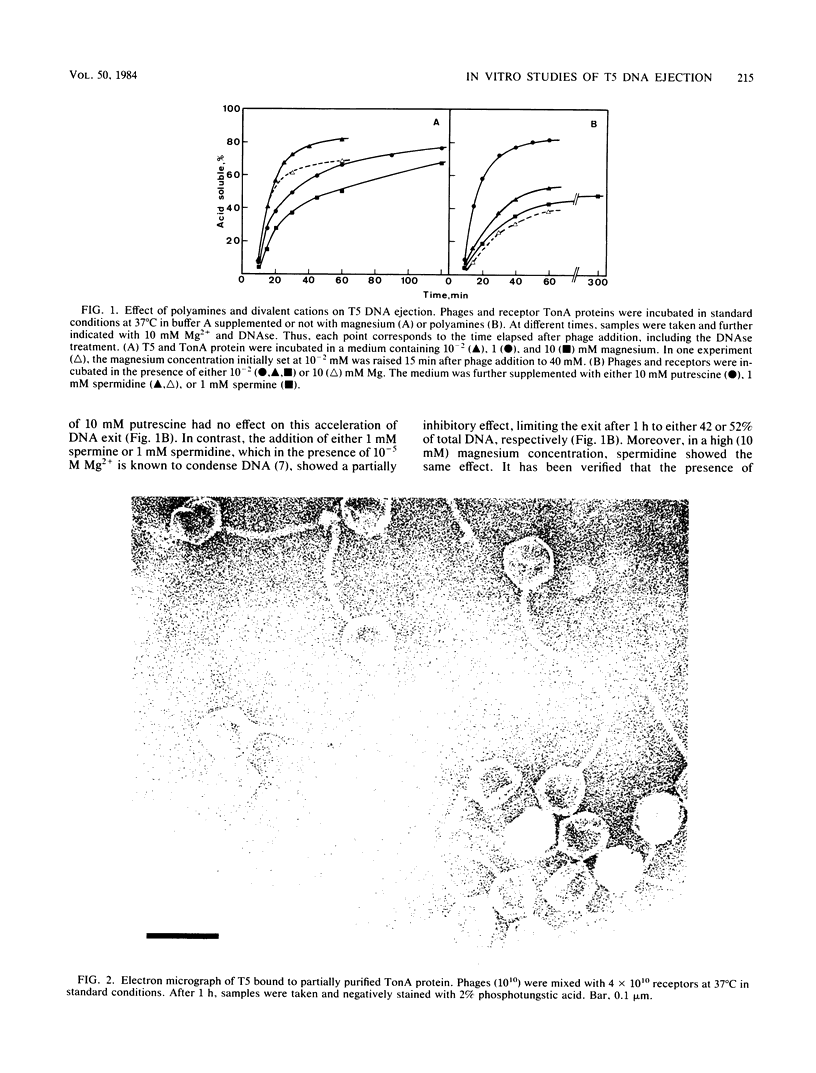
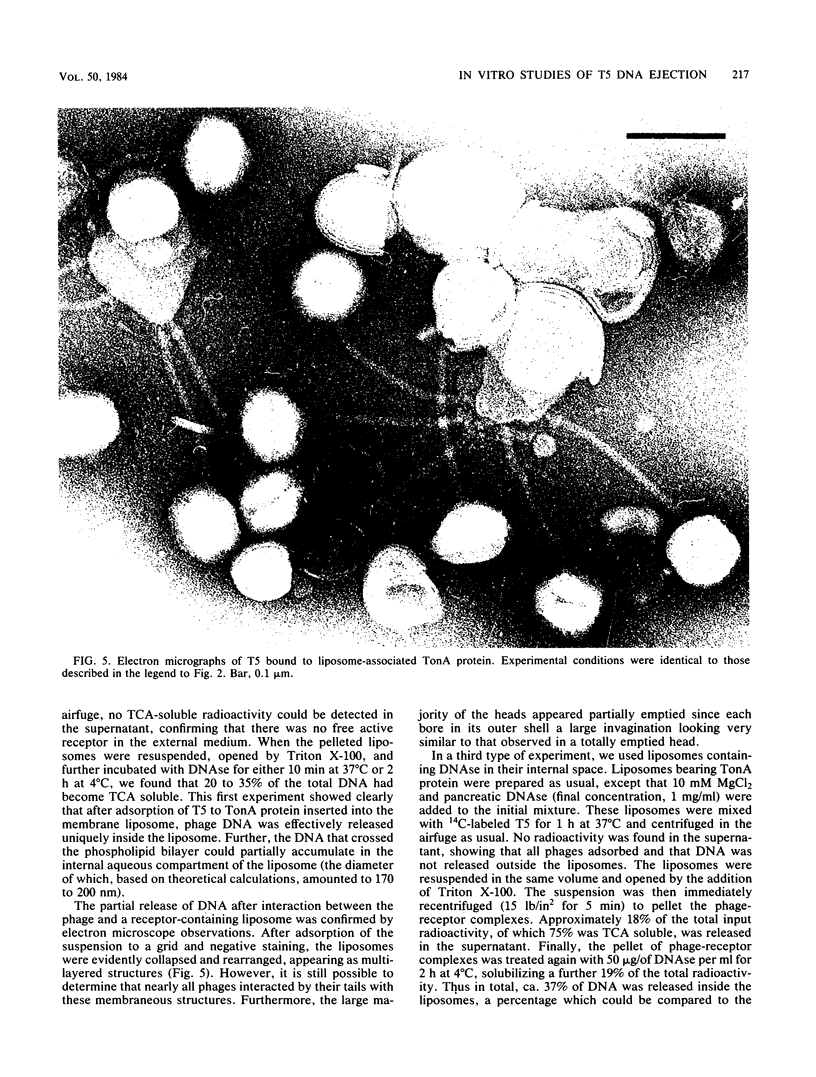

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok M. C., van Deenen L. L., De Gier J. Effect of the gel to liquid crystalline phase transition on the osmotic behaviour of phosphatidylcholine liposomes. Biochim Biophys Acta. 1976 Apr 16;433(1):1–12. doi: 10.1016/0005-2736(76)90172-3. [DOI] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Filali Maltouf A., Labedan B. Host cell metabolic energy is not required for injection of bacteriophage T5 DNA. J Bacteriol. 1983 Jan;153(1):124–133. doi: 10.1128/jb.153.1.124-133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen W. J., Verkley A. J., Zwaal R. F., Van Deenen L. L. Freeze-fracture appearance and disposition of band 3 protein from the human erythrocyte membrane in lipid vesicles. Eur J Biochem. 1978 Apr;85(1):255–261. doi: 10.1111/j.1432-1033.1978.tb12234.x. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Harrison D. P., Bode V. C. Putrescine and certain polyamines can inhibit DNA injection from bacteriophage lambda. J Mol Biol. 1975 Aug 15;96(3):461–470. doi: 10.1016/0022-2836(75)90173-4. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Hulen C., Labedan B., Legault-Demare J. Evidence for heterogeneity in populations of T5 bacteriophage. II. Some particles are unable to inject their second-step-transfer DNA. J Virol. 1980 Dec;36(3):633–638. doi: 10.1128/jvi.36.3.633-638.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B. A very early step in the T5 DNA injection process. Virology. 1976 Dec;75(2):368–375. doi: 10.1016/0042-6822(76)90035-0. [DOI] [PubMed] [Google Scholar]
- Labedan B. In vitro study of the phage T5 DNA injection process: use of columns of Escherichia coli membranes immobilized on kieselguhr. Virology. 1978 Apr;85(2):487–493. doi: 10.1016/0042-6822(78)90455-5. [DOI] [PubMed] [Google Scholar]
- Labedan B., Legault-Demare J. Evidence for heterogeneity in populations of T5 bacteriophage. J Virol. 1974 May;13(5):1093–1100. doi: 10.1128/jvi.13.5.1093-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichi B., Buu A. Integration of the overproduced bacteriophage T5 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1983 Apr;154(1):130–138. doi: 10.1128/jb.154.1.130-138.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner C. A., Struck D. K., Ihler G. M. Injection of DNA into liposomes by bacteriophage lambda. J Biol Chem. 1983 Jan 10;258(1):643–648. [PubMed] [Google Scholar]
- WEIDEL W., KELLENBERGER E. The E. coli B-receptor for the phage T5. II. Electron microscopic studies. Biochim Biophys Acta. 1955 May;17(1):1–9. doi: 10.1016/0006-3002(55)90314-0. [DOI] [PubMed] [Google Scholar]
- Zarybnicky V., Zarybnicka A. Infection process of T5 phages. I. Ejection of T5 DNA on isolated T5 receptors. Virology. 1973 Aug;54(2):318–329. doi: 10.1016/0042-6822(73)90146-3. [DOI] [PubMed] [Google Scholar]
- Zárybnický V. Mechanism of T-even DNA ejection. J Theor Biol. 1969 Jan;22(1):33–42. doi: 10.1016/0022-5193(69)90078-2. [DOI] [PubMed] [Google Scholar]