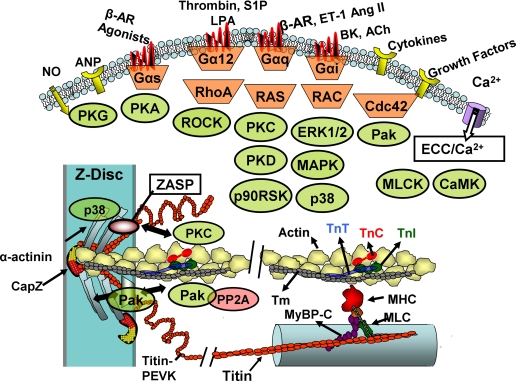
The transient change in tension development and length in a working cardiac myocyte during the heart beat reflects the integrated effects of kinases in signaling cascades regulating mechanisms controlling the dynamics and intensity of a transient increase in cytoplasmic Ca2+ as well as the responsiveness of the sarcomeres to Ca2+. Kinases modifying regulatory membrane proteins represent a major mechanism for controlling the coupling of transmembrane voltage to the release of Ca2+ into the cytoplasm that triggers contraction by binding to TnC2 and for regulating dynamics of the return of Ca2+ to the diastolic state by membrane transporters and exchangers (1). Binding of Ca2+ to TnC triggers a strong reaction of sarcomere thick filament cross-bridges with the thin filament actins and the promotion of force development and shortening (2, 3). Sarcomeres are not passive responders to these transient changes in cytoplasmic Ca2+. Protein-protein interactions downstream of Ca2+-TnC are subject to functionally significant modifications by signaling cascades that modify the number and kinetics of actin-cross-bridge reactions (Fig. 1).
FIGURE 1.
Kinases affecting sarcomeric proteins. Major substrates for these kinases are illustrated in a region of overlap between thin actin-containing and thick myosin-containing filaments. Also shown is a portion of the network of proteins at the Z-disk, which houses docking sites (e.g. ZASP) for kinases. Thin filaments terminate in the Z-disk and are capped by CapZ; thick filaments connect to the Z-disk network via the giant elastic (PEVK region) protein, titin. See text for details. ANP, atrial natriuretic peptide; β-AR, β-adrenergic receptor; LPA, lysophosphatidic acid; S1P, sphingosine 1-phosphate; ET-1, endothelin-1; Ang II, angiotensin II; BK, bradykinin; ACh, acetylcholine; ECC, excitation-contraction coupling; CaMK, calmodulin kinase; MHC, myosin heavy chain; MLC, myosin light chain.
I focus here on control mechanisms at the level of the sarcomere and on kinases immediately upstream of sarcomeric protein substrates. Major substrates are (i) thin filament proteins TnI, TnT, and Tm, which are important in transducing the Ca2+-TnC signal (4, 5); (ii) MyBP-C (6) and RLC (7), which control the radial movement of cross-bridges from the thick filament backbone; and (iii) titin, a giant third filament controlling diastolic tension as well as length-dependent radial movement of cross-bridges (8, 9). Detailed discussion of how phosphorylation modifies the function of these proteins has been reviewed elsewhere (2, 4–9). In general, phosphorylation of thin filament proteins controls sarcomere Ca2+ sensitivity, kinetics of Ca2+ binding to TnC (related to dynamics of relaxation), and the number and kinetics of cross-bridges reacting with the thin filament (related to levels and rates of rise and fall of tension). Phosphorylations of MyBP-C and RLC control Ca2+ sensitivity and rates of contraction/relaxation by modifying the local concentration of cross-bridges at the interface with actins. MyBP-C may also interact with and affect thin filament activation. Cardiac but not skeletal isoforms of titin contain phosphorylation sites within a unique sequence, located in the elastic segment. Phosphorylation of a unique cardiac titin reduces passive tension (8).
To appreciate the potential role of how kinases modify sarcomeric function, it is important to consider the working cardiac myocyte operating in an environment influenced by immediate prevailing mechanical (load and length), neural, endocrine, autocrine, and paracrine control mechanisms and by the short- and long-term history of this environment. Beat-to-beat control mechanisms, which occur, for example, as hemodynamic load rises with exercise or falls with rest, are related to the immediate prevailing regulatory mechanisms. Mechanisms occurring over the time scale of hours, days, and longer are related to growth and remodeling in response to chronic changes in load or chemical environment as occur with sustained bouts of exercise, hypertension, or ischemia. Kinases and phosphorylations play a significant role in compensation and adaptation to beat-to-beat and chronic changes in hemodynamic load. However, maladaptive kinase activation may induce remodeling and phosphorylations of sarcomeric proteins with cardiovascular disorders, leading to heart failure (10, 11).
Multiple Kinases and Hierarchical Phosphorylation of Sarcomeric Proteins Control Beat-to-Beat Changes of Cardiac Dynamics
Kinases acting via G protein-coupled receptors are among the most extensively studied in control of short-term cardiac dynamics (Fig. 1). PKA is the most studied and understood, but other significant kinases are PKG, calmodulin kinase, and MLCK as well as PKC. PKA-dependent phosphorylation of TnI and MyBP-C appears to be dominant in control of sarcomeric function by β-adrenergic stimulation. The special role of these proteins is emphasized by the insertion of sequences with phosphorylation motifs that are unique to the cardiac variants (4–8). TnI has an N-terminal extension of some 30 amino acids that houses Ser23/Ser24; Ser24 is more rapidly phosphorylated by PKA, but both Ser23/Ser24 sites must be phosphorylated to depress sarcomere sensitivity to Ca2+ and to enhance the off-rate for Ca2+ binding to TnC. This sort of hierarchy in kinase-dependent phosphorylation is poorly understood in other sarcomeric proteins. Cardiac MyBP-C has a unique insertion at its N-terminal region that has multiple phosphorylation sites. Together with PKA phosphorylation of membrane proteins, PKA phosphorylation of both TnI and MyBP-C at these sites is critical to the reduction in cycle time of the heart beat required to tune the cycle to the increased frequency in exercise (12–14). What is not clear is the exact role of the multiple phosphorylations and the multiple kinases potentially involved.
Beat-to-beat regulation of cardiac function is likely to involve paracrine effects involving NO. Effects of NO on cardiac myocytes provide an interesting example of kinase signaling to cardiac sarcomeres in which the same signal engages distinct cAMP- and cGMP-dependent pathways. Whereas low levels of NO increase cardiac contraction, relatively high levels of NO activate guanylate cyclase and induce activation of PKG, which in turn is responsible for a Ca2+-independent depression in cellular mechanics (15). The mechanism involves depression of the response of the sarcomeres to Ca2+, but the substrates modified remain unclear. One likely substrate is TnI, which has been known for some time as a substrate for PKG at Ser23/Ser24 (4). The PKG pathway is also downstream of natriuretic peptide receptors, and recent data support a role of the NO-cGMP-PKG pathway in effects of β3-adrenoreceptors in controlling cardiac relaxation dynamics; a role for sarcomeric protein phosphorylation remains unclear (16).
The role of other kinases in short-term regulation of cardiac dynamics appears likely, but remains poorly understood. G protein signaling through α1-adrenergic receptors with activation of PKC potentially induces phosphorylation of a number of sarcomeric proteins, including TnI, TnT, RLC, MyBP-C, and titin. The PKC sites in TnI are the best studied and serve to illustrate what may be general properties of proteins with multisite isoform-specific phosphorylations by different kinases. PKC cross-phosphorylates TnI Ser23/Ser24 and induces the same functional effects as PKA phosphorylation (17, 18). There is also PKC phosphorylation of TnI Thr144, which may sensitize (19) or desensitize (20) the myofilaments to Ca2+, and TnI Ser43/Ser45, which depresses maximum tension and desensitizes the sarcomeres to Ca2+. Thr144 is the most accessible of the sites, but site-specific phosphorylation has not been determined under physiological conditions during short-term control of cardiac function. Isoform specificity in the phosphorylation of these sites was demonstrated by Noland et al. (17), who reported that PKCδ preferentially phosphorylated TnI Ser23/Ser24, whereas PKCα preferentially phosphorylated TnI Ser43/Ser45. Moreover, the substrate specificity of TnI Ser23/Ser24 for PKC isoforms may depend on the state of downstream phosphorylation sites. In vitro studies with reconstituted preparations demonstrated enhanced kinetics of phosphorylation of Ser23/Ser24 in Tn complexes containing TnI T144A (17). In transgenic mice expressing TnI S43A/S45A in the heart, there was significantly enhanced phosphorylation of TnI Ser23/Ser24 (21). A hierarchy in the phosphorylation of TnI has been demonstrated in experiments testing the effects of endothelin on dynamics of contraction of cardiac myocytes. Westfall et al. (22) reported that activation of PKC by endothelin induced an acute and prolonged enhancement of relaxation rate associated with a time-dependent phosphorylation of different sites on TnI. Thr144 was phosphorylated early after administration of endothelin, and Ser23 and Ser24 were phosphorylated after prolonged administration of endothelin-1. Phosphorylation at specific Tyr residues associated with oxidative stress-related activation of Src induces a change in substrate specificity of PKCδ (23). Studies in our laboratories indicate that the specificity for phosphorylation of sites on TnI is also subject to PKCδ Tyr phosphorylation (24).
Although likely to be highly significant, the functional significance of PKC-dependent phosphorylation of sarcomeric proteins other than TnI remains poorly understood. MyBP-C has long been known to be a substrate for PKC, but the functional significance of these sites in short-term control of cardiac function remains unclear (4). Cross-phosphorylation by PKA and PKC sites of MyBP-C appears possible, and interplay among the phosphorylation sites is likely. These possibilities have not been rigorously investigated. Myosin RLC2 is also a substrate for PKC (4), but its role in beat-to-beat regulation is not apparent. Andersen et al. (25) reported that the positive inotropic effect of acute α1-adrenergic stimulation involved PKC-independent phosphorylation of RLC. In their studies, Ca2+-calmodulin-dependent phosphorylation through MLCK was the main factor responsible for the increase in left ventricular function. However, Wang et al. (26) reported that, unlike the case with left ventricular muscle preparations, inhibitors of MLCK did not abrogate the response to α1-adrenergic stimulation in right ventricular preparations. This result agreed with earlier studies (4) using right ventricular muscle preparations from transgenic mouse models, which indicated that α1-adrenergic and PKC-mediated phosphorylation of Ser43/Ser45 of TnI plays an important role in regulating force development in the intact myocardium. Species-specific differences and variations in signaling complexity need to be taken into account when assessing the role of α1-adrenergic agonists.
Kinases Engaged in Cardiac Stress and Growth Signaling Phosphorylate Sarcomeric Proteins
Kinases that couple extracellular stimuli to the promotion of cell growth or maladaptive responses with stresses on the heart also have the potential to modify function by phosphorylating sarcomeric proteins. Common long-term stresses on the heart include ischemia/hypoxia, hypertension, altered redox environment, and elaboration of cytokines. These stresses activate PKC isoforms differentially in the same animal models and may activate PKC isoforms differentially in different animal models (27, 28). For example, in guinea pig hearts, ischemia/hypoxia activated PKCα, PKCβ2, PKCγ, and PKCζ. Oxidative stress simulated by H2O2 induced activation of PKCα, PKCβ2, and PKCζ, which, in contrast to the case with ischemia/hypoxia, was not blocked by inhibitors of tyrosine kinase or phospholipase C (27). Although it is certain that there are multiple effects of the activation of these isoforms of PKC, they all apparently affect sarcomere response to Ca2+. Interest in PKCα and PKCβ2 increased greatly with the demonstration that these isoforms are up-regulated in end-stage heart failure (29). Other PKC isoforms reported to be expressed in human myocardium are PKCα, PKCβ1, PKCδ, and PKCζ, with PKCθ and PKCγ missing (27) Whatever the case, the effects of PKCβ2 activation are not clear. Compared with controls, skinned fiber preparations isolated from hearts harboring an inducible PKCβ2 that was turned on at 10 weeks of age and remained on for 10 months demonstrated a reduction in maximum tension with no change in Ca2+ sensitivity (30). This result agreed with data of Takeishi et al. (28), who concluded that TnI phosphorylation was associated with depression in cardiac function in a mouse model overexpressing PKCβ2, yet one study has concluded that PKCβ2 phosphorylation of TnI enhanced myofilament Ca2+ sensitivity (19). However, detailed studies of sites of phosphorylation were not carried out.
The results of studies on the role of altered kinase activity in disease models and on human heart samples are conflicting and, in some cases, difficult to interpret. Studies on detergent-extracted (skinned) myocytes from rat models of hypertrophy/failure (myocardial infarction and pressure overload) demonstrated a depression in maximum tension and Ca2+ sensitivity and indicated a causal role of Tn phosphorylation (31). Moreover, in these models, these effects appear likely to be induced not by activation of PKCβ2 but by PKCα. Thus, the relative role of PKCβ2 and PKCα in human heart failure requires further study. PKCα, as well as heart failure, induces an increase in highly charged TnI species, but the exact sites have not been determined. It is also significant that PKCα preferentially phosphorylates a TnT site (Thr206, mouse sequence) over PKCβ2. Phosphorylation of TnT Thr206 significantly depresses force and Ca2+ sensitivity of skinned fibers (32). Notably, there are other PKC sites on TnT, and phosphorylation of these sites influences the functional effects of Thr206 phosphorylation.
The role of sarcomeric protein alterations associated with the effects of novel Ca2+-independent isozymes PKCε and PKCδ has been studied, and although there is much additional work to be done, the results appear significant in relation to the complex effects of these kinases. Constitutive, relatively long-term activation of both PKCε and PKCδ induces a hypertrophy, and eventually dilated cardiomyopathy, that is apparently indistinguishable with regard to histology and markers (33). Evidence that the effects of long-term activation of PKC may involve sarcomeric protein phosphorylation comes from studies demonstrating a resistance to development of dilated cardiomyopathy in hearts of double-transgenic mice expressing mutant TnI S43A/S45A as well as elevated levels of PKCε (34), yet acute activation of PKCε protects against, whereas PKCδ exacerbates, injury associated with ischemia/reperfusion (33). Phosphorylation of sarcomeric proteins, which may be important in these differential responses, has not been studied in detail, but multiple effects of these kinases may be involved. Phosphorylation levels of MyBP-C have been explicitly identified as a significant factor in acute (35) and chronic (36) low flow ischemia, with a calmodulin kinase site appearing to be of significance (37).
Although there is much work to be done to understand the role of the atypical PKCζ in control of cardiac function, there are data providing clues as to its significance. Signaling to PKCζ occurs by various extracellular stimuli such as nerve growth factor and interleukin-1 (38), and there is evidence that stresses on the myocardium such as myocardial infarction and volume overload lead to increased expression of PKCζ (39). We (40) reported that the atypical PKCζ isoform associates specifically with TnI in adult rat ventricular cardiac myocytes and modulates myofilament protein phosphorylation. Using mutant forms that mimic PKCζ activation, we demonstrated an increase in Tm phosphorylation and MyBP-C, but, surprisingly, there was a dephosphorylation of Thr residues in TnI and TnT in cardiac myocytes. One active form of PKCζ exists in complex with Pak1 (p21-activated kinase 1) and PP2A (protein phosphatase 2A).
In addition to evidence of direct phosphorylation of sarcomeric proteins by PKCs, downstream effectors of PKCs are also involved in multiple signaling cascades, which modify phosphorylation of sarcomeric proteins (Fig. 1). These downstream kinases include p38 MAPK (41) and PKD (42). Constitutive activation of p38 MAPK in conditional transgenic mouse hearts induces a depression in myocyte mechanics with no effects on Ca2+ fluxes. Skinned fibers from these hearts demonstrated a depression in tension and a decrease in Tm phosphorylation apparently due to activation of a phosphatase by p38 (41). Identification of TnI as a prominent binding partner of PKD led to the demonstration that PKD phosphorylates TnI Ser23/Ser24. Although PKD activation is poorly understood, there is evidence in rat ventricular myocytes that it is downstream of PKC activation, particularly PKCε (42). Moreover, endothelin-1-induced activation of PKD appears to be suppressed by concomitant activation of PKA (42). This finding, which indicates a counter-regulatory role for these two kinases in modification of cardiac Tn function, provides evidence of the complexity of control mechanisms of sarcomeric function. Along these lines, PKD activation was demonstrated to be increased in association with the increased glucose uptake and increased GLUT4 activation associated with contractile activity of cardiac myocyte suspensions (43). This change occurred independently of AMP kinase activation and suggests a potential role for PKC/PKD in linking increased energy demands to increased energy supply. PKD may also coordinate cell growth and function through its phosphorylation of HDAC5 (44). In neonatal rat ventricular myocytes, PKC activation appears sufficient and apparently necessary for phosphorylation and nuclear export of HDAC5. Export of HDAC5 releases its effects on chromatin and its repression of genes that induce growth and maladaptive remodeling.
As illustrated in Fig. 1, kinases activated through small G protein (Rac, RhoA, and Ras) signaling also modify sarcomeric protein phosphorylation. RhoA signaling through ROCK (Rho-activated kinase) has been reported to not only influence RLC phosphorylation through inhibition of myosin phosphatase (4) but also to directly phosphorylate TnI and TnT (45). Evidence still evolving indicates that Pak1, which is downstream of Rac and Cdc42, induces dephosphorylation of TnI and MyBP-C by PP2A (46). Ras signaling through ERK1/2 to p90RSK (ribosomal S6 kinase) induces phosphorylation of TnI Ser23/Ser24 (47). Specific inhibitors of PKCβ2 also suppressed the effects of H2O2 in activating p90RSK and in phosphorylation of TnI Ser23/Ser24.
Sarcomeric Proteins Serve as Scaffolds for Kinases That May Serve to Link Kinase Activity and Localization to Mechanical State
Sarcomeric proteins not only are substrates for kinases activated by remote receptor-activated signaling cascades but also participate in instigation of local signaling. Kinases and phosphatases are known to dock at sarcomeric sites, notably the Z-disk protein network and its linkage to costameres, and to be released from these sites in a process likely to be related to mechanical state (48, 49). Fig. 1 indicates a few examples. There is limited appreciation in the literature of the localization of PKCs and MAPKs in non-membrane structures of the sarcomere.
Major Challenges and Therapeutic Implications
Apart from what kinases do in the heart to regulate contractility, we need to know when and where they do it. This brief review reflects our ignorance of these aspects of the role of kinases in cardiac contractility. Together with complex hierarchical phosphorylations controlling sarcomeric function, the complexity of potential intramolecular and intermolecular interactions among multisite- and multikinase-dependent phosphorylations in sarcomeric proteins poses considerable challenges to the quest to understand relations between normal and disordered cardiac function and protein phosphorylation. The significance of kinase activation in the heart beat needs to be studied in preparations that are as close to the physiological state and as close to the physiological signaling environment as possible. We need to fully integrate functional effects of phosphorylation of sarcomeric and membrane proteins into adaptive signaling as well as maladaptive signaling that triggers and sustains the growth and remodeling processes leading to heart failure and sudden death. This understanding is especially critical in the search for therapies for heart failure and sudden death (50).
This work was supported, in whole or in part, by National Institutes of Health Grants HL 62426, HL 64035, and HL 22231. This minireview will be reprinted in the 2008 Minireview Compendium, which will be available in January, 2009.
Footnotes
The abbreviations used are: Tn, troponin; Tm, tropomyosin; MyBP-C, myosin-binding protein C; RLC, myosin regulatory light chain; PK, protein kinase; MLCK, myosin light chain kinase; MAPK, mitogen-activated protein kinase.
References
- 1.Bers, D. M. (2001) Excitation-Contraction Coupling and Cardiac Contractile Force, 2nd Ed., Kluwer Academic Publishers, Norwell, MA
- 2.Kobayashi, T., and Solaro, R. J. (2005) Annu. Rev. Physiol. 67 39–67 [DOI] [PubMed] [Google Scholar]
- 3.Hinken, A., and Solaro, R. J. (2007) Physiology 22 73–80 [DOI] [PubMed] [Google Scholar]
- 4.Solaro, R. J. (2001) in Handbook of Physiology (Page, E., Fozzard, H., and Solaro, R. J., eds) Section 2, Vol. 1, pp. 264–300, Oxford University Press, New York [Google Scholar]
- 5.Metzger, J. M., and Westfall, M. V. (2004) Circ. Res. 94 146–158 [DOI] [PubMed] [Google Scholar]
- 6.Moss, R. L., and Fitzsimons, D. P. (2006) Circ. Res. 99 225–227 [DOI] [PubMed] [Google Scholar]
- 7.Flashman, E., Redwood, C., Moolman-Smook, J., and Watkins, H. (2004) Circ. Res. 94 1279–1289 [DOI] [PubMed] [Google Scholar]
- 8.Granzier, H., and Labeit, S. (2007) Muscle Nerve 36 740–755 [DOI] [PubMed] [Google Scholar]
- 9.Linke, W. A. (2008) Cardiovasc. Res. 77 637–648 [DOI] [PubMed] [Google Scholar]
- 10.Solaro, R. J., and de Tombe, P. P. (2008) Cardiovasc. Res. 77 616–618 [DOI] [PubMed] [Google Scholar]
- 11.Hamdani, N., Kooij, V., van Dijk, S., Merkus, D., Paulus, W. J., Remedios, C. D., Duncker, D. J., Stienen, G. J., and van der Velden, J. (2008) Cardiovasc. Res. 77 649–658 [DOI] [PubMed] [Google Scholar]
- 12.Kentish, J. C., McCloskey, D. T., Layland, J., Palmer, S., Leiden, J. M., Martin, A. F., and Solaro, R. J. (2001) Circ. Res. 88 1059–1065 [DOI] [PubMed] [Google Scholar]
- 13.Cazorla, O., Szilagyi, S., Vignier, N., Salazar, G., Krämer, E., Vassort, G., Carrier, L., and Lacampagne, A. (2006) Cardiovasc. Res. 69 370–380 [DOI] [PubMed] [Google Scholar]
- 14.Stelzer, J. E., Patel, J. R., Walker, J. W., and Moss, R. L. (2007) Circ. Res. 101 503–511 [DOI] [PubMed] [Google Scholar]
- 15.Vila-Petroff, M. G., Younes, A., Egan, J., Lakatta, E. G., and Sollott, S. J. (1999) Circ. Res. 84 1020–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelone, T., Filice, E., Quintieri, A. M., Imbrogno, S., Recchia, A., Pulerà, E., Mannarino, C., Pellegrino, D., and Cerra, M. C. (2008) Acta Physiol. 193 229–239 [DOI] [PubMed] [Google Scholar]
- 17.Noland, T. A., Jr., Guo, X., Raynor, R. L., Jideama, N. M., Averyhart-Fullard, V., Solaro, R. J., and Kuo, J. F. (1995) J. Biol. Chem. 270 25445–25454 [DOI] [PubMed] [Google Scholar]
- 18.Noland, T. A., Jr., Raynor, R. L., Jideama, N. M., Guo, X., Kazanietz, M. G., Blumberg, P. M., Solaro, R. J., and Kuo, J. F. (1996) Biochemistry 35 14923–14931 [DOI] [PubMed] [Google Scholar]
- 19.Wang, H., Grant, J. E., Doede, C. M., Sadayappan, S., Robbins, J., and Walker, J. W. (2006) J. Mol. Cell. Cardiol. 41 823–833 [DOI] [PubMed] [Google Scholar]
- 20.Burkart, E. M., Sumandea, M. P., Kobayashi, T., Nili, M., Homsher, E., and Solaro, R. J. (2003) J. Biol. Chem. 278 11265–11272 [DOI] [PubMed] [Google Scholar]
- 21.Roman, B. B., Goldspink, P. H., Spaite, E., Urboniene, D., McKinney, R., Geenen, D. L., Solaro, R. J., and Buttrick, P. M (2004) Am. J. Physiol. 286 H2089–H2095 [DOI] [PubMed] [Google Scholar]
- 22.Westfall, M. V., Lee, A. M., and Robinson, D. A. (2005) J. Biol. Chem. 280 41324–41331 [DOI] [PubMed] [Google Scholar]
- 23.Steinberg, S. F. (2004) Biochem. J. 384 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumandea, M. P., Rybin, V. O., Hinken, A. C., Wang, C., Kobayashi, T., Harleton, E., Sievert, G., Balke, C. W., Feinmark, S. J., Solaro, R. J., and Steinberg, S. F. (2008) J. Biol. Chem. 283 22680–22689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen, G. ø., Qvigstad, E., Schiander, I., Aass, H., Osnes, J. B., and Skomedal, T. (2002) Am. J. Physiol. 283 H1471–H1480 [DOI] [PubMed] [Google Scholar]
- 26.Wang, G. Y., McCloskey, D. T., Turcato, S., Swigart, P. M., Simpson, P. C., and Baker, A. J. (2006) Am. J. Physiol. 291 H2013–H2017 [DOI] [PubMed] [Google Scholar]
- 27.Shin, H. G., Barnett, J. V., Chang, P., Reddy, S., Drinkwater, D. C., Pierson, R. N., Wiley, R. G., and Murray, K. T. (2000) Cardiovasc. Res. 48 285–299 [DOI] [PubMed] [Google Scholar]
- 28.Takeishi, Y., Chu, G., Kirkpatrick, D. M., Li, Z., Wakasaki, H., Kranias, E. G., King, G. L., and Walsh, R. A. (1998) J. Clin. Investig. 102 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowling, N., Walsh, R. A., Song, G., Estridge, T., Sandusky, G. E., Fouts, R. L., Mintze, K., Pickard, T., Roden, R., Bristow, M. R., Sabbah, H. N., Mizrahi, J. L., Gromo, G., King. G. L., and Vlahos, C. J. (1999) Circulation 99 384–391 [DOI] [PubMed] [Google Scholar]
- 30.Huang, L., Wolska, B. M., Montgomery, D. E., Burkart, E., Buttrick, P. M., and Solaro, R. J. (2001) Am. J. Physiol. 280 C1114–C1120 [DOI] [PubMed] [Google Scholar]
- 31.Belin, R. J., Sumandea, M. P., Allen, E. A., Schoenfelt, K., Wang, H., Solaro, R. J., and de Tombe, P. P. (2007) Circ. Res. 101 195–204 [DOI] [PubMed] [Google Scholar]
- 32.Sumandea, M. P., Pyle, W. G., Kobayashi, T., de Tombe, P. P., and Solaro, R. J. (2003) J. Biol. Chem. 278 35135–35144 [DOI] [PubMed] [Google Scholar]
- 33.Chen, L., Hahn, H., Wu, G., Chen, C. H., Liron, T., Schechtman, D., Cavallaro, G., Banci, L., Guo, Y., Bolli, R., Dorn, G. W., II, and Mochly-Rosen, D. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scruggs, S. B., Walker, L. A., Lyu, T., Geenen, D. L., Solaro, R. J., Buttrick, P. M., and Goldspink, P. H. (2006) J. Mol. Cell. Cardiol. 40 465–473 [DOI] [PubMed] [Google Scholar]
- 35.Sadayappan, S., Osinska, H., Klevitsky, R., Lorenz, J. N., Sargent, M., Molkentin, J. D., Seidman, C. E., Seidman, J. G., and Robbins, J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16912–16923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decker, R. S., Decker, M. L., Kulikovskaya, I., Nakamura, S., Lee, D. C., Harris, K., Klocke, F. J., and Winegrad, S. (2005) Circulation 111 906–912 [DOI] [PubMed] [Google Scholar]
- 37.Yuan, C., Guo, Y., Ravi, R., Przyklenk, K., Shilkofski, N., Diez, R., Cole, R. N., and Murphy, A. M. (2006) Proteomics 6 4176–4186 [DOI] [PubMed] [Google Scholar]
- 38.Hirai, T., and Chida, K. (2003) J. Biochem. (Tokyo) 133 1–7 [DOI] [PubMed] [Google Scholar]
- 39.Wang, J., Liu, X., Sentex, E., Takeda, N., and Dhalla, N. S. (2003) Am. J. Physiol. 284 H2277–H2287 [DOI] [PubMed] [Google Scholar]
- 40.Wu, S. C., and Solaro, R. J. (2007) J. Biol. Chem. 282 30691–30698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vahebi, S., Ota, A., Li, M., Warren, C. M., de Tombe, P. P., Want, Y., and Solaro, R. J. (2007) Circ. Res. 100 408–415 [DOI] [PubMed] [Google Scholar]
- 42.Avkiran, M., Rowland, A. J., Cuello, F., and Haworth, R. S. (2008) Circ. Res. 102 157–163 [DOI] [PubMed] [Google Scholar]
- 43.Luiken, J. J., Vertommen, D., Coort, S. L. M., Habets, D. D. J., El Hasnaoui, M. E., Pelsers, M. M. L., Viollet, B., Bonen, A., Hue, L., Rider, M. H., and Glatz, J. F. C. (2008) Cell. Signal. 20 543–556 [DOI] [PubMed] [Google Scholar]
- 44.Vega, R. B., Harrison, B. C., Meadows, E., Roberts, C. R., Papst, P. J., Olson, E. N., and McKinsey, T. A. (2004) Mol. Cell. Biol. 24 8374–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vahebi, S., Kobayashi, T., Warren, C. M., de Tombe, P. P., and Solaro, R. J. (2005) Circ. Res. 96 740–747 [DOI] [PubMed] [Google Scholar]
- 46.Sheehan, K. A., Ke, Y., and Solaro, R. J. (2007) Am. J. Physiol. 293 R963–R973 [DOI] [PubMed] [Google Scholar]
- 47.Itoh, S., Ding, B., Bains, C. P., Wang, N., Takeishi, Y., Jalili, T., King, G. L., Walsh, R. A., Yan, C., and Abe, J. (2005) J. Biol. Chem. 280 24135–24142 [DOI] [PubMed] [Google Scholar]
- 48.Pyle, W. G., and Solaro, R. J. (2004) Circ. Res. 94 296–305 [DOI] [PubMed] [Google Scholar]
- 49.Hoshijima, M. (2006) Am. J. Physiol. 290 H1313–H1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mudd, J. O., and Kass, D. A. (2008) Nature (2008) 451 919–928 [DOI] [PubMed] [Google Scholar]