Abstract
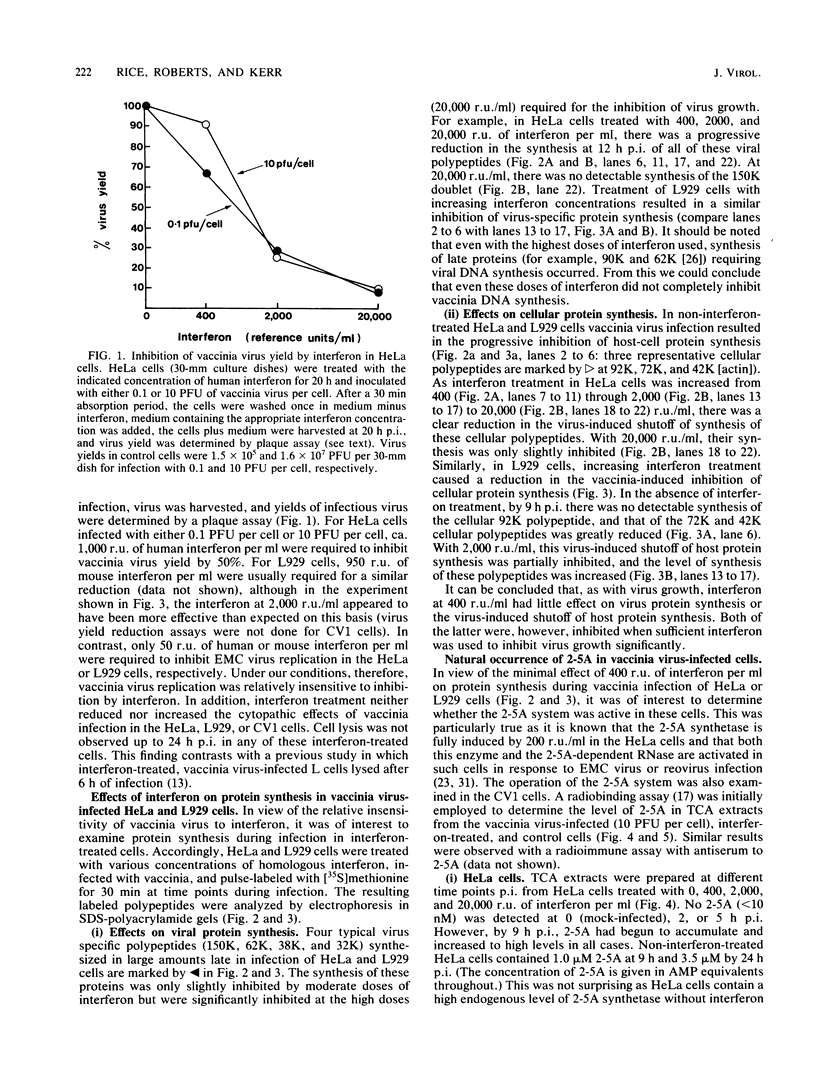
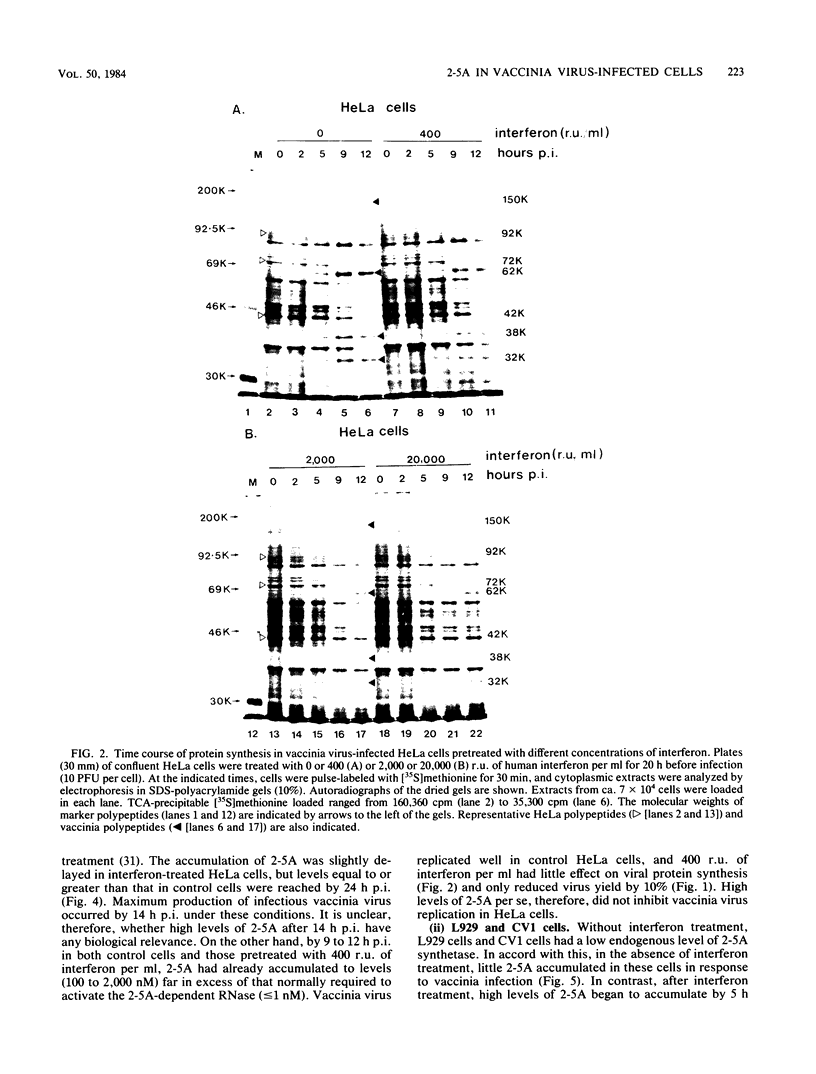
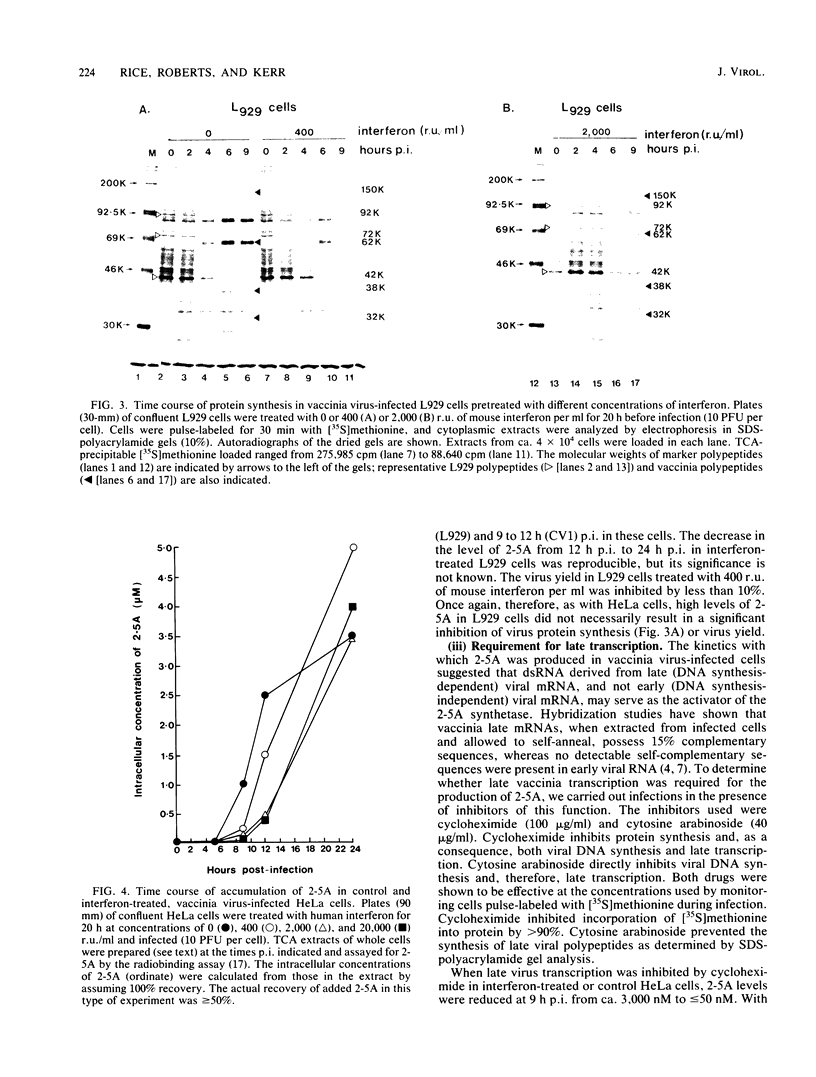
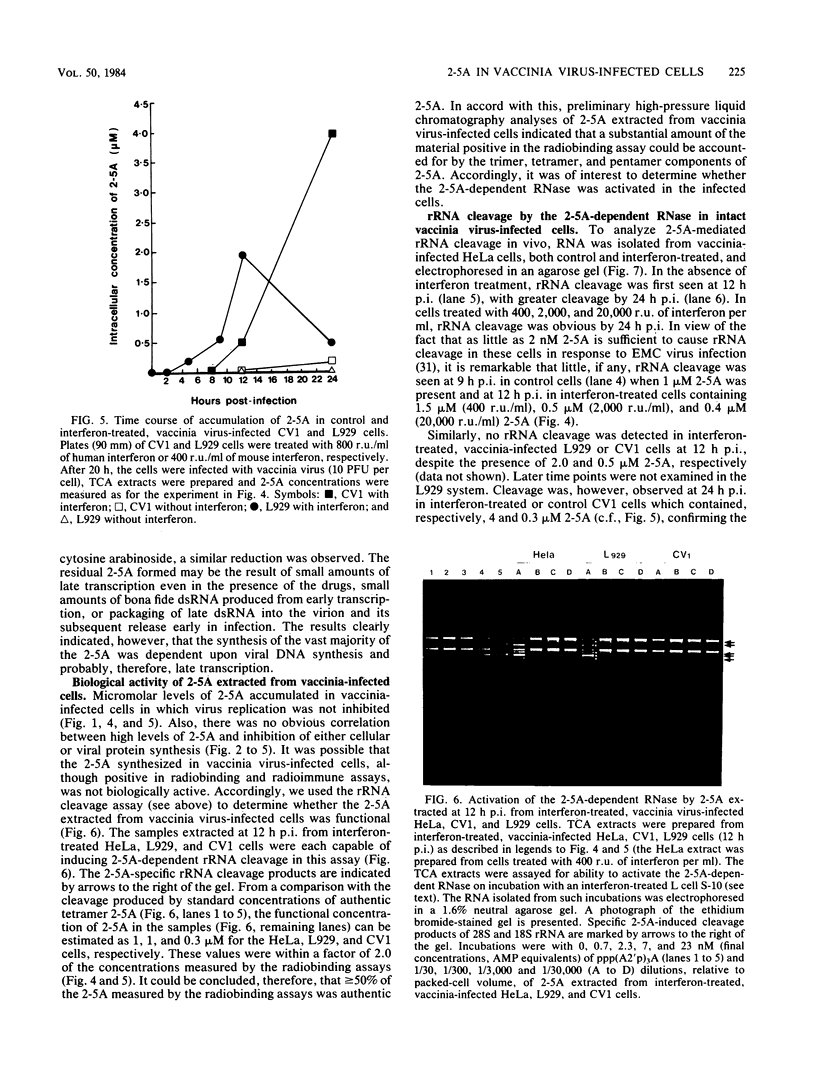
We investigated the effects of interferon treatment on virus yield, protein synthesis, and the 2-5A system in vaccinia virus-infected HeLa, L929, and CV1 cells. Under the culture conditions used, vaccinia virus replication was relatively insensitive to the antiviral effects of interferon. In L929 and HeLa cells, interferon at 400 reference units (r.u.) per ml had little effect on viral protein synthesis, the virus-induced inhibition of host protein synthesis, or virus yield: 2,000 to 20,000 r.u./ml were required to inhibit these. Despite this, high levels (up to 5 microM) of 2-5A [ppp(A2'p)nA; n greater than or equal to 2] were found during vaccinia infection of all of these types of cells treated with 400 r.u. of interferon per ml, i.e., at interferon concentrations too low to inhibit significantly virus growth. High levels (up to 5 microM) were also found in non-interferon-treated HeLa cells (which have a high constitutive level of 2-5A synthetase) in which vaccinia virus replicates perfectly well. It can be concluded that high levels of 2-5A per se have no necessary antiviral effect on vaccinia virus in these systems. These results are in marked contrast to those obtained here and previously with encephalomyocarditis virus. For example, in HeLa cells less than 20 nM 2-5A accumulated, but virus replication was inhibited by 50 r.u. of interferon per ml (Silverman et al., Eur. J. Biochem. 124:131-138, 1982). The characteristic cleavage of rRNA by the 2-5A-dependent RNase was delayed relative to 2-5A accumulation in the vaccinia virus-infected cells. This delay was not the result of either defective 2-5A or of a stable virus-induced inhibition of the 2-5A-dependent RNase; 2-5A extracted from the cells had full biological activity when assayed by activation of the 2-5A-dependent RNase in cell extract, and the 2-5A-dependent RNase extracted from the vaccinia virus-infected cells was fully active in vitro. The basis for the delay remains to be determined. High levels of 2-5A were not observed when late (DNA synthesis-dependent) vaccinia transcription was inhibited by either cycloheximide or cytosine arabinoside. The only known activator of the 2-5A synthetase is double-stranded RNA. The presence of 2-5A therefore implies the natural occurrence of double-stranded structures in late viral RNA in intact vaccinia virus-infected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Fantes K. H., Burke D. C., Morser J. Analysis and purification of human lymphoblastoid (Namalwa) interferon using a monoclonal antibody. J Gen Virol. 1982 Nov;63(Pt 1):207–212. doi: 10.1099/0022-1317-63-1-207. [DOI] [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Bialy H. S., Colby C. Inhibition of early vaccinia virus ribonucleic acid synthesis in interferon-treated chicken embryo fibroblasts. J Virol. 1972 Feb;9(2):286–289. doi: 10.1128/jvi.9.2.286-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone R. F., Parr R. P., Moss B. Intermolecular duplexes formed from polyadenylylated vaccinia virus RNA. J Virol. 1979 Apr;30(1):365–374. doi: 10.1128/jvi.30.1.365-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayley P. J., Knight M., Kerr I. M. Virus-mediated inhibition of the ppp(A2'p)nA system and its prevention by interferon. Biochem Biophys Res Commun. 1982 Jan 29;104(2):376–382. doi: 10.1016/0006-291x(82)90647-7. [DOI] [PubMed] [Google Scholar]
- Cayley P. J., White R. F., Antoniw J. F., Walesby N. J., Kerr I. M. Distribution of the ppp(A2'p)nA-binding protein and interferon-related enzymes in animals, plants, and lower organisms. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1243–1250. doi: 10.1016/0006-291x(82)92133-7. [DOI] [PubMed] [Google Scholar]
- Colby C., Duesberg P. H. Double-stranded RNA in vaccinia virus infected cells. Nature. 1969 Jun 7;222(5197):940–944. doi: 10.1038/222940a0. [DOI] [PubMed] [Google Scholar]
- DALES S., KAJIOKA R. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. I. UPTAKE AND PENETRATION. Virology. 1964 Nov;24:278–294. doi: 10.1016/0042-6822(64)90167-9. [DOI] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Inhibition of early vaccinia virus protein synthesis in interferon-treated chicken embryo fibroblasts. J Gen Virol. 1973 Jul;20(1):111–115. doi: 10.1099/0022-1317-20-1-111. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen H., Czarniecki C. W., Krause D., Friedman R. M., Silverman R. H. Interferon-induced synthesis of 2-5A-dependent RNase in mouse JLS-V9R cells. Virology. 1983 Mar;125(2):496–501. doi: 10.1016/0042-6822(83)90222-2. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAJIOKA R., SIMINOVITCH L., DALES S. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. II. INITIATION OF DNA SYNTHESIS AND MORPHOGENESIS. Virology. 1964 Nov;24:295–309. doi: 10.1016/0042-6822(64)90168-0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Ball L. A. Increased sensitivity of cell-free protein synthesis to double-stranded RNA after interferon treatment. Nature. 1974 Jul 5;250(461):57–59. doi: 10.1038/250057a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Friedman R. M., Brown R. E., Ball L. A., Brown J. C. Inhibition of Protein Synthesis in Cell-Free Systems from Interferon-Treated, Infected Cells: Further Characterization and Effect of Formylmethionyl-tRNA(F). J Virol. 1974 Jan;13(1):9–21. doi: 10.1128/jvi.13.1.9-21.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Cayley P. J., Silverman R. H., Wreschner D. H., Gilbert C. S., Brown R. E., Kerr I. M. Radioimmune, radiobinding and HPLC analysis of 2-5A and related oligonucleotides from intact cells. Nature. 1980 Nov 13;288(5787):189–192. doi: 10.1038/288189a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J Biol Chem. 1982 Dec 25;257(24):14593–14596. [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Maintenance of protein synthesis in spite of mRNA breakdown in interferon-treated HeLa cells infected with reovirus. Mol Cell Biol. 1983 Jan;3(1):64–69. doi: 10.1128/mcb.3.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Kerr I. M. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984 Apr;50(1):229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Rösel J., Jungwirth C. Isolation of early viral proteins from poxvirus-infected chick embryo fibroblasts by DNA-cellulose chromatography and inhibition of their synthesis by chicken interferon. Eur J Biochem. 1983 May 2;132(2):361–367. doi: 10.1111/j.1432-1033.1983.tb07371.x. [DOI] [PubMed] [Google Scholar]
- Sharma O. K., Goswami B. B. Inhibition of vaccinia mRNA methylation by 2',5'-linked oligo(adenylic acid) triphosphate. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2221–2224. doi: 10.1073/pnas.78.4.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. H., Cayley P. J., Knight M., Gilbert C. S., Kerr I. M. Control of the ppp(a2'p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem. 1982 May;124(1):131–138. doi: 10.1111/j.1432-1033.1982.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Dower W. J., Schimke R. T., Brown R. E., Kerr I. M. 2-5A synthetase: assay, distribution and variation with growth or hormone status. Nature. 1979 Mar 29;278(5703):471–473. doi: 10.1038/278471a0. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Brown R. E., Gilbert C. S., Kerr I. M. Natural occurrence of 2-5A in interferon-treated EMC virus-infected L cells. Nature. 1979 Dec 6;282(5739):582–586. doi: 10.1038/282582a0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]