Abstract
Trehalose 6,6′-dimycolate (TDM) is a glycolipid component of the mycobacterial cell wall that causes immune responses in mice similar to Mycobacterium tuberculosis (MTB) infection, including granuloma formation with production of proinflammatory cytokines. The precise roles of tumour necrosis factor (TNF)-α, complement C5 and interleukin (IL)-6 in the molecular events that lead to the initiation and maintenance of the granulomatous response to TDM have not been fully elucidated. Macrophage proinflammatory responses from wild-type and complement-deficient mice after infection with MTB were assessed, and compared to responses from organisms in which surface TDM had been removed. Removal of TDM abolished proinflammatory responses, markedly so in the complement-deficient macrophages. Mice deficient in TNF-α, C5a and IL-6, along with wild-type C57BL/6 controls, were intravenously injected with TDM in a water-in-oil emulsion, and analysed for histological response and cytokine production in lungs. Wild-type C57BL/6 mice formed granulomas with increased production of IL-1β, IL-6, TNF-α, macrophage inflammatory protein-1α (MIP-1α), IL-12p40, interferon-γ (IFN-γ), and IL-10 protein and mRNA. TNF-α-deficient mice failed to produce a histological response to TDM, with no increases in cytokine production following TDM administration. While C5a-deficient mice exhibited inflammation, they did not form structured granulomas and initially had decreased production of proinflammatory mediators. IL-6-deficient mice initiated granuloma formation, but failed to maintain the granulomas through day 7 and demonstrated decreased early production of proinflammatory mediators in comparison to wild-type mice. These data suggest that TNF-α is critical for initiation of the granulomatous response, C5a is necessary for formation of cohesive granulomas, and IL-6 plays a key role in the granuloma maintenance response to mycobacterial TDM.
INTRODUCTION
Mycobacteria cause a wide range of infectious pathologies that are a significant cause of morbidity and mortality. Disease due to Mycobacterium tuberculosis (MTB) is responsible for an estimated two million deaths each year (WHO, 2003). Infection is classically described as a pulmonary pathology in which the formation of granulomas results in containment of organisms. However, disseminated disease may also occur in which any organ system can be infected, including the kidneys, bones, central nervous system and those of the digestive tract (Cagatay et al., 2004). The mechanisms underlying protective granuloma formation which lead to the prevention of disseminated disease have not been fully elucidated. In light of the increasing number of therapeutics being developed to target cytokines in the treatment of chronic immune-mediated disease, it is clinically relevant to understand the role these molecules play in the initiation and maintenance of the granulomatous response.
Tumour necrosis factor-α (TNF-α) is thought to be the major cytokine responsible for the formation and maintenance of mycobacterial antigen-induced granulomas (Chensue et al., 1995). More importantly, TNF-α is of high importance in triggering molecular mechanisms that provide protection against mycobacterial disease (Algood et al., 2005). TNF-α exhibits a wide range of biological functions, including the activation of immune and endothelial cells, induction of apoptosis, and thymocyte proliferation (Hernandez-Caselles & Stutman, 1993). Depletion of TNF using neutralizing antibodies prevented granuloma formation and the ability to contain infection with bacille Calmette–Guérin (BCG) in mice (Kindler et al., 1989). Mice lacking the TNF receptor, as well as wild-type mice treated with a TNF-α neutralizing antibody, were rapidly killed upon infection with MTB (Flynn et al., 1995). Furthermore, TNF-deficient mice failed to form granulomas in response to mycobacterial infection, exhibiting delayed expression of C-C and C-X-C chemokines and delayed recruitment of CD11b+ cells (Roach et al., 2002). TNF has also been shown to be critical to control long-term and persistent infections, with depletion resulting in failure to maintain granuloma pathology (Botha & Ryffel, 2003).
Complement C5 is another critical component in the granulomatous response. Its cleavage product, C5a, is a potent anaphylotoxin that recruits cells to inflammatory sites and induces the production of cytokine subsets (Schulman et al., 1988). Complement C5-deficient A/J mice exhibit increased mortality and a markedly increased inflammatory response in the absence of granuloma formation in a murine model of MTB infection (Actor et al., 2001). The complement-deficient A/J mice also exhibited exacerbation in an induced chronic infection, significantly earlier reactivation of drug-cured disease, and their infected macrophages showed a reduction in secretion of cytokines and chemokines compared to complement-sufficient mice (Jagannath et al., 2000). An investigation by Borders et al. (2005) demonstrated that mice lacking the C5a receptor exhibited an exacerbated inflammatory response with failure to form granulomas. Thus, complement C5 likely plays an important role in early maturation of the granulomatous response, related in part to mediation of chemotaxis of regulating proinflammatory cells to the site of inflammation.
Interleukin (IL)-6 is a proinflammatory cytokine produced by monocytes, fibroblasts, T-cells and B-cells that is hypothesized to play a role in mycobacterial infection through its involvement in adaptive cell activation, acute-phase protein production and immunoglobulin production (Van Snick, 1990). In vitro studies suggest a pathological role for IL-6 in mycobacterial infection, but these experiments are in need of further definition. IL-6 production by macrophages infected with MTB suppresses the T-cell response (VanHeyningen et al., 1997) as well as blocking transcriptional activation of interferon-γ (IFN-γ) in nearby, uninfected macrophages (Nagabhushanam et al., 2003). In vivo studies yielded mixed results. One investigation found that MTB infection was lethal for mice deficient in IL-6 (Ladel et al., 1997); however, another study using IL-6-deficient mice found that the deficient mice had delayed production of IFN-γ but were still able to contain the infection (Saunders et al., 2000). Clearly IL-6 plays a role in the response, but to date the precise function of IL-6 in mycobacterial infection and granuloma formation is unclear.
Trehalose 6,6′-dimycolate (TDM) is a glycolipid component of the mycobacterial cell wall. In mice, purified TDM causes immunopathology that mimics in part MTB infection, including the formation of granulomas and proinflammatory cytokines (Geisel et al., 2005; Perez et al., 1994). TDM is emerging as important in multiple roles during the development of pathogenesis during MTB infection (Hunter et al., 2006b; Rao et al., 2006). Recent findings implicate this molecule in the development of caseation in the lung post-experimental infection of mice (Hunter et al., 2006a). Taken together, the TDM model system provides a useful tool to dissect the factors required for granulomatous response. Because the exact roles for TNF-α, C5 and IL-6 remain unknown in the initiation and maintenance of granuloma formation to isolated TDM, experiments were performed to analyse responses in mice deficient in these critical immune factors.
METHODS
Animals
Three- to five-week-old, female C57BL/6 mice, A/J mice (C5a deficient), and mice deficient in TNF-α (B6;129S6-Tnftm1Gkl/J), C5a (B10.D2-Hc0H2dH2-T18c/oSnJ), or IL-6 (B6.129S2-Il6tm1Kopf/J) were obtained from Jackson Laboratories. Animal studies were conducted under the approval of the UTHHSC Institutional Review Board, document AWP 04-065. Four to six mice were used per group, per time point indicated.
Bone marrow-derived macrophage (BMM) isolation, challenge with TDM-coated beads, and infection with mycobacteria
BMMs from C57BL/6 and complement C5a-deficient (A/J) mice were established as previously described (Indrigo et al., 2002). Femurs were flushed with PBS and 3 × 107 cells were added to 75 cm2 tissue culture flasks (Corning). Cells were grown in Eagle’s minimal essential medium (EMEM; Sigma) containing 10 % fetal bovine serum (FBS), 2 mM glutamine, 10 ng ml−1 recombinant murine granulocyte/macrophage colony stimulating factor (GM-CSF; Chemicon), 100 U penicillin (Sigma) ml−1 and 100 μg streptomycin (Sigma) ml−1. Cells were incubated at 37 °C in 5 % CO2 overnight. Non-adherent cells were collected, incubated for 7 days and given two additional media changes containing GM-CSF. Finally, adherent cells were removed, washed and resuspended in EMEM containing 2 % FBS. Cells were then adjusted to 1 × 106 cells ml−1 and added to 24-well tissue culture plates (Corning) in 1 ml total volume.
TDM-coated beads were prepared by previously detailed methods, as were control BSA-coated beads (Indrigo et al., 2003). Beads were washed thoroughly and sonicated before use in experiments, and were added to cells at a ratio of 10 beads per cell. TNF-α and IL-6 protein production was assayed in the supernatants after 72 h using an ELISA (see below).
Mycobacterium tuberculosis (MTB) (Erdman, ATCC 35801) was cultured to exponential phase in Dubos broth (Difco) supplemented with 5 % BSA and 7.5 % glucose. For delipidated MTB, mycobacterial surface lipids were extracted with petroleum ether, as previously described (Indrigo et al., 2002; Silva et al., 1985). After addition of petroleum ether (Sigma), the bacteria were vortexed vigorously for 2 min, followed by 5 min incubation at room temperature. The culture was centrifuged at 500 g for 10 min. The supernatant was removed and the process was repeated twice more and suspended in PBS. Petroleum ether extraction by these methods does not affect viability of the bacteria after 14 days’ growth in Dubos broth and has no impact on the acid-fastness of the organisms (Indrigo et al., 2002; Silva et al., 1985). HPLC and TLC analyses have demonstrated that TDM is the primary extracted component (Indrigo et al., 2002; Silva et al., 1985). Delipidated MTB was reconstituted by addition of a 0.01 % (50 μg ml−1) solution of purified TDM (Sigma; 100 % pure by TLC, >98 % as 6,6′-mycolate esters) in petroleum ether (Indrigo et al., 2002; Silva et al., 1985). Solvent was evaporated, and the bacteria were resuspended in PBS. The amount of surface glycolipids recovered is indistinguishable from untreated bacteria by 7 days post-extraction (Indrigo et al., 2002).
Bacteria were adjusted to 5 × 106 bacteria ml−1 in DMEM containing 2 % FBS and sonicated to disperse clumps. Serial dilutions were plated on Middlebrook 7H11 agar (Remel) to confirm infectious dose. Colony-forming units were enumerated after incubation of plates at 37 °C for 21 days. Matured BMMs were infected 24 h after seeding (m.o.i. 5 : 1). Immediately before use, monolayers were washed extensively with PBS. One millilitre of native MTB, delipidated MTB, delipidated MTB reconstituted with purified TDM, or medium alone was added, and the infection was allowed to proceed for 4 h at 37 °C with gentle rotation. Cells were infected in triplicate wells; they were then washed to remove extracellular bacteria, and fresh DMEM containing 2 % FBS was added.
Administration of TDM, processing and histology
A TDM water-in-oil emulsion was prepared as previously described (Perez et al., 2000), with modifications discussed below. C57BL/6 mice, or mice deficient in TNF-α (B6;129S6-Tnftm1Gkl/J), C5a (B10.D2-Hc0H2dH2-T18c/oSnJ), or IL-6 (B6.129S2-Il6tm1Kopf/J), were injected intravenously in the tail vein with 50 μl emulsion, prepared by dissolving 25 μg purified TDM (Sigma) in 9 : 1 (v/v) hexane/ethanol followed by evaporation of the solvent. Then 1 μl Drakeol (Penreco) was added and homogenized. Finally, 48 μl 0.2 % Tween 80 (Mallinckrodt) in PBS (Mediatech) was admixed. Mice were sacrificed at days 0, 4, 7 and 14 post-TDM challenge. Lung tissues were immediately aseptically removed, weighed, and processed for cytokine analysis or fixed for histological studies. Calculation of a lung weight index (LWI) was performed as a measure of inflammatory intensity (Borders et al., 2005; Guidry et al., 2004; Pelletier et al., 1982) using the following equation:
Approximately 30 mg lung tissue was homogenized and placed into 2 ml Dulbecco’s modified Eagle’s medium (Sigma) containing 0.01 % L-arginine (Sigma), 0.01 % HEPES (Sigma), 10 % FBS (Sigma), 100 μg penicillin (Sigma) ml−1 and 50 μg gentamicin (Sigma) ml−1. Samples were incubated for 4 h at 37 °C with 5 % CO2. The resulting supernatants were stored at −20 °C for later analysis by ELISA. For histological analysis, the left lung was fixed in 10 % buffered formalin, sectioned (5 μm thick) and stained with haematoxylin and eosin by standard procedures. The remaining lung section was snap-frozen in RNAzol B (TelTest) and stored at −70 °C for RNA extraction and analysis by real-time RT-PCR. No significant difference in histopathology or lung weight index was seen in the emulsion-alone treated controls when compared to naive controls, as previously detailed (Guidry et al., 2004). Experiments were repeated three times through 7 days, and once through 14 days.
ELISA analysis of lung cytokines and chemokines
Levels of IFN-γ, IL-1β, IL-6, IL-10, IL-12p40, macrophage inflammatory protein-1α (MIP-1α) and TNF-α in cell supernatants were measured by a sandwich ELISA according to the manufacturer’s instructions (R&D Systems). Briefly, capture antibody coated Costar 96-well plates (Corning) were washed (0.5 % Tween-20 in PBS) and blocked (1 % BSA, 5 % sucrose, 0.05 % NaN3 in PBS). Supernatants were incubated for 2 h, followed by detection using biotin-conjugated secondary antibodies, with visualization using streptavidin-horseradish peroxidase (R&D) and TMB Microwell Peroxidase Substrate (KPL). Reactions were halted using 1 M H2SO4, and the absorbance was read at 570 nm and 450 nm on an ELISA plate reader (Molecular Devices). The mean of duplicate wells was calculated based on a standard curve generated for each assay using manufacturer supplied recombinant molecules (R&D). The lower range limit for detection sensitivity was 15–32 pg ml−1.
Isolation and purification of mRNA, reverse transcription and quantitative PCR
Lung tissue was homogenized in 1 ml RNAzol, and RNA extracted as previously described (Guidry et al., 2006). cDNA was synthesized from 2 μg RNA in buffer (250 mM Tris/HCl, pH 8.3, 375 mM KCl, 15 mM MgCl2) (Invitrogen), with final concentrations of 0.1 M DTT (Invitrogen), 2.5 mM deoxynucleotide triphosphates (dNTPs) (Invitrogen), 80 U random hexamer oligo-nucleotides (Roche Diagnostics), and 20 U RNase inhibitor (Promega). SuperScript II reverse transcriptase (200 U; Invitrogen) was added after heating to 70 °C for 5 min; incubation continued at 42 °C for 50 min after which the reaction was terminated by heating to 70 °C for 15 min. The sample was diluted 1 : 8 with distilled water prior to analysis.
Quantification of cDNA was performed using the Taqman assay with fluorogenic probes that were FAM (6-carboxifluorescein) reporter and BHQ-1 (black hole quencher) quencher dyes (Biosearch). The sequences of the primers and probes used are shown in Table 1. The reaction mix contained 200 nM dNTPs (Invitrogen), 1 × ROX Reference Dye (Invitrogen), 400 nM of each primer (Integrated DNA Technologies), 1 × PCR buffer (20 mM Tris/HCl pH 8.0, 100 mM KCl, 0.1 mM EDTA, 1 mM DTT, 50 % glycerol, 0.5 % Tween 20, 0.5 % Igepal CA-630) (5 Prime), 1 U/45 μl Taq DNA polymerase (5 Prime), and 100 nM probe. Amplification in the ABI Prism 7700 Sequence Detection System (Applied Biosystems) was achieved by heating to 95 °C for 1 min, followed by 40 cycles of a 12 s step at 95 °C and a 1 min step at 60 °C. Data were analysed by the −2ΔΔCT method as previously described (Livak & Schmittgen, 2001) using β-actin message as the calibrator.
Table 1.
Oligonucleotide primers and probes
| Protein | Primer (5′–3′) | Probe (5′–3′) | Product size (bp) | |
|---|---|---|---|---|
| β-Actin | S* | TCTGGCTCCTAGCACCATGA | ATCAAGATCATTGCTCCTCCTGAGCGC | 72 |
| AS* | CCACCGATCCACACAGAGTACT | |||
| IFN-γ | S | AGCAACAGCAAGGCGAAA | TCAAACTTGGCAATACTCATGAATGCATCCT | 72 |
| AS | CTGGACCTGTGGGTTGTTGA | |||
| IL-1β | S | CTCATTGTGGCTGTGGAGAA | TGGCAGCTACCTGTGTCTTTCCCG | 78 |
| AS | GGTGCTCATGTCCTCATCCT | |||
| IL-6 | S | CCCAATTTCCAATGCTCTC | TAGCCACTCCTTCTGTGACTCCAGCT | 77 |
| AS | TGAATTGGATGGTCTTGGTC | |||
| IL-10 | S | CAGCCGGGAAGACAATAACTG | CCCACTTCCCAGTCGGCCAG | 67 |
| AS | CCGCAGCTCTAGGAGCATG | |||
| IL-12p40 | S | AAGTGTGAAGCACCAAATTACTC | ACGGTTCACGTGCTCATGGCT | 71 |
| AS | TTCAAGTCCATGTTTCTTTGC | |||
| MIP-1α | S | ACTAAGAGAAACCGGCAGAT | TGCGCTGACTCCAAAGAGACC | 77 |
| AS | TTCAGTTCCAGGTCAGTGAT | |||
| TNF-α | S | CCGATGGGTTGTACCTTGTCT | TCTTCAAGGGACAAGGCTGCCCC | 76 |
| AS | TGGGTGAGGAGCACGTAGTC |
S, sense; AS, antisense.
Statistics
Data are presented as the mean ± 1 SD. Normally distributed data were analysed by an unpaired t-test where the difference between two means was compared within groups. Two-way ANOVA was used for statistical analysis between strains for results of in vivo experimentation. Differences between means were considered significant at a level of P < 0.05.
RESULTS
Inflammatory response to TDM in normal and C5a-deficient bone marrow-derived macrophages
TDM is recognized as a major inducer of proinflammatory cytokines from monocytes (Perez et al., 2000). The levels of TNF-α and IL-6 were significantly elevated in culture supernatants of both C5a-sufficient (C57BL/6) and C5a-deficient (A/J) BMMs treated with TDM-coated beads (Table 2). Previous investigations demonstrated that removal of TDM from the surface of MTB led to significantly less proinflammatory response (Indrigo et al., 2002). Recent reports identified the importance of complement factors in this response (Actor et al., 2001; Borders et al., 2005); therefore, TNF-α and IL-6 were also measured in C5a-deficient BMMs infected with native M. tuberculosis (Mtb), or organisms treated to remove surface TDM (delipidated; dMtb). Comparisons were made to responses in the C5a-sufficient derived cells. For both groups, delipidation led to significantly diminished magnitude of response. Of interest, the response from C5a-deficient derived cells was always significantly less than that from matched C5a-sufficient derived cells. The response was nearly completely restored when cells were challenged with organisms reconstituted with purified TDM (r-dMtb), confirming the relative importance of the TDM glycolipid in the initial proinflammatory response to mycobacteria.
Table 2. TDM-induced cytokine production in C5a-sufficient and C5a-deficient bone marrow-derived macrophages.
Production of TNF-α and IL-6 by BMMs in response to BSA-coated beads, TDM-coated beads, native Mtb, delipidated Mtb (dMtb), delipidated Mtb reconstituted with TDM (r-dMtb), or untreated cells. Values were measured by ELISA and expressed as mean pg protein per 106 BMMs (±SD); three replicates per time point.
| BSA beads | TDM beads | Mtb | dMtb | r-dMtb | Uninfected | |
|---|---|---|---|---|---|---|
| TNF-α | ||||||
| C5a-sufficient | 24 (2) | 1294 (173)* | 541 (70) | 253 (39)† | 461 (44) | 19 (5) |
| C5a-deficient | 14 (1) | 322 (19)* | 303 (50) | 166 (32)† | 339 (50) | 10 (1) |
| IL-6 | ||||||
| C5a-sufficient | 7 (1) | 245 (4)* | 335 (75) | 102 (6)† | 227 (14) | 5 (1) |
| C5a-deficient | <10 | 24 (4) | 30 (1) | <10 | <10 | <10 |
P < 0.05 between treatment groups (TDM-coated vs BSA-coated or untreated cells) as analysed by Student’s t test.
P < 0.05 between groups compared against dMtb or against TDM-reconstituted organisms; all responses from C5a-deficient (A/J) derived BMMs were significantly lower (P < 0.05) than the C5a-sufficient (C57BL/6) BMMs treated in the identical manner. Experiments were repeated two or three times with similar results.
Inflammatory response to TDM in TNF-α-, C5a-and IL-6-deficient mice
LWIs were calculated as a measure of general inflammatory response for wild-type and deficient mice following intravenous administration of TDM (Table 3). The wild-type, C5a-deficient (OSN) and IL-6-deficient mice demonstrated significantly (P < 0.05) elevated LWI at 4 and 7 days post-TDM administration in comparison to untreated control mice, or compared to emulsion-alone controls (not shown; Actor et al., 2001). In contrast, the mean LWIs of the TNF-α-deficient mice were 0.99 ± 0.01 on day 4 and 1.01 ± 0.01 on day 7, compared to wild-type mice that had LWIs of 1.31 ± 0.07 and 1.63 ± 0.23 on days 4 and 7, respectively, suggesting the TNF-α-deficient mice failed to initiate a significant inflammatory response to TDM.
Table 3. Lung weight indices following TDM administration.
LWIs were calculated at days 0, 4, 7 and 14 post-TDM administration. Individual values are mean responses (±SD) and compared to wild-type C57BL/6 mice at each indicated time point.
| Day 0 | Day 4 | Day 7 | Day 14 | |
|---|---|---|---|---|
| Wild- type | 1.00 ± 0.033 | 1.31 ± 0.073 | 1.63 ± 0.232 | 1.06 ± 0.154 |
| TNF-α −/− | 0.98 ± 0.026 | 0.99 ± 0.012* | 1.01 ± 0.010* | 0.96 ± 0.035 |
| C5a −/− | 0.93 ± 0.144 | 1.32± 0.156 | 1.53 ± 0.157 | 1.04 ± 0.196 |
| IL-6 −/− | 1.02 ± 0.048 | 1.30 ± 0.051 | 1.55 ± 0.082 | 1.19 ± 0.216 |
P < 0.05.
TNF-α-, C5a- and IL-6-deficient mice demonstrate altered TDM-induced lung histopathology compared to control challenged mice
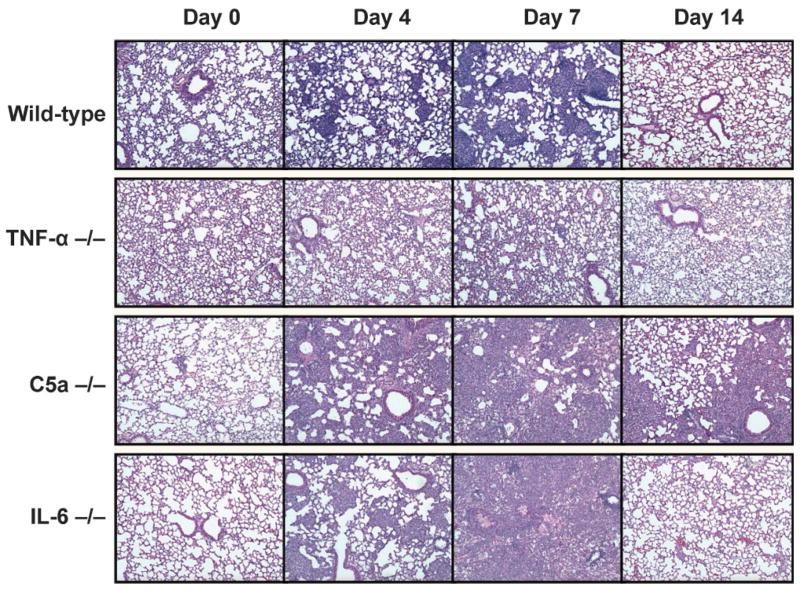
Wild-type C57BL/6 mice formed cohesive and transient granulomas after a single intravenous injection of TDM (Fig. 1). Focal, small, histiocytic clusters were evident at day 4 post-TDM administration. The clusters became more complex at day 7, increasing in both size and number. The granulomas were located within the lung parenchyma, with no obvious occlusion of blood vessels or lymphatics. Responses were primarily monocytic, with larger phenotypic responders containing activated intracellular vesicles. By day 14, there was complete resolution of the granulomatous response.
Fig. 1.
Granulomatous response to TDM in mice deficient in TNF-α, C5a, or IL-6. Wild-type mice challenged with mycobacterial TDM demonstrate small focal pulmonary granulomas by 4 days post-TDM administration, which peak at day 7 and resolve by day 14. In comparison, challenge of TNF-α-deficient mice did not elicit significant histopathology, with failure to mount significant inflammation in the lung. Mice with deficiency in C5a demonstrated a non-focal inflammatory response by day 4, and with no cellular aggregation of accompanying lymphocytic and monocytic infiltration or true resolution by 14 days post-challenge. The IL-6-deficient mice initiated granuloma histopathology, but by day 7 exhibited marked monocytic infiltration, lymphocytic cuffing around occluded vesicles, and oedema with accompanied alveolar cell wall thickening. Sections representative of 4–6 mice per group. Haematoxylin and eosin staining; magnification ×40.
In contrast to the wild-type mice, the TNF-α-deficient mice largely failed to demonstrate a histological response to TDM. Inflammation was not apparent at 4 days after TDM administration. Examination of tissue at day 7 revealed slight accumulation of cellular infiltrates without the formation of granulomas; infiltrating cells were monocytic, with few or no visible intracellular inclusions. There was no obvious occlusion of vasculature, nor was there evidence of oedema or pneumonitis.
C5a-deficient mice also failed to form granulomas in response to isolated TDM. However, the pathology was different from that of the TNF-α-deficient mice. The complement-deficient mice showed a marked non-focal inflammatory response at day 4. By 7 days, they completely lacked cohesive or structured cellular aggregates and had histological evidence of considerable lymphocytic and monocytic infiltration. The lung parenchyma was distorted and demonstrated some vessel occlusion with small pockets of pulmonary oedema. This reaction appeared to be transient, although resolution of the response remained incomplete at day 14.
The IL-6-deficient mice initially formed granulomas in response to TDM with focal monocytic clusters apparent at day 4. However, they failed to maintain this response. By day 7 post-challenge, acute inflammation was increased with marked monocytic infiltration. Lymphocytic cuffing around occluded vesicles was evident, as was oedema and alveolar cell-wall thickening. Of interest, the response was transient, and largely resolved by day 14.
Altered proinflammatory cytokine and chemokine protein and mRNA profiles in deficient mice after TDM challenge
Cytokine and chemokine protein patterns were examined in the lungs of mice challenged with TDM (Figs 2 and 3). Challenged wild-type mice had significantly elevated levels (P < 0.05) of IL-1β, IL-6, TNF-α, MIP-1α, IL-12p40 and IL-10 on both days 4 and 7 in comparison to non-injected mice. In addition, there was a significant, but transient, rise in IFN-γ on day 4. Cytokine protein returned to near baseline levels by day 14 post-TDM administration.
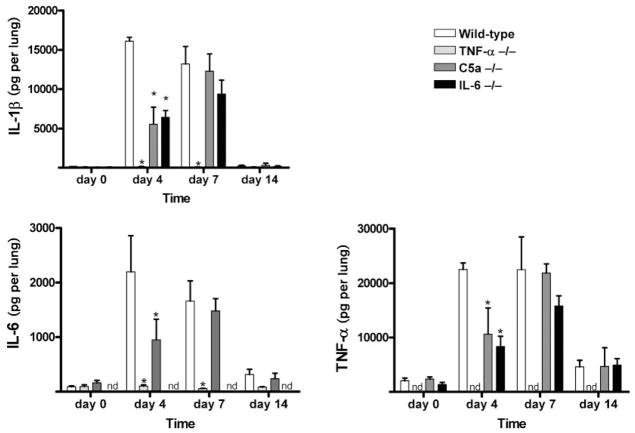
Fig. 2.
TDM-elicited proinflammatory mediator production from lungs of deficient and wild-type mice. Levels of pulmonary proinflammatory mediators IL-1β, IL-6 and TNF-α were quantified post-challenge in the lungs of mice administered TDM. Mean values per lung are shown for mice deficient in TNF-α, C5a or IL-6 prior to challenge (day 0) and at 4, 7 and 14 days post-TDM challenge. Comparisons are made to the wild-type control animals administered TDM. Data are represented as the mean ± SD for duplicate wells per mouse (n = 4–6 mice per group per time point). *P < 0.05; nd, none detected.
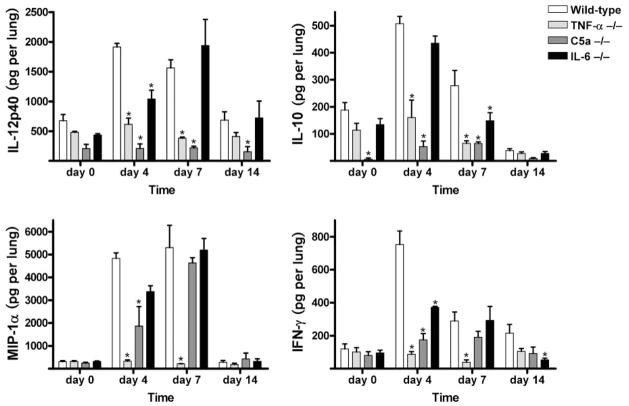
Fig. 3.
TDM-elicited cytokine production from lungs of deficient and wild-type mice. Levels of pulmonary cytokines IL-12p40, IL-10, MIP-1α and IFN-1γ were quantified post-challenge in the lungs of mice administered TDM. Mean values per lung are shown for mice deficient in TNF-α, C5a or IL-6 prior to challenge (day 0) and at 4, 7 and 14 days post-TDM challenge. Comparisons are made to the wild-type control animals administered TDM. Data are represented as the mean ± SD for duplicate wells per mouse (n = 4–6 mice per group per time point). *P < 0.05.
The differences in protein production in the lung between controls and knockout strains were readily apparent on day 4 post-TDM administration. The TNF-α-deficient mice did not produce an elevation in cytokine or chemokine levels in response to TDM. The levels of IL-1β, IL-6, MIP-1α, IFN-γ, IL-12p40 and IL-10 did not differ from that of non-injected mice, and were thus significantly below levels produced by wild-type mice at those time points (Figs 2 and 3). TNF-α was not detectable in the TNF-α-deficient mice (data not shown).
The complement C5-deficient mice had significantly decreased production of IL-1β, IL-6, TNF-α, MIP-1α and IFN-γ in lungs on day 4, compared to the wild-type challenged mice. However, protein levels were comparable to the wild-type mice on day 7. Specifically, amounts of the proinflammatory cytokines IL-1β, IL-6 and TNF-α on day 4 were 5536.43 ± 4316.35, 950.57 ± 758.49 and 10 602.18 ± 9694.66 pg per lung, respectively, in the C5a-deficient mice compared to 16 113.19 ± 941.15, 2199.75 ± 1319.66 and 22 510.79 ± 2384.84 pg per lung in the wild-type mice. MIP-1α, a chemokine that controls the migration of numerous effector cells, was significantly reduced in the C5a-deficient mice at day 4, with levels of 1867.43 ± 1701.89 pg per lung compared to wild-type levels of 4835.34 ± 480.04 pg per lung. IL-12p40 was also significantly decreased on days 0, 4, 7 in comparison to the complement-sufficient mice.
The IL-6-deficient mice also demonstrated reduced production of IL-1β, TNF-α, IFN-γ and IL-12p40 in lung tissue at day 4 post-TDM challenge. TNF-α was 8330.44 ± 2384.84 pg per lung in the IL-6-deficient mice whereas it was 22 510.79 ± 2384.84 in the wild-type mice at day 4. The amount of IL-12p40 in the IL-6-deficient mice was 1040.28 ± 253.31 pg per lung in comparison to wild-type levels of 1912.53 ± 124.45 pg per lung at day 4. The amount of these cytokines became similar to that of wild-type mice on day 7. IL-10 levels significantly decreased at day 7 in the IL-6-deficient mice, with 148.79 ± 51.30 pg per lung compared to wild-type levels of 278.03 ± 112.82 pg per lung. Levels of MIP-1α did not differ significantly from the wild-type mice on any days tested. Protein production of IL-6 in the IL-6-deficient mice was not detectable (data not shown).
Evaluation of mRNA in the wild-type and knockout mice treated with TDM yielded similar results to the protein levels identified within lung tissue (Table 4). The wild-type C57BL/6 mice had a marked increase (20-fold or greater) in message for the proinflammatory mediators IL-1β, IL-6, TNF-α and MIP-1α message compared to untreated or emulsion-alone controls (not shown, Guidry et al., 2004). A modest increase in expression of IL-12p40 occurred on days 4 and 7. There was an early increase in expression of IFN-γ and IL-10 that decreased by day 7. The deficient mice had decreased expression of all cytokines tested, except IL-12p40, compared to the wild-type mice. Significant differences in individual message levels for each knockout strain compared to the wild-type control mice (P < 0.05) are indicated in Table 4. Expression of TNF-α or IL-6 in the TNF-α-deficient or IL-6-deficient mice, respectively, was not observed (data not shown).
Table 4. Relative change in mRNA following TDM administration.
Proinflammatory mediator mRNAs in lungs of mice deficient in TNF-α, C5a, or IL-6 were evaluated by RT-PCR and compared to wild-type controls, after challenge with TDM. Results shown are normalized to β-actin, and represented as fold-change from non-injected mice. Data are expressed as means ± SD.
| IL-1β | IL-6 | TNF-α | MIP-1α | IFN-c | IL-12p40 | IL-10 | |
|---|---|---|---|---|---|---|---|
| Day 4 | |||||||
| Wild-type | 23.89 ± 9.37 | 32.01 ± 7.71 | 20.71 ± 3.67 | 29.57 ± 10.59 | 23.09 ± 14.60 | 2.99 ± 0.32 | 29.17 ± 17.71 |
| TNF-α −/− | 3.79 ± 2.24* | 5.45 ± 3.41* | – | 4.61 ± 1.87* | 4.12 ± 1.76* | 3.21 ± 1.74 | 7.10 ± 4.02* |
| C5a −/− | 15.25 ± 1.94* | 16.57 ± 6.76 | 31.34 ± 13.99 | 19.59 ± 20.04 | 12.43 ± 2.10* | 1.35 ± 0.98 | 11.45 ± 7.86* |
| IL-6 −/− | 5.02 ± 1.42* | – | 5.72 ± 1.50* | 5.54 ± 2.00* | 7.02 ± 2.48* | 2.04 ± 1.02 | 7.02 ± 3.22* |
| Day 7 | |||||||
| Wild-type | 32.96 ± 10.66 | 27.87 ± 26.62 | 43.59 ± 13.60 | 42.51 ± 16.84 | 9.63 ± 5.95 | 2.19 ± 0.72 | 11.84 ± 3.34 |
| TNF-α −/− | 2.54 ± 1.73* | 1.08 ± 0.88* | – | 2.32 ± 1.77* | 2.31 ± 1.36 | 0.89 ± 0.68 | 2.49 ± 1.64 |
| C5a −/− | 15.94 ± 4.17* | 3.28 ± 0.97 * | 18.44 ± 10.55* | 16.36 ± 10.88* | 10.57 ± 5.37 | 1.79 ± 0.45 | 5.73 ± 1.20 |
| IL-6 −/− | 5.04 ± 1.77* | – | 5.97 ± 2.21* | 7.04 ± 1.89* | 2.18 ± 0.87 | 0.95 ± 0.55 | 2.07 ± 0.81 |
| Day 14 | |||||||
| Wild-type | 0.61 ± 0.20 | 0.53 ± 0.05 | 10.29 ± 15.73 | 0.72 ± 0.56 | 1.75 ± 1.04 | 1.09 ± 0.29 | 0.67 ± 0.30 |
| TNF-α −/− | 2.30 ± 1.25 | 1.50 ± 1.53 | – | 2.14 ± 1.14 | 3.42 ± 1.97 | 1.13 ± 0.69 | 2.16 ± 1.39 |
| C5a −/− | 0.68 ± 0.55 | 0.44 ± 0.22 | 0.63 ± 0.60 | 0.94 ± 0.55 | 1.37 ± 1.48 | 1.58 ± 1.19 | 1.79 ± 2.24 |
| IL-6 −/− | 2.01 ± 1.32 | – | 3.29 ± 2.72 | 2.65 ± 1.05 | 2.56 ± 1.71 | 1.35 ± 0.60 | 3.41 ± 2.29 |
P < 0.05.
DISCUSSION
Roughly one-third of the world’s population is latently infected with MTB (WHO, 2003). The formation of granulomas is a critical host defence mechanism for containment of organisms and is a process that requires on-going concerted regulation of proinflammatory mediators (Russell, 2007). The growing number of therapeutics that target cytokines to treat chronic immune-mediated disease makes it clinically relevant to identify how these molecules are involved in the initiation and maintenance of the granulomatous response. For example, patients treated with infliximab, a TNF-α neutralizing antibody, experienced altered inflammatory patterns that led to reactivation of latent pulmonary tuberculosis, resulting in greater occurrence of disseminated disease (Keane et al., 2001). In addition, the use of cytokines as adjunct therapeutics to augment antibiotic efficacy is being considered for tuberculosis due to the emergence of multidrug-resistant strains and in treating immunodeficient patients (Bermudez & Kaplan, 1995; Murray et al., 1996). Understanding the implications of dysregulation of these mediators is paramount when considering treatment in these individuals.
Numerous investigations implicate TDM as a major immunomodulatory component of the mycobacterial cell wall. Remarkably similar proinflammatory molecules are induced in mice and BMMs challenged with TDM as observed in MTB infection (Actor et al., 2000; Perez et al., 1994, 2000). We demonstrate that removal of MTB surface lipids diminished macrophage TNF-α and IL-6 production, with restoration of this response upon reconstitution with TDM. These data confirm the critical involvement of surface glycolipids in the macrophage response to MTB. The studies outlined here extend these findings to investigate the role of these particular cytokines, as well as complement C5a, in development of the granulomatous response against purified TDM.
TNF-α is a key mediator involved in the initiation of the granulomatous response. TNF-α-deficient mice failed to form granulomas in response to MTB infection, with subsequent development of necrotic lesions devoid of epithelioid cells (Kaneko et al., 1999). The TNF-α-deficient mice in the studies reported here failed to produce a histological response to TDM, with severely limited inflammatory protein or message production. Of interest, these knockout mice also demonstrated decreased levels of the TH1 cytokines INF-γ and IL-12p40. An investigation by Kindler et al. (1989) noted that TNF neutralization inhibited granuloma formation in response to BCG and dissolved established granulomas. Transgenic mice that express high levels of the human soluble TNF receptor 1 also failed to form granulomas in response to BCG and had delayed production of both IFN-γ and IL-12p40 (Guler et al., 2005). The lack of granuloma formation in TNF-α-deficient mice may be due to an inability to produce an inflammatory cascade that includes chemokine production to recruit monocytes and T-cells to the lung. Neutralization of TNF in macrophages infected with MTB caused a decrease in CCL5, CXCL9 and CXCL10 (Algood et al., 2004). This same study found that CD11b+ cells isolated from mice lacking the 55 kDa TNF receptor infected with MTB had delayed production of inflammatory chemokines. Another investigation found that TNF-α was essential for TDM to prime peritoneal macrophages (Oswald et al., 1999), perhaps indicating an autocrine mechanism towards initiation of responses observed in this study. Furthermore, TNF-α is a critical regulator of the TH1 immune response essential for the control of mycobacterial infections. Macrophages treated with a TNF-neutralizing antibody and macrophages lacking the TNF receptor 1 failed to produce IL-12 after infection with BCG (Flesch et al., 1995). TNF-α was required for induction of the IL-12-mediated TH1 response in BALB/c mice (Shibuya et al., 1998). In addition, Ahlers et al. (2001) noted that a synergism between IL-12 and TNF-α was necessary to upregulate IFN-γ and the IL-12Rβ2 chain to promote development of TH1 cells. The overall importance of T-cells in response to TDM-induced granulomas has recently been investigated (Guidry et al., 2006; Yamagami et al., 2001), with T-helper cells of major importance for development of hypersensitive immunopathology (Guidry et al., 2006; Oiso et al., 2005).
The experiments described here indicate that complement C5 is essential for both initiation of the granulomatous response and continued development of cohesive granuloma formation in response to the mycobacterial glycolipid TDM, once initiated. TDM activates the alternative pathway of complement, and complement component C5a has been found to increase transcription and translation of TNF-α, IL-6 and IL-1β (Gross & Andus, 1992; Ramanathan et al., 1980; Schindler et al., 1990). C5 is secreted by numerous cell types, including macrophages, and is cleaved to C5a by extracellular proteases to activate macrophages by autocrine binding to C5aR (Czermak et al., 1999). The hypothesis that macrophages from C5-deficient mice would have defective responses to MTB and TDM was explored. BMMs derived from C5a-deficient mice had diminished levels of TNF-α and IL-6 in response to MTB infection and stimulation with TDM-coated beads compared to BMMs derived from complement-sufficient C57BL/6 mice, implicating complement in the initial induction of proinflammatory mediators that lead to granuloma formation. Deficiency of C5 resulted in the development of a pneumonitis with monocytic infiltration and oedema with delayed resolution of the response after in vivo challenge with TDM, suggesting the importance of C5 in the maintenance and further resolution of granulomas. In addition, the complement-deficient mice had delayed production of proinflammatory mediators concurrent with consistently lowered production of IL-12p40. The histology and cytokine response of the C5a-deficient mice challenged with TDM have been identified as mediating chemokine profiles that moderate cellular infiltrates in the lung (Borders et al., 2005), studies that correlate very well with deficient mouse models of MTB infection. A/J mice, which lack the fifth component of complement, failed to form granulomas and rapidly succumbed to MTB infection (Jagannath et al., 2000). An investigation utilizing the same C5a-deficient (OSN) mice as used in this study found enhanced pulmonary growth of MTB as well as lack of productive granulomatous response (Actor et al., 2001). Both studies noted early decreases in cytokine and chemokine protein and mRNA, similar to data presented here for the β chemokine MIP-1α. Complement C5 is essential for a number of additional or synergistic processes that may be involved in the formation and maintenance of granulomas. C5a induces NFκB, which regulates expression of numerous inflammatory molecules (Hsu et al., 1999). C5a and C5b induce expression of adhesion molecules on macrophages, neutrophils and endothelial cells (Fleming et al., 2003; Foreman et al., 1994). Finally, C5 appears to be essential for IL-12-induced cell-mediated immunity (Karp & Wills-Karp, 2001).
The results presented here also suggest a role for IL-6 in granuloma maintenance, but not in initiation of response. The IL-6-deficient mice formed granulomas upon TDM challenge at day 4, similar to wild-type mice. Of interest, this occurred despite lowered levels of inflammatory cytokines. However, granuloma cohesiveness was not maintained through day 7, even in the presence of cytokine levels comparable to that of the wild-type mice. Studies on the role of IL-6 in the control of mycobacterial diseases have yielded conflicting results, with even less understood regarding the role of IL-6 in protective granuloma formation during MTB infection. Ladel et al. (1997) demonstrated that IL-6-deficient mice had much higher bacterial burdens and succumbed to infection, while Saunders et al. (2000) found that mice deficient in IL-6 were ultimately able to contain the infection despite higher bacterial load and decreased early production of IFN-γ. The discrepancies between these studies may be the result of the route and number of organisms used for the infectious process. To further complicate interpretation, treatment of Mycobacterium avium-infected mice with IL-6 neutralizing antibodies resulted in increased bacterial growth; however, no differences in granuloma number or size were noted (Appelberg et al., 1994). Infection of IL-6-deficient mice with M. avium produced fewer necrotic lesions compared to wild-type mice (Florido et al., 2002). Infection of IL-6-deficient mice with Rhodococcus aurantiacus, a short-chain TDM-producing organism that induces TH1 granulomas similar to mycobacterial agents, found considerable inflammatory infiltrates following challenge, with larger granulomas that had central necrosis compared to wild-type mice (Yimin et al., 2003). IL-6 is generally considered a TH2 cytokine (Rincon et al., 1997; Van Snick, 1990); however, this is not a stand-fast rule in all intracellular infection models (Romani et al., 1996). IL-6 is identified with the development of a T-cell response against M. avium (Appelberg et al., 1994). It is clear that maintenance of granulomas requires antigen-specific T-cells (Dannenberg, 1991). Therefore, IL-6 may be a regulator of the T-cell responses critical for the maintenance of granulomas, especially during infection when persistent antigen is present.
The TDM-induced granulomatous response mimics in part many aspects of the mycobacterial immunopathology identified early during aerosol infections of mice. As such, this model system is ideal for investigation of the roles of complement and cytokine components towards early response to mycobacterial antigens. The studies described here indicate that TNF-α is a critical mediator involved in initiation of the granulomatous response to the TDM. Once the granulomatous process has begun, factors such as complement C5 and IL-6 mediate secondary responses. C5 most likely is a critical mediator of responses culminating in a cohesive and structured granuloma immunopathology, while IL-6 regulates the maintenance of granulomas once established. The end result of deficiency of any of these components is an inability to effectively regulate the early granulomatous response, and would therefore also likely be critical in control during mycobacterial infections.
Acknowledgments
This work was supported by NIH grants 1R21AI058247-1 and R01HL068537. We thank C. W. Kan of the University of Texas-Houston Graduate School of Biomedical Sciences for confirmation of experimental results.
Abbreviations
- BCG
bacille Calmette–Guérin
- BMM
bone marrow-derived macrophage
- IFN-γ
interferon-γ
- IL
interleukin
- LWI
lung weight index
- MIP-1α
macrophage inflammatory protein-1α
- MTB
Mycobacterium tuberculosis
- TDM
trehalose 6,6′-dimycolate
- TNF
tumour necrosis factor
References
- Actor JK, Leonard CD, Watson VE, Wells A, Jagannath C, Hunter RL, Jr, Dasgupta A. Cytokine mRNA expression and serum cortisol evaluation during murine lung inflammation induced by Mycobacterium tuberculosis. Comb Chem High Throughput Screen. 2000;3:343–351. [PubMed] [Google Scholar]
- Actor JK, Breij E, Wetsel RA, Hoffmann H, Hunter RL, Jr, Jagannath C. A role for complement C5 in organism containment and granulomatous response during murine tuberculosis. Scand J Immunol. 2001;53:464–474. doi: 10.1046/j.1365-3083.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- Ahlers JD, Belyakov IM, Matsui S, Berzofsky JA. Signals delivered through TCR instruct IL-12 receptor (IL-12R) expression: IL-12 and tumor necrosis factor-alpha synergize for IL-12R expression at low antigen dose. Int Immunol. 2001;13:1433–1442. doi: 10.1093/intimm/13.11.1433. [DOI] [PubMed] [Google Scholar]
- Algood HM, Lin PL, Yankura D, Jones A, Chan J, Flynn JL. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J Immunol. 2004;172:6846–6857. doi: 10.4049/jimmunol.172.11.6846. [DOI] [PubMed] [Google Scholar]
- Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(Suppl 3):S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- Appelberg R, Castro AG, Pedrosa J, Minoprio P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994;82:361–364. [PMC free article] [PubMed] [Google Scholar]
- Bermudez LE, Kaplan G. Recombinant cytokines for controlling mycobacterial infections. Trends Microbiol. 1995;3:22–27. doi: 10.1016/s0966-842x(00)88864-2. [DOI] [PubMed] [Google Scholar]
- Borders CW, Courtney A, Ronen K, Pilar Laborde-Lahoz M, Guidry TV, Hwang SA, Olsen M, Hunter RL, Jr, Hollmann TJ, et al. Requisite role for complement C5 and the C5a receptor in granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate. Scand J Immunol. 2005;62:123–130. doi: 10.1111/j.1365-3083.2005.01643.x. [DOI] [PubMed] [Google Scholar]
- Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol. 2003;171:3110–3118. doi: 10.4049/jimmunol.171.6.3110. [DOI] [PubMed] [Google Scholar]
- Cagatay AA, Caliskan Y, Aksoz S, Gulec L, Kucukoglu S, Gagatay Y, Berk H, Ozsut H, Eraksoy H, Calangu S. Extrapulmonary tuberculosis in immunocompetent adults. Scand J Infect Dis. 2004;36:799–806. doi: 10.1080/00365540410025339. [DOI] [PubMed] [Google Scholar]
- Chensue SW, Warmington KS, Ruth JH, Lincoln P, Kunkel SL. Cytokine function during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Local and regional participation of IFN-γ, IL-10, and TNF. J Immunol. 1995;154:5969–5976. [PubMed] [Google Scholar]
- Czermak BJ, Sarma V, Bless NM, Schmal H, Friedl HP, Ward PA. In vitro and in vivo dependency of chemokine generation on C5a and TNF-α. J Immunol. 1999;162:2321–2325. [PubMed] [Google Scholar]
- Dannenberg AM., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Anderson J, Wilson F, Shea-Donohue T, Tsokos GC. C5 is required for CD49d expression on neutrophils and VCAM expression on vascular endothelial cells following mesenteric ischemia/reperfusion. Clin Immunol. 2003;106:55–64. doi: 10.1016/s1521-6616(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Flesch IE, Hess JH, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann SH. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florido M, Cooper AM, Appelberg R. Immunological basis of the development of necrotic lesions following Mycobacterium avium infection. Immunology. 2002;106:590–601. doi: 10.1046/j.1365-2567.2002.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, Eddy SM, Ward PA. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisel RE, Sakamoto K, Russell DG, Rhoades ER. In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guerin is due principally to trehalose mycolates. J Immunol. 2005;174:5007–5015. doi: 10.4049/jimmunol.174.8.5007. [DOI] [PubMed] [Google Scholar]
- Gross V, Andus T. Human recombinant C5a enhances lipopolysaccharide-induced synthesis of interleukin-6 by human monocytes. Eur J Clin Invest. 1992;22:271–276. doi: 10.1111/j.1365-2362.1992.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Guidry TV, Olsen M, Kil KS, Hunter RL, Jr, Geng YJ, Actor JK. Failure of CD1D−/− mice to elicit hypersensitive granulomas to mycobacterial cord factor trehalose 6,6′-dimycolate. J Interferon Cytokine Res. 2004;24:362–371. doi: 10.1089/107999004323142222. [DOI] [PubMed] [Google Scholar]
- Guidry TV, Hunter RL, Jr, Actor JK. CD3+ cells transfer the hypersensitive granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate in mice. Microbiology. 2006;152:3765–3775. doi: 10.1099/mic.0.29290-0. [DOI] [PubMed] [Google Scholar]
- Guler R, Olleros ML, Vesin D, Parapanov R, Garcia I. Differential effects of total and partial neutralization of tumor necrosis factor on cell-mediated immunity to Mycobacterium bovis BCG infection. Infect Immun. 2005;73:3668–3676. doi: 10.1128/IAI.73.6.3668-3676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Caselles T, Stutman O. Immune functions of tumor necrosis factor. I. Tumor necrosis factor induces apoptosis of mouse thymocytes and can also stimulate or inhibit IL-6-induced proliferation depending on the concentration of mitogenic costimulation. J Immunol. 1993;151:3999–4012. [PubMed] [Google Scholar]
- Hsu MH, Wang M, Browning DD, Mukaida N, Ye RD. NF-κB activation is required for C5a-induced interleukin-8 gene expression in mononuclear cells. Blood. 1999;93:3241–3249. [PubMed] [Google Scholar]
- Hunter RL, Olsen M, Jagannath C, Actor JK. Trehalose 6,6′-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am J Pathol. 2006a;168:1249–1261. doi: 10.2353/ajpath.2006.050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006b;36:371–386. [PubMed] [Google Scholar]
- Indrigo J, Hunter RL, Jr, Actor JK. Influence of trehalose 6,6′-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology. 2002;148:1991–1998. doi: 10.1099/00221287-148-7-1991. [DOI] [PubMed] [Google Scholar]
- Indrigo J, Hunter RL, Jr, Actor JK. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149:2049–2059. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- Jagannath C, Hoffmann H, Sepulveda E, Actor JK, Wetsel RA, Hunter RL. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component (C5) Scand J Immunol. 2000;52:369–379. doi: 10.1046/j.1365-3083.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, Sugawara I. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest. 1999;79:379–386. [PubMed] [Google Scholar]
- Karp CL, Wills-Karp M. Complement and IL-12: yin and yang. Microbes Infect. 2001;3:109–119. doi: 10.1016/s1286-4579(00)01358-7. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci U S A. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-γ. J Immunol. 2003;171:4750–4757. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- Oiso R, Fujiwara N, Yamagami H, Maeda S, Matsumoto S, Nakamura S, Oshitani N, Matsumoto T, Arakawa T, Kobayashi K. Mycobacterial trehalose 6,6′-dimycolate preferentially induces type 1 helper T cell responses through signal transducer and activator of transcription 4 protein. Microb Pathog. 2005;39:35–43. doi: 10.1016/j.micpath.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Oswald IP, Dozois CM, Fournout S, Petit JF, Lemaire G. Tumor necrosis factor is required for the priming of peritoneal macrophages by trehalose dimycolate. Eur Cytokine Netw. 1999;10:533–540. [PubMed] [Google Scholar]
- Pelletier M, Forget A, Bourassa D, Gros P, Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982;129:2179–2185. [PubMed] [Google Scholar]
- Perez RL, Roman J, Staton GW, Jr, Hunter RL. Extravascular coagulation and fibrinolysis in murine lung inflammation induced by the mycobacterial cord factor trehalose-6,6′-dimycolate. Am J Respir Crit Care Med. 1994;149:510–518. doi: 10.1164/ajrccm.149.2.8306054. [DOI] [PubMed] [Google Scholar]
- Perez RL, Roman J, Roser S, Little C, Olsen M, Indrigo J, Hunter RL, Actor JK. Cytokine message and protein expression during lung granuloma formation and resolution induced by the mycobacterial cord factor trehalose-6,6′-dimycolate. J Interferon Cytokine Res. 2000;20:795–804. doi: 10.1089/10799900050151067. [DOI] [PubMed] [Google Scholar]
- Ramanathan VD, Curtis J, Turk JL. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect Immun. 1980;29:30–35. doi: 10.1128/iai.29.1.30-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, Gao F, Chen B, Jacobs WR, Jr, Glickman MS. Trans-cyclopropanation of mycolic acids on trehalose dimycolate suppresses Mycobacterium tuberculosis-induced inflammation and virulence. J Clin Invest. 2006;116:1660–1667. doi: 10.1172/JCI27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000;68:3322–3326. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- Schulman ES, Post TJ, Henson PM, Giclas PC. Differential effects of the complement peptides, C5a and C5a des Arg on human basophil and lung mast cell histamine release. J Clin Invest. 1988;81:918–923. doi: 10.1172/JCI113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Robinson D, Zonin F, Hartley SB, Macatonia SE, Somoza C, Hunter CA, Murphy KM, O’Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-γ in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- Silva CL, Ekizlerian SM, Fazioli RA. Role of cord factor in the modulation of infection caused by mycobacteria. Am J Pathol. 1985;118:238–247. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- VanHeyningen TK, Collins HL, Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol. 1997;158:330–337. [PubMed] [Google Scholar]
- WHO. The World Health Report 2003. Geneva, Switzerland: World Health Organization; 2003. Global Tuberculosis Control. Surveillance, Planning, Financing. [Google Scholar]
- Yamagami H, Matsumoto T, Fujiwara N, Arakawa T, Kaneda K, Yano I, Kobayashi K. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces foreign-body- and hypersensitivity-type granulomas in mice. Infect Immun. 2001;69:810–815. doi: 10.1128/IAI.69.2.810-815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimin Kohanawa M, Minagawa T. Up-regulation of granulomatous inflammation in interleukin-6 knockout mice infected with Rhodococcus aurantiacus. Immunology. 2003;110:501–506. doi: 10.1111/j.1365-2567.2003.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]