Abstract
The chloride/anion channels that have been so far identified in cultured astrocytes and those that have been confirmed in situ by a combination of mRNA identification, immunocyto-chemistry, and biophysical studies are reviewed. It is emphasized that we are just beginning to describe such channels and analyze their functions in astrocytes. The best-studied anion channels studied so far are those known as volume-regulated anion channels (VRACs). These, as for most channels, have been mainly studied in cultured astrocytes, but some correlative studies have been done in situ, because these channels have been emphasized as release routes for transmitters; namely, excitatory amino acids and ATP. They are activated by cell shape changes and cell swelling, and the release of amino acids and ATP and chloride currents, measured by whole cell clamping, by these processes has been well described, as is also their activation by low concentrations of extracellular ATP. However, the identity of these channels in astrocytes, as in all other cells, remains elusive. The potential involvement of VRACs in pathological states such as stroke, metastasis, and spreading depression is also discussed.
Keywords: volume-regulated anion channels, excitatory amino acid release, ATP effects, ATP release, spreading dispersion
INTRODUCTION
Cl- is the major strong anion in all physiological saline environments for vertebrates, with bicarbonate as the major (anionic) buffer, but is partially replaced by organic anions in many invertebrates (Prosser and Brown, 1961), such as malate in the medicinal leech (Hildebrandt and Zerbst-Boroffka, 1992). All living cells express a combination of transporters and ion channels to move Cl-, bicarbonate, and other anions across cell membranes. In the mammalian brain, dramatic changes in osmolarity or water content occur during some diseases and present as brain edema. However, water movements and changes in the extracellular space also occur during normal activity (Nicholson and Sykova, 1998), and it has been proposed that buffering of K+ by glial cells may also be associated with channel-mediated Cl- fluxes (e.g. Walz, 2002). The early biophysical studies of Kuffler and colleagues (Kuffler et al., 1966) failed to identify any contribution of Cl- to the glial resting conductance. This was due to technical limitations, and that the physiological context can determine whether or not astrocytic anion channels become activated. These channels are only beginning to be studied at the molecular and biophysical level, and thus, our knowledge regarding Cl- channels in glial cells is more tentative than that of other channels. There are a number of technical limitations, including a lack of channel-specific pharmacological reagents and, until recently, channel-specific antibodies. The lack of specific channel antagonists makes it difficult to rigorously link cloned Cl- channels to actual Cl- currents expressed in cells. Nevertheless, some exciting recent studies indicate that Cl- channels may be important contributors to the physiology and pathophysiology of glia. This review concerns itself with more recent studies on Cl- and anion channels in a particular group of glial cells, the astrocytes. We therefore purposively exclude transporters that provide important parallel nonconductive pathways for cotransport of anions with cations and exchange of anions, such as Cl- with bicarbonate (Brookes, 2005; Kimelberg and Bourke, 1982).
Anion channels, measured electrophysiologically with Cl- as the current carrying anion, serve to alter cell shape and cell volume changes in migratory cells (Ransom et al., 2001) and may be an important pathway for the release of neurotransmitters and ATP in the normal brain (Mongin and Kimelberg, 2004). Under pathophysiological conditions, these channels may assist in brain volume homeostasis, specifically contributing to or ameliorating brain edema (Kimelberg, 2000).
ANION/CL- CHANNELS IN ASTROCYTES
Cl- outnumbers any other available negatively charged mobile anion, and hence Cl- ions are the primary charge carriers through typical anion channels. It is important to note that most anion channels are also permeable to other anions, including amino acids and other organic and inorganic anions, and hence may function to release intracellular pools of these molecules, as discussed later. However, in biophysical studies, channels that mediate anion flux are typically referred to as Cl- channels, because the measurements are made with solutions where Cl- is the major anion.
Our molecular understanding of Cl- channels in astrocytes is in its infancy. Cl- channels cloned to date can be grouped into four molecular super families (Valverde, 1999): the CFTR channels, Ca2+-activated Cl- channels (Agnel et al., 1999), voltage-dependent anion-selective channels (VDACs) (Dermietzel et al., 1994; Dolder et al., 1999; Wunder and Colombini, 1991), and the ClC channels (Jentsch et al., 1999; Valverde, 1999). Only a few of these have thus far been identified in any type of astrocyte preparation. For example, PCR studies have demonstrated the presence of ClC-2 and ClC-3 transcripts in cultured cortical astrocytes (Parkerson and Sontheimer, 2004), and these two channels are also prominent in astrocyte-derived tumors (Olsen et al., 2003).
As illustrated in Figs. 1A—C, ClC-2 and ClC-3 channels can be detected by both PCR and immunolabeling in cultured astrocytes. Electrophysiological recordings show outward currents that are reduced by a replacement of Cl- with I- (Fig. 1D), consistent with functional channels of the ClC family. Both ClC-2 and ClC-3 appear to be engaged in regulatory volume decrease (RVD) following hypotonic swelling (Parkerson and Sontheimer, 2003, 2004). BR1-VDAC, a member of the VDAC family has been cloned from bovine brain and shown to constitute a porin in astrocytes (Dermietzel et al., 1994). ClC-1 iso-forms have also been identified in primary astrocyte cultures by RT-PCR (Zhang et al., 2004b). VDAC and weak CFTR transcript expression have been found in cultured astrocytes (Parkerson and Sontheimer, 2004).
Fig. 1.
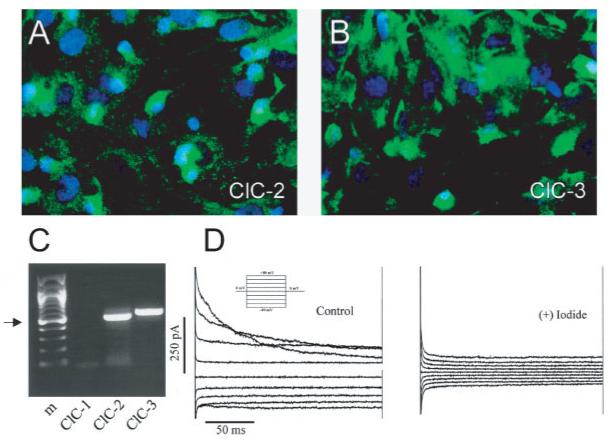
Cultured cortical rat astrocytes express ClC channels. A,B. Immunostaining with specific antibodies to ClC-2 and ClC-3 show prominent, membrane-associated staining. C. Transcripts for these channels are also found by RTPCR. Arrow indicates 500-bp band. D. Representative whole-cell recording of voltage steps from -80 to +80 mV for 200 ms from a 0-mV holding potential (inset) were obtained in choline—chloride bath and pipette solution following a 2-min hypotonic challenge. Currents were inhibited by substitution of 140 mM choline I for choline Cl-. (Adapted from Parkerson and Sontheimer, 2004.)
The majority of our current biophysical knowledge regarding Cl- channel expression in astrocytes stems from recordings in cultured cells. Using patch-clamp recordings, Ritchie and colleagues (Gray and Ritchie, 1986) first demonstrated outwardly rectifying currents from cultured astrocytes that persisted when extra-cellular Cl- was replaced with acetate, but these currents disappeared when Cl- was replaced by gluconate, suggesting that these outwardly rectifying currents were mediated by Cl- ions. However, astrocytes do not show a significant resting Cl- conductance (Walz, 2002). Indeed, only cell swelling or changes in the morphology of the astrocyte or receptor activation activates these otherwise silent Cl- channels. Another factor in studying these channels is that the dominant K+ conductance has to be inhibited to see the anion channels.
Lascola et al. (1998) demonstrated that changes in the cell cytoskeleton were necessary and sufficient to activate Cl- channels in cultured astrocytes. Rounding up cells by brief exposure to trypsin or by exposure to serum-free Ringer’s solution, as well as swelling cells by exposure to hypo-osmotic solution, were each able to induce the same outwardly rectifying Cl- currents. One possibility is that astrocyte Cl- channels are intrinsically connected to the cell’s cytoskeleton, which will change with shape or volume changes. This may explain why Barres and colleagues (Barres et al., 1988) were only able to observe Cl- channels in cultured rat optic nerve astrocytes upon excision of the channel-containing patch from the cell membrane. However, the cytoskeleton does not act alone, as MacVicar and colleagues showed that hypotonic challenge activate outwardly rectifying Cl- currents in cultured cortical astrocytes, but that the development of currents was dependent on theactivation of mitogen-activated protein kinases (Crépel et al., 1998).
In addition to these volume or shape-regulated outwardly rectifying Cl- channels, astrocytes also express inwardly rectifying Cl- currents under certain conditions. For example, Ferroni and colleagues treated astrocytes for 2 weeks with dBcAMP, a drug frequently used to round up these cells (Ferroni et al., 1997). Under these conditions, they were able to record Cl- currents mediated by small conductance (3–6 pS) channels that were inhibited by Cd2+ . Similar small conductance Cl- currents had previously been demonstrated in cultured mouse cerebral astrocytes (Nowak et al., 1987). The currents were potentiated by hyperpolarization, a hallmark for ClC-2 Cl- channels, and showed a Cl- = Br- = I- > F- > cyclamate > or = gluconate permeability sequence. External application of the Cl- channel blockers, 4,4-diisothiocyanatostilbene-2,2-disulphonic acid or 4-acetamido-4-isothiocyanatostilbene-2,2-disulphonic acid, did not affect these currents, but anthracene-9-carboxylic acid and Cd2+ or Zn2+ inhibited it. These features are consistent with those of recombinant ClC-2 channels expressed in oocytes (Furukawa et al., 1998). Interestingly, as with outwardly rectifying Cl- channels in astrocytes, ClC-2 in oocytes has been demonstrated to be normally inhibited by intact cytoskeletal actin (Ahmed et al., 2000).
The presence of ClC-2 in astrocytes in vivo is now supported by immunohistochemical studies that demonstrate ClC-2 protein in hippocampal lamina that also contains GABAergic neuronal terminals (Sik et al., 2000). The most compelling evidence, however, for the expression of ClC-2 channels in astrocytes in vivo, comes from studies in mouse brain slices comparing astrocytes in ClC-2 knockout mice to wild-type controls. The former showed hyperpolarization-activated Cd2+ -sensitive Cl- channels that were absent in astrocytes recorded in ClC-2 KO animals (Makara et al., 2003).
ClC-2 channels are inwardly rectifying and therefore ideally suited to aid Cl- secretion. (Note that the flow of current is defined as flow of positive charge and is thus opposite to the flow of negatively charged Cl- ions). In addition, ClC-2 currents are active near the cell’s resting potential and currents are often modulated by cell volume changes (Duan et al., 2000; Grunder et al., 1992; Jordt and Jentsch, 1997; Roman et al., 2001; Xiong et al., 1999). Hence ClC-2 may be a channel that supports the release of osmotically active Cl- following cell swelling. Alternatively, ClC-2 channels may support the Cl- efflux in the context of cell shape changes that occur as cells migrate. Such a requirement has been documented in astrocyte-derived tumor cells where cell invasion is accompanied by marked cell shrinkage to accommodate cells to fit through narrow extracellular spaces in the brain (Ransom et al., 2001).
Both cell shrinkage and cell invasion can be inhibited using blockers that inhibit Cl- channel function, including drugs that presumably inhibit ClC-2 and ClC-3 (Olsen et al., 2003). Importantly, cultured astrocytes and glioma cells actively accumulate intracellular Cl-, such that the equilibrium potential for Cl- in these cells is always positive (∼-40 mV) to the resting membrane potential (∼-80 mV) (Kettenmann, 1987; Kimelberg, 1981), and hence any opening of Cl- channels causes an efflux of Cl- from the cell. The resulting efflux of Cl- in conjunction with K+ and obligated water represents an effective mechanism for cells to shrink. Indeed, such a role for cell shape and cell volume changes may apply more broadly to migratory cells, including immature neurons, which also accumulate intracellular Cl- (Andersen et al., 1980). Of note, chlorotoxin, a peptide presumed to be a Cl- channel-specific inhibitor (DeBin et al., 1993), is currently in phase II clinical testing for patients with malignant glioma. While the mode of action of this peptide and its specificity for Cl- channels is somewhat controversial, it has recently been shown to induce the endocytosis of ClC Cl- channels into caveoli (McFerrin and Sontheimer, 2005).
A significant body of work in cell cultures supports the notion that cell swelling induced by hypotonicity or elevated Ko+ activates Cl- channels that are normally silent, and numerous neurological conditions have been reported to present with edema and show marked swelling of astrocytes (Kimelberg, 1995, 2000). In vitro, astrocytes respond to hypotonic swelling with a characteristic RVD (Kimelberg and Frangakis, 1986), and in brain slices, RVD has been observed when tissue taurine levels are maintained, suggesting that similar mechanisms are at play in intact tissue (Kreisman et al., 2003). The RVD process is still not entirely understood, but clearly involves the extrusion of osmotically active anions, Cl- and taurine amongst them, to aid the secretion of water and may regulate the cell volume back to a normal value. These volume-regulated anion channels (VRACs) will now be more fully discussed.
VRACS AND THE RELEASE OF AMINO ACIDS
VRACs are found in essentially all cells, and have mainly been studied in culture. These channels have also been termed VRACs, volume-sensitive outwardly rectifying anion channels, volume-sensitive organic anion channels and ICl-,vol or ICl-, swell, as they were initially defined by the characteristic outwardly rectifying chloride current that develops in cells swollen by exposure to hypotonic media (Eggermont et al., 2001; Jentsch et al., 2002; Okada, 1997; Strange et al., 1996). The only properties on which most investigators agree is the electrophysiological signature of this current. The channel protein (or proteins) responsible for this current remains elusive (Jentsch et al., 2002; Nilius and Droogmans, 2003; Okada, 1997; Strange, 1998). Figures 2D and 2E show the signature features of VRAC currents. When primary astrocyte cultures are exposed to hypotonic medium, VRAC currents are typically inhibited by tamoxifen and DCPIB (Figs. 2B,D). Above the current traces (Figs. 2A,C), we illustrate the typical time courses of the efflux of preloaded 3H-D-aspartate, as an indicator of excitatory amino-acid release, due to exposure to hypotonic medium. D-Aspartate was used instead of L-glutamate, because it is not metabolized. There is a rapid rise of aspartate release followed by a subsequent decline attributable to the reduction in cell volume as the cell regains its control volume through the solute release via the swelling activated anion channels. These function together with K+ channels for efflux of KCl plus other anions in conjunction with osmotically obligated water (Pasantes-Morales et al., 1994). Also shown is the marked potentiation of the efflux by addition of 10 μM ATP, which is massively released in the CNS initially during ischemia and head injury. The relationship of ATP to anion channels is discussed further later. Both efflux and current are inhibited by tamoxifen and DCPIB, which are very effective blockers of VRACs. In the case of tamoxifen, marked inhibition of efflux is only seen for the ATP-stimulated component, while for DCPIB the inhibition is around 90% in both conditions (see bar graph in Fig. 2F). Tamoxifen has a number of other effects (Kimelberg, 2005; Mongin and Kimelberg, 2004) but, at least currently, DCPIB is considered a specific blocker, in the sense that at the same concentrations it does not block a variety of other anion channels (Decher et al., 2001).
Fig. 2.
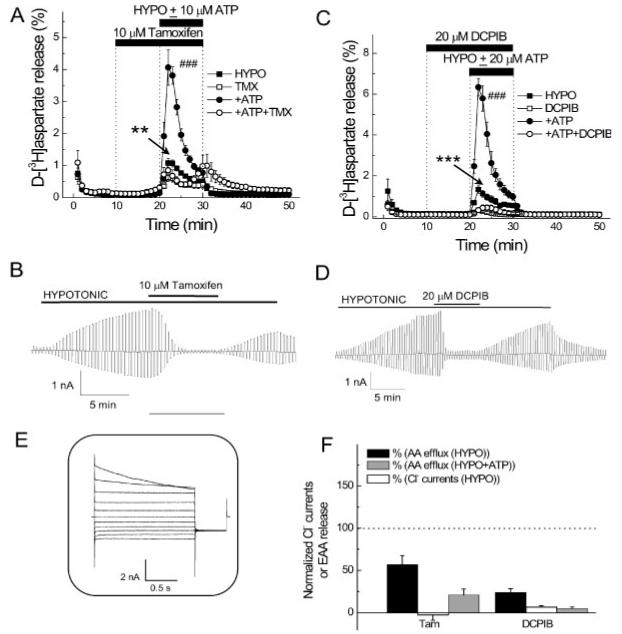
VRAC blockers DCPIB and tamoxifen potently inhibit swelling-activated D-[3H]aspartate release and Cl- currents in primary astrocyte cultures. A. Effect of 10 μM tamoxifen on swelling-activated D-[3H]aspartate release in the presence or absence of 10 μM ATP. Cells were exposed to hypo-osmotic medium (30% reduction in osmolarity for all experiments) as indicated, in the presence (□, ○) or absence (■, •) of 10 μM tamoxifen, applied as indicated. ATP was present in hypo-osmotic ∼ medium only (○, •). Data are means ±SEM of 5–7 experiments. **P = 0.002, tamoxifen vs. control; ###P < 0.001, ATP vs. ATP plus tamoxifen, repeated measures ANOVA. B. Effect of 10 μM tamoxifen on swelling-activated Cl- currents. Cells were held at 0 mV and step pulses to + and -40 mV were applied. Representative of six electrophysiological recordings. C. Effect of 20 μM DCPIB on swelling-activated D-[3H] aspartate release in the presence or absence of 20 μM ATP, in the presence (□, ○)or absence (■, •) of DCPIB, which was given 10 min before and during application of hypo-osmotic medium, as indicated. Data are means ± SEM of five experiments. ***P < 0.001, DCPIB vs. control; ***P < 0.001; ATP vs. ATP plus DCPIB, repeated measures ANOVA. D. Effect of 20-μM DCPIB on swelling-activated Cl- currents. Representative of six electrophysiological recordings. E. shows Cl- current responses to step pulses from -100 to +100 mV in 20-mV increments from 0-mV holding potential after exposure to hypotonic medium. F. Normalized release values relative to 100 for control swelling-activated excitatory amino acid release, with and without ATP and normalized VRAC currents in the absence of ATP in presence of tamoxifen or DCPIB. For B, D-F the isoosmotic external solution contained (in mM): 110 CsCl, 2 CaCl2, 1 MgSO4, 5 glucose, 10 Hepes, and 60 mannitol (pH 7.4, 290 mosmol). The hypoosmotic solution was made by omitting mannitol from isotonic solution and had an osmolarity of 230 mosmol. The pipette solution contained (in mM): 110 CsCl, 1 MgSO4, 1 Na2-ATP, 0.3 Na2-GTP, 15 Na-Hepes, 10 Hepes, and 1 EGTA (pH 7.3, 255 mosmol). The osmolarity of the pipette solution was set lower than that of the isotonic bath solution in order to prevent spontaneous cell swelling after attaining the whole-cell mode (from Abdullaev et al., 2006).
For astrocytes, VRACs have been predominantly studied in primary cultures of these cells prepared from neonatal pups. The initial suggestion that such channels occur in astrocytes came from the work of Pasantes-Morales and Schousboe (1989) studying the release of radiolabeled taurine from swollen primary astrocyte cultures. Kimelberg et al. (1990) then made the observation that swelling of primary astrocyte cultures released preloaded radiolabeled glutamate and aspartate as well as taurine. This led to the hypothesis that the release of glutamate via VRACs could contribute to the initial increase in extracellular glutamate leading to excitotoxicity. This may occur in the context of acute pathologies such as stroke and closed head injury where marked early swelling of astrocytes is observed. (Kimelberg, 1995, 2000; Mongin and Kimelberg, 2004), and this topic has been specifically covered in another special issue of Glia (Kimelberg, 2005). The present special issue deals mainly with the release of transmitters from astrocytes under nonpathological conditions, with implications for astrocytic modulation of synaptic activity and other effects, depending on where, when, and how much of the transmitter type molecules are released, as envisioned in the tripartite synapse concept (Araque et al., 1999). Recent work has shown that preloaded D-[3H]aspartate can be released from primary astrocyte cultures with very modest swelling, for example in response to a decrease of ∼5% in osmolarity when a low concentration of ATP (10 μM) is also present (Mongin and Kimelberg, 2002, 2005 and Fig. 3A). Is this ATP effect additive, or potentiation of an obligatory swelling response? Some activation of VRACs by slight swelling cannot be eliminated even if the bath solution is considered nominally isosmotic, so that a direct effect of ATP without prior activation of VRACs is very difficult to establish. However, an important finding is that co-application of ATP causes around a 10-fold increase in the otherwise small release (Figs. 3A,B) of aspartate, and also for taurine (Fig.3C). When[Ca2+]i is reduced to lower than resting levels by BAPTA-AM, the release is also inhibited (Mongin and Kimelberg, 2002). A smaller release was seen with nominally isosmotic media, but the effect was not seen when the osmolarity of the media was increased by 5–10% (Mongin and Kimelberg, 2002 and Figs. 3E,F.). Recently, Takano et al. (2005) have reported that this osmolarity-dependent release of glutamate by ATP was associated with an increase in volume of the primary astrocyte cultures in nominally isosmotic medium, and this increase as well as the glutamate efflux depended on an increase in intracellular Ca2+. The increase could therefore also be indirect due to activation of a K+Ca2+, channel leading to hyperpolarization and an increased outward driving force on aspartate, or activation of vesicular release pathways (Haydon and Carmignoto, 2006). However, the pharmacology of the inhibition of the release (Mongin and Kimelberg, 2002) argues, to some extent, against this latter possibility.
Fig. 3.
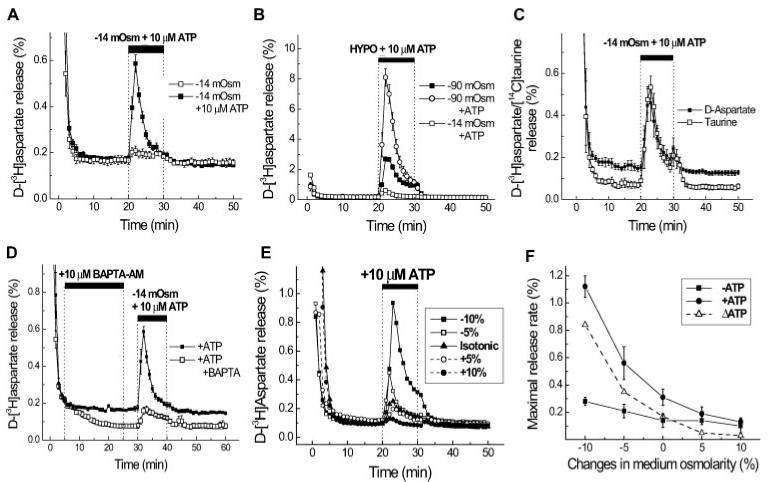
Activation of D-3H aspartate release by ATP with moderate and substantial swelling and effects of Ca2+ and changes in osmolarity. A. Moderate cell swelling was induced by 5% reduction in medium osmolarity (-14.5 mosmol). Ten micromoles of ATP was applied simultaneously with hypoosmotic medium. The data are the mean values ±SEM of seven experiments performed on three different cell culture preparations. B. Substantial cell swelling was induced by a 30% reduction in medium osmolarity (-90 mosmol). Ten micromolar ATP was applied simultaneously with hypoosmotic medium. The open squares show the ATP-induced D-[3H]aspartate release values in moderately swollen cells for comparison. The data are the mean values ±SEM of five experiments performed on two cell culture preparations. When not indicated, SEMs were less than symbols. C. Simultaneous measurements of the ATP-induced D-[3H]aspartate and [14C]taurine release from moderately swollen cultured astrocytes. Astrocytes were preloaded overnight with D-[3H]-aspartate and [14C]taurine. The data are means ±SEM of five experiments. D. ATP-induced organic osmolyte release in astrocytes is dependent on intracellular [Ca2+]. Cells were preincubated with 10 μM BAPTA-AM for 20 min, followed by a 5-min wash to remove extracellular BAPTA-AM. Then, astrocytes were exposed to a 5% reduction in medium osmolarity plus 10 μM ATP. The data are the means ±SEM of five experiments performed on two cell culture preparations. E. ATP-induced release is inhbited by an increase in medium osmolarity. Cells were exposed to 10 μM ATP and the simultaneous changes in medium osmolarity shown during 21st to 30th min of superfusion. F. Summary of the osmotic dependence of D-[3H]aspartate release in the presence or absence of 10 μM ATP shown in E. Open triangles represent an ATP-induced increment in EAA release. Data are the means ±SEM of 3–10 experiments performed on 2–4 different astrocyte culture preparations (from Mongin and Kimelberg, 2002, 2005).
Thus, in principle, a small degree of swelling coupled with astrocyte receptor activation that increases intracellular Ca2+ can release EAAs and other compounds that permeate VRACs. The volume-dependent release of transmitters from astrocytes could lead to effects on transmitter release from synaptic terminals (Araque et al., 1999). To further establish that such effects do occur in situ, one needs to be able to test its occurrence and determine its functional consequences in situ or in vivo. Studies have been done that show that fusion-dependent release of EAAs occurs in astrocytes in situ (Pascual et al., 2005; Zhang et al., 2004a), and that interfering with such events causes changes in synaptic strength and LTP (Pascual et al., 2005). These effects are always ascribed to effects on fusion of intracellular EAA-filled vesicles and release of the EAAs from astrocytes. However, it is quite possible that intracellular vesicles are fusing with the astrocyte plasma membrane, to insert channels such as VRACs, as this is a ubiquitous mechanism for inserting channels, transporters, and other proteins into the plasma membrane (Brown et al., 1997). Figure 3D shows that in astrocytic cultures the hypoosmotic release mediated by VRACs is also dependent on intracellular Ca2+ , so that Ca2+ dependence does not distinguish these two mechanisms. There has been no work to date on the existence of VRACs on astrocytes in situ, and this is clearly a gap that needs to be closed. There is the intriguing observation that some cultured neurons do not swell when exposed to hypotonic medium (Aitken et al., 1998 and the following section related to Fig. 4). Again, does this translate to neurons in vivo and correspond to the marked rapid astrocytic, but lack of neuronal swelling, after stroke and head injury (Kimelberg, 1995)? It would also predict that neurons do not express VRACs but astrocytes do. These questions are pressing and need to be answered.
Fig. 4.

Hyposmotic solutions cause swelling of astrocytes but not neurons. Two photon laser scanning microscopy of green fluorescent labeled (GFP) neurons and GFP astrocytes in cortical brain slices showed that osmotic changes in solution caused IOSs (intrinsic optical signals) that were not associated with any changes in neuronal volume but were associated with astrocytic swelling. The panels in column (A) show the changes in light transmittance as hippocampal brain slices swell or shrink in changing osmolarity. The GFP-labeled (B) soma, (C) dendrites, and (D) axon terminals of CA1 pyramidal neurons did not change their volume. In contrast (E) astrocytes showed swelling in hypo-osmotic solution and shrinkage in hyper-osmotic solution. The light transmittance and fluorescence images were obtained at 10 min after beginning perfusion with the solutions of different osmolarities, to ensure that a complete change had occurred in the slice (from Andrew et al., 2006).
Recently, we have found considerable similarity between hypotonic media induced ICl- measured electro-physiologically, and D-[3H]aspartate release, in terms of the profiles of inhibition by a number of different anion channel blockers (Fig. 2F and Abdullaev et al., 2006). There are apparent differences in time courses of activation under these different conditions. For example, the current does not decline in the whole cell voltage clamp approach. This maybedue to the lack of volume regulatory decrease in the whole cell patch clamp technique, because solute continuously diffuses from the normosmotic pipette fluid to replace K+ Cl-, and as they are organic anions are lost via VRACs from the cell cytoplasm. Thus there will be a continuous swelling and activation of the VRAC, rather than the activation followed by inactivation that occurs in an intact cell, which loses solutes and returns to its normal volume in the volume regulatory process, thus inhibiting VRAC’s.
ATP RELEASE VIA ANION CHANNELS
The modulation by ATP of swelling-activated Cl- currents and volume changes suggests an important role for ATP in modifying the release of glutamate via volume-activated anion channels via intracellular signaling and other mechanisms. However, the relationships between ATP and this anion channel activation are complex. ATP appears to be released by anion channels, but at the same time, it appears to regulate their activity. In several cell types, swelling itself has been shown to induce the release of ATP (Wang et al., 1996), which can act in an autocrine fashion to modify the response to swelling. These different pathways add further levels of complexity to the links between swelling, Cl- channel activation, and the release of neurotransmitters.
The original suggestion that ATP efflux was via P-glycoprotein Cl- channels has been questioned (Grygorczyk and Guyot, 2001; Hazama et al., 2000; Roman et al., 1997). There appears to be several mechanisms contributing to the efflux of ATP as a result of swelling. MacVicar’s laboratory reported that swelling of astrocyte cultures induced an efflux of ATP (Darby et al., 2003) and seemed to involve a multidrug resistance protein (MRP), because it was sensitive to MRP transport inhibitors such as MK571. The efflux of ATP then in turn increased the activation of the swelling-activated Cl- current. In mouse airway epithelial cells, ATP release was shown to be caused in part by another pathway via VDAC channels (Okada et al., 2004), but later work using deletion of the three genes encoding the VDAC isoforms showed no effect on the maxianion channel activity held to be responsible for ATP release (Sabirov et al., 2006).
FUNCTIONAL IMPACT OF ATP RELEASE THROUGH VOLUME ACTIVATED CHANNELS IN ASTROCYTES
The first step in linking the work in cell culture to the intact CNS is to show the circumstances under which astrocyte swelling actually occurs in intact tissue such as brain slices or in vivo. There is surprisingly little work on this subject, considering the pathological importance of brain cellular edema (Kimelberg 2000). It is clear that cells can rapidly change their volume in intact brain tissue. This has been elegantly demonstrated by the analysis of volume fraction and tortuosity of extra-cellular space using techniques pioneered by Nicholson and colleagues (Nicholson and Phillips, 1981; Nicholson and Sykova, 1998). Increased synaptic activity decreases the extracellular volume as a result of cell swelling in the hippocampus (McBain et al., 1990). In addition, ischemia causes very rapid and dramatic cellular swelling as indicated by large decreases in the extracellular volume (Perez-Pinzon et al., 1995). A good dynamic method for examining volume changes in tissue are intrinsic optical signals (IOSs) (MacVicar and Hochman, 1991). When cells swell, there is decreased light scattering, and brain slices show increased light transmittance that can easily be detected using digital imaging techniques (Andrew and MacVicar, 1994; Holthoff and Witte, 1996; Niermann et al., 2001). A recent study using two photon laser scanning microscopy has directly shown that astrocytes swell in hypo-osmotic solutions, but neurons don’t (Andrew et al., 2006). This is shown in Fig. 4, where the hypo-osmotically induced IOS (shown as increased light transmittance) is associated with increased astrocyte volume with no changes in neuronal size measured at the soma, dendrites, and axonal projections. This corroborates, and to some extent validates, the extensive work on isolated systems. It is certainly possible that the neurons had swollen but volume regulated (RVD) more rapidly than the astrocytes. However, there is no evidence to support this hypothesis, the measurements showing no neuronal swelling were made within 3 min of the exposure to hypotonic media (Anderson et al., 2006), and other work on freshly isolated CA1 pyramidal neurons has shown that some of them are resistant to hypotonic media-induced swelling when volume measurements were made simultaneously with the osmolarity changes (Aitken et al., 1998). The reasons why some neurons do not appear to swell upon exposure to hypotonic medium is an interesting question. Some possibilities are an unusually low permeability of their membranes to water together with a low level of aquaporins (Kimelberg, 2005), an unusually strong and rigid cytoskeleton that restricts swelling but then would cause an osmotic pressure across the cell membrane (Aitken et al., 1998), as well as the possibility of the aforementioned very rapid RVD.
SPREADING DEPRESSION AND THE RELEASE OF GLUTAMATE THROUGH VOLUME ACTIVATED CL- CHANNELS
Spreading depression (SD) is a slowly propagating wave of neuronal and astrocyte depolarization that leads to neuronal inactivation and synaptic depression (Martins-Ferreira et al., 2000). There are dramatic increases in cell volume and decreases in the extracellular space as cells swell during SD. These changes can be imaged by monitoring the IOS, which can be used to map the spread of the SD wavefront (Basarsky et al., 1998). The increase in light transmittance occurs coincidently with the extracellular negative potential shift that is generated by the widespread depolarization. Basarsky et al. (1999) showed that the SD wavefront also correlated with a wave of increased intracellular calcium. However, only the intracellular calcium wave was reduced by removing extracellular calcium and not the SD, a finding recently confirmed using confocal imaging (Peters et al., 2003). The profound cellular swelling during SD suggested that if glutamate release occurs during cell swelling in intact tissue, it should occur during SD. This was tested in MacVicar’s laboratory by measuring glutamate release during SD in calcium-free solution (Basarsky et al., 1999). In the presence of calcium and normal exocytosis, it is difficult to see the contribution of swelling-mediated release, because of the high degree of exocytotic release of transmitters from nerve terminals. However, when synaptic release was blocked by calcium-free EGTA solution, SD as measured by IOS and field recordings still occurred, and SD-induced glutamate release was measured in the effluent using HPLC (Basarsky et al., 1999 and Fig. 5). The release of glutamate during SD was blocked by NPPB (Figs. 5E,F), a nonvoltage dependent blocker of volume-activated Cl channels, in cultured astrocytes (Abdullaev et al., 2006; Crepel et al., 1998). Thus, the release of glutamate through volume-activated Cl- channels appears to increase the rate of propagation of SD by acting on NMDA receptors (Basarsky et al., 1999).
Fig. 5.
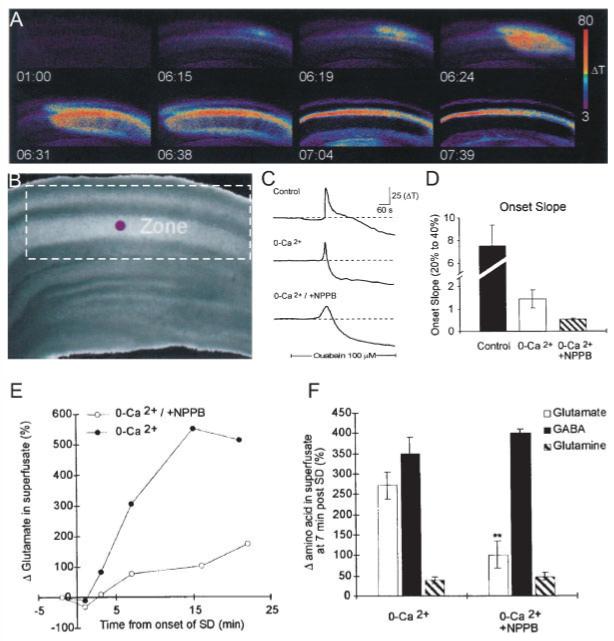
The release of glutamate during SD is reduced by NPPB, a blocker of volume-activated chloride channels. The release of glutamate during SD is reduced by NPPB, a blocker of volume-activated chloride channels, supporting a role for amino acid release due to astrocyte swelling. SD was triggered by inhibiting Na,K ATPase with ouabain. Imaging IOSs showed the progressive propagation of the depolarization and swelling during SD (A) (time in min; ΔT indicates increased light transmittance in arbitrary digital units) through the CA1 region (B) of the hippocampal brain slice. (C,D) SD still propagated in 0 external calcium, and NPPB in the absence of calcium reduced the onset slope of SD. The onset slope is the rate of change of transmittance during the propagation of SD. (E,F) NPPB also significantly reduced the efflux of glutamate during SD but did not alter GABA or glutamine efflux rates (from Basarsky et al., 1999). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
CONCLUSIONS
It is evident that few anion or Cl- channels are well characterized in astrocytes and even less is known regarding their expression and function in astrocytes in intact tissue. Hence, the first order of business would call for a careful and comprehensive description of anion channel expression and activity in astrocytes in brain slices and, to the extents, it can reliably be done in vivo. The relative paucity of such studies over the past few decades may, in part, stems from the early physiological recordings in glial cells, presenting no evidence for any measurable Cl- conductance in glia. Such studies started with the sharp electrode recordings of Kufflerand colleagues (Kuffler et al., 1966) in the optic nerve of the amphibian Necturus maculosus. They impaled the large glial cells of the optic nerve and recorded I-V curves with two sharp electrodes and obtained resistances of <1–7 MΩ. This low resistance indicates that either the cells have a high density of open channels or a very large membrane area, perhaps augmented by extensive cell—cell coupling and low resistance gap junctions. This also makes the study of the electrophysiology of more minor channels difficult, i.e., the dominant K+ conductance has to be selectively inhibited, which requires one to know the identity of all the K+ channels. In contrast to a lack of changes when bath Na+ and Cl- were changed, dramatic changes in the very negative membrane potential of around -90 mV occurred when bath K+ was modified, giving a Nernstian relation for varying [Ko+] down to 1.5 mM. Mammalian CNS glia blindly impaled with sharp electrodes, and then identified as nonexcitable, also gave very negative membrane potentials and showed a Nernstian response to the more limited changes in [Ko+] (3 to ∼10 mM) that could be achieved by stimulation of the brain in the intact animals (reviewed in Somjen, 1995). Thus arose the concept of the plasma membranes of glia, originally astroglia and later oligodendroglia, as having plasma membranes that exclusively contained K+ selective leak channels and therefore exhibited large negative membrane potentials with Em ≈ EK+. However, Kuffler et al. (1966) could not exclude passive anion channels that could serve to rapidly equilibrate the transmembrane chloride gradient to the new EK as they noted; “Other ions such as Na+ and Cl- had little influence on the membrane potential recorded 10–50 min after changing solutions.... We did not look for transient changes.”
From these studies in the 1960s to 1970s arose the concept of glial cells, especially astroglia, as cells that controlled [K+o] by a process termed spatial K+ buffering (Orkand et al., 1966) in the mammalian brain by virtue of the fact that their plasma membranes showed an exclusive permeability to [K+o] by unknown channels that behave ohmically. These are often called “leak” channels, but these are K+-specific channels and not a nonspecific “leak,” and therefore ohmic would seem a preferable word to avoid any confusion on this. As pointed out by Barres et al., in a review in 1990 (Barres et al., 1990), newer work up to that time called many of these “tenets” of glial cells into question. However, recent whole cell patch clamp studies on astrocytes in mammalian hippocampal slices from older rats seems to have reconfirmed the viewpoint that mature astrocytes show predominantly ohmic K+ currents, at least for the CA1 hippocampal region, until other regions are similarly studied (Zhou et al., 2006).
As covered in this review, the implication that glial cells have no anion (chloride) channels has now also been questioned. As pointed out earlier, however, activation of anion channels typically requires a stimulus, be it release of ATP, swelling, or shape changes. Few studies have attempted to search for currents under such conditions in situ, yet given the importance of anion channels in the context of brain volume homeostasis and change neutralization to allow significant fluxes of cations, such as K+, NA+ and H+ such studies are vitally needed. Also, the recent finding that even very small perturbations in extracellular osmolarity or changes in ATP can activate anion channels, which in turn may release amino-acid transmitters challenges us to consider the role of these channels in normal brain function as well as in pathology.
Acknowledgments
Grant sponsors: The work by the authors reported in this review was partly supported by NS 35205 (HKK), NS 36692 (HS) and the Canadian Institutes for Health Research and the Heart and Stroke Foundation for BC and Yukon (BAM).
REFERENCES
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in rat cultured astrocytes. J Physiol. 2006;572(Part 3):677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnel M, Vermat T, Culouscou JM. Identification of three novel members of the calcium-dependent chloride channel (CaCC) family predominantly expressed in the digestive tract and trachea. FEBS Lett. 1999;455:295–301. doi: 10.1016/s0014-5793(99)00891-1. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Ramjeesingh M, Wong S, Varga A, Garami E, Bear CE. Chloride channel activity of ClC-2 is modified by the actin cytoskeleton. Biochem J. 2000;352(Part 3):789–794. [PMC free article] [PubMed] [Google Scholar]
- Aitken PG, Borgdorff AJ, Juta AJA, Kiehart DP, Somjen GG, Wadman WJ. Volume changes induced by osmotic stress in freshly isolated rat hippocampal neurons. Pflugers Arch. 1998;436:991–998. doi: 10.1007/s004240050734. [DOI] [PubMed] [Google Scholar]
- Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Laursen AM. Two different responses of hippocampal pyramidal cells to application of γ-amino butyric acid. J Physiol. 1980;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA.Physiological evidence that pyramidal neurons lack functional water channels Cereb Cortex[Epub ahead of print].2006July 12 [DOI] [PubMed] [Google Scholar]
- Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. doi: 10.1016/0306-4522(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Barres BA, Chun LLY, Corey DP. Ion channel expression by white matter glia. I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1:10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barres BA, Chun LLY, Corey DP. Ion channels in vertebrate glia. Ann Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Basarsky TA, Duffy SN, Andrew RD, MacVicar BA. Imaging spreading depression and associated intracellular calcium waves in brain slices. J Neurosci. 1998;18:7189–7199. doi: 10.1523/JNEUROSCI.18-18-07189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci. 1999;19:6439–6445. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N. Mechanisms of solute transport in glia. In: Kettenmann HO, Ransom BR, editors. Neuroglia. 2nd Oxford University Press; New York: 2005. pp. 163–176. [Google Scholar]
- Brown D, Sabolic I, Breton S. Polarized expression of membrane proteins in renal epithelial cells: Involvement of specialized transport vesicles and intracellular pathways. Adv Nephrol Necker Hosp. 1997;27:297–315. [PubMed] [Google Scholar]
- Crépel V, Panenka W, Kelly MEM, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol. 2003;89:1870–1877. doi: 10.1152/jn.00510.2002. [DOI] [PubMed] [Google Scholar]
- DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264(2)(Part 1):C361–C369. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of ICl, swell and prevents swelling- induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Hwang TK, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes FP, Fishman GI, Spray DC, Siemen D. Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc Natl Acad Sci USA. 1994;91:499–503. doi: 10.1073/pnas.91.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder M, Zeth K, Tittmann P, Gross H, Welte W, Wallimann T. Crystallization of the human, mitochondrial voltage-dependent anion-selective channel in the presence of phospholipids. J Struct Biol. 1999;127:64–71. doi: 10.1006/jsbi.1999.4141. [DOI] [PubMed] [Google Scholar]
- Duan D, Ye L, Britton F, Horowitz B, Hume JR. A novel anionic inward rectifier in native cardiac myocytes. Circ Res. 2000;86:E63–E71. [PubMed] [Google Scholar]
- Eggermont J, Trouet D, Carton I, Nilius B. Cellular function and control of volume-regulated anion channels. Cell Biochem Biophys. 2000;35:263–274. doi: 10.1385/CBB:35:3:263. [DOI] [PubMed] [Google Scholar]
- Ferroni S, Marchini C, Nobile M, Rapisarda C. Characterization of an inwardly rectifying chloride conductance expressed by cultured rat cortical astrocytes. Glia. 1997;21:217–227. doi: 10.1002/(sici)1098-1136(199710)21:2<217::aid-glia5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ogura T, Katayama Y, Hiraoka M. Characteristics of rabbit ClC-2 current expressed in Xenopus oocytes and its contribution to volume regulation. Am J Physiol. 1998;274(2)(Part 1):C500–C512. doi: 10.1152/ajpcell.1998.274.2.C500. [DOI] [PubMed] [Google Scholar]
- Gray PT, Ritchie JM. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986;228:267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Grunder S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Guyot A. Osmotic swelling-induced ATP release: A new role for tyrosine and Rho-kinases? J Physiol. 2001;532:582. doi: 10.1111/j.1469-7793.2001.0582e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl- conductances in murine C127 cells. J Physiol. 2000;523(Part 1):1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt JP, Zerbst-Boroffka I. Osmotic and ionic regulation during hypoxia in the medicinal leech, Hirudo medicinalis L. J Exp Zool. 1992;263:374–381. doi: 10.1002/jez.1402630405. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Witte OW. Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J Neurosci. 1996;16:2740–2749. doi: 10.1523/JNEUROSCI.16-08-02740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Friedrich T, Schriever A, Yamada H. The CLC chloride channel family. Pflügers Arch. 1999;437:783–795. doi: 10.1007/s004240050847. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H. K+ and Cl- uptake by cultured oligodendrocytes. Can J Physiol Pharmacol. 1987;65:1033–1037. doi: 10.1139/y87-163. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Active accumulation and exchange transport of chloride in astroglial cells in culture. Biochim Biophys Acta. 1981;646:179–184. doi: 10.1016/0005-2736(81)90285-6. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Cell volume in the CNS: Regulation and implications for nervous system function and pathology. Neuroscientist. 2000;6:13–24. [Google Scholar]
- Kimelberg HK. Water homeostasis in the brain: Basic concepts. Neuroscience. 2004;129:851–860. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia. 2005;50:389–397. doi: 10.1002/glia.20174. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Bourke RS. Anion transport in the nervous system. In: Latjtha A, editor. Handbook of neurochemistry. 2nd Vol. 1. New York: Plenum: 1982. pp. 31–67. [Google Scholar]
- Kimelberg HK, Frangakis MV. Volume regulation in primary astrocyte cultures. Adv Biosci. 1986;61:177–186. [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman NR, Olson JE. Taurine enhances volume regulation in hippocampal slices swollen osmotically. Neuroscience. 2003;120:635–642. doi: 10.1016/s0306-4522(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Lascola CD, Nelson DJ, Kraig RP. Cytoskeletal actin gates a Cl- channel in neocortical astrocytes. J Neurosci. 1998;18:1679–1692. doi: 10.1523/JNEUROSCI.18-05-01679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Hochman D. Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J Neurosci. 1991;11:1458–1469. doi: 10.1523/JNEUROSCI.11-05-01458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara JK, Rappert A, Matthias K, Steinhauser C, Spat A, Kettenmann H. Astrocytes from mouse brain slices express ClC-2-mediated Cl- currents regulated during development and after injury. Mol Cell Neurosci. 2003;23:521–530. doi: 10.1016/s1044-7431(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Martins-Ferreira H, Nedergaard M, Nicholson C. Perspectives on spreading depression. Brain Res Rev. 2000;32:215–234. doi: 10.1016/s0165-0173(99)00083-1. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Traynelis SF, Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990;249:674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glial Biol. 2005;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol. 2002;283:C569–C578. doi: 10.1152/ajpcell.00438.2001. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. Astrocytic swelling in neuropathology. In: Kettenmann HO, Ransom BR, editors. Neuroglia. Oxford University Press; New York, NY: 2004. pp. 550–562. [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Phillips JM. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP. A novel role of vasopressin in the brain: Modulation of activity-dependent water flux in the neocortex. J Neurosci. 2001;21:3045–3051. doi: 10.1523/JNEUROSCI.21-09-03045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Amazing chloride channels: An overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- Nowak L, Ascher P, Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci. 1987;7:101–109. doi: 10.1523/JNEUROSCI.07-01-00101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl- channel: Fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol. 2004;124:513–526. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Schade S, Lyons SA, Amarillo MD, Sontheimer H. Expresssion of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–5582. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Contribution of chloride channels to volume regulation of cortical astrocytes. Am J Physiol Cell Physiol. 2003;284:C1460–C1467. doi: 10.1152/ajpcell.00603.2002. [DOI] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419–436. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Lilja L, Moran J. Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability Am J Physiol Cell Physiol. 1994;266:C165–C175. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A. Release of taurine from astrocytes during potassium-evoked swelling. Glia. 1989;2:45–50. doi: 10.1002/glia.440020105. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Tao L, Nicholson C. Extracellular potassium, volume fraction, and tortuosity in rat hippocampal CA1, CA3, and cortical slices during ischemia. J Neurophysiol. 1995;74:565–573. doi: 10.1152/jn.1995.74.2.565. [DOI] [PubMed] [Google Scholar]
- Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci. 2003;23:9888–9896. doi: 10.1523/JNEUROSCI.23-30-09888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser CL, Brown FA. Comparative animal physiology. 2nd Philadelphia: Saunders: 1961. pp. 57–80. [Google Scholar]
- Ransom CB, O’Neal J, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci. 2001;21:7674–7683. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RM, Smith RL, Feranchak AP, Clayton GH, Doctor RB, Fitz JG. ClC-2 chloride channels contribute to HTC cell volume homeostasis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G344–G353. doi: 10.1152/ajpgi.2001.280.3.G344. [DOI] [PubMed] [Google Scholar]
- Roman RM, Wang Y, Lidofsky SD, Feranchak AP, Lomri N, Scharschmidt BF, Fitz JG. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J Biol Chem. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem. 2006;281:1897–1904. doi: 10.1074/jbc.M509482200. [DOI] [PubMed] [Google Scholar]
- Sik A, Smith RL, Freund TF. Distribution of chloride channel-2-immunoreactive neuronal and astrocytic processes in the hippocampus. Neurosci. 2000;101:51–65. doi: 10.1016/s0306-4522(00)00360-2. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Electrophysiology of mammalian glial cells in situ. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University Press; New York: 1995. pp. 319–331. [Google Scholar]
- Strange K. Molecular identity of the outwardly rectifying, swelling-activated anion channel: Time to reevaluate pICln. J Gen Physiol. 1998;111:617–622. doi: 10.1085/jgp.111.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M. Receptor-mediated glutamate release from volume-sensitive channels in astrocytes. Proc Natl Acad Sci USA. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde MA. ClC channels: Leaving the dark ages on the verge of a new millennium. Curr Opin Cell Biol. 1999;11:509–516. doi: 10.1016/s0955-0674(99)80074-x. [DOI] [PubMed] [Google Scholar]
- Walz W. Chloride/anion channels in glial cell membranes. Glia. 2002;40:1–10. doi: 10.1002/glia.10125. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder UR, Colombini M. Patch clamping VDAC in liposomes containing whole mitochondrial membranes. J Mem Biol. 1991;123:83–91. doi: 10.1007/BF01993966. [DOI] [PubMed] [Google Scholar]
- Xiong H, Li C, Garami E, Wang Y, Ramjeesingh M, Galley K, Bear CE. ClC-2 activation modulates regulatory volume decrease. J Mem Biol. 1999;167:215–221. doi: 10.1007/s002329900485. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004a;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Morishima S, Ando-Akatsuka Y, Takahashi N, Nabekura T, Inoue H, Shimizu T, Okada Y. Expression of novel isoforms of the CIC-1 chloride channel in astrocytic glial cells in vitro. Glia. 2004b;47:46–57. doi: 10.1002/glia.20024. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: Mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]


