Abstract
The design and synthesis of analogues of diadenosine 5′,5′″-P1,P3-triphosphate that are resistant to pyrophosphate hydrolysis is described in relation to their role in signaling and tumorigenesis involving the Fhit protein, the human fragile histidine triad protein, which is a novel Ap3A binding/cleaving protein.
Dinucleoside polyphosphates (DNPPs) occur ubiquitously in both prokaryotic and eukaryotic cells.1 Naturally occurring species have linear polyphosphate chains ranging from 3 to 6 phosphate residues, among which the major forms are the dinucleoside triphosphates (Np3Ns) and dinucleoside tetraphosphates (Np4Ns) with adenosine as the dominant nucleoside. The precise biological function of these nucleotides has been a subject of conjecture and exploration since the discovery of the diguanosine polyphosphates, GpnGs, by Warner2 in 1963 and of the diadenosine polyphosphates, ApnAs, by Zamecnik3 and coworkers in 1966. Inter alia, it has been observed that levels of DNPPs rise markedly in vivo under condition of cell stress, especially for Ap4A, which has excited strong interest and has been well studied.4
It has been suggested that levels of Ap4A may be related to cell proliferation and environmental stress in prokaryotes and lower eukaryotes, as well as to have a function in extracellular signaling in higher eukaryotes. In addition, there is considerable current interest in an extracellular rôle for Ap4A and Ap3A in blood platelet physiology. Both molecules are stored in high concentration in the metabolically inactive, dense granules of platelets5 and are released when platelets are stimulated to undergo aggregation.6 Indeed, Ap3A has been shown gradually to induce platelet aggregation, most likely through its hydrolysis in plasma to ADP. While Ap4A strongly inhibits platelet aggregation induced by ADP, it too can be degraded to ADP, which is a known agonist of platelet aggregation. In response to this situation, we have evaluated7 Ap4A analogues as antithrombotic agents that cannot be cleaved to ADP.
The major problem frustrating accurate definition of the cellular function of DNPPs, especially of the ApnAs, is their rapid degradation by both specific and non-specific hydrolases and phosphorylases8 which leads to the maintenance of DNPPs at submicromolar levels in resting cells, notwithstanding their continuous biosynthesis. Chemical synthesis of DNPP analogues that are stable to enzymatic cleavage has provided useful tools which have been deployed alike in the elucidation of some of their biological functions and in the mechanism of action of some specific DNPP pyrophosphohydrolases.9 Much excitement has attached to the recent identification of the human Fhit (Fragile histidine triad) protein, encoded by a gene located on the short arm of chromosome 3, which spans the fragile site FRA3B and is disrupted in many human tumours.10 This putative tumour suppressor is a DNPP hydrolase dependent on Mn2+ that prefers Ap3A (kcat/KM = 2 × 106 s-1 M-1) to Ap4A (6.7 × 103 s-1 M-1).11 These results strongly suggest that Ap3A or similar dinucleotide polyphosphates may be factors in tumorigenesis.10 Moreover, the high degree of protein homology between the sequence of Fhit and that of the Ap4A hydrolase from S. pombe, a known asymmetric Ap4A hydrolase,12 suggests that Fhit may be related in activity to the eukaryotic Ap3A hydrolase from lupin whose mechanism of action we have already characterised.9
We have previously described syntheses of a range of phosphonate analogues13,14 of Ap3A and Ap4A as part of a programme to investigate the mechanism of action the corresponding, specific dinucleoside polyphosphate hydrolases and to uncover the biological function of such dinucleoside polyphosphates. This paper describes the design and synthesis of two novel analogues of Ap3A, programmed to be resistant to hydrolysis by Fhit, and generated for use in X-ray crystal structure and NMR solution structure determinations of binary complexes with that protein.
Results and Discussion
The pattern of cleavage of Ap3A by the eukaryotic specific Ap3A hydrolase involves attack of water at P1 with cleavage of the P1,P2 bridge (FIG. 1).9 As we have shown earlier,15 such cleavage can be blocked either by the use of an isosteric carbon function in the P1,P2-bridge locus or by the introduction of sulfur with R configuration at P1. Because of the C2 symmetry of the Ap3A substrate, the 1-RP,3-RP isomer of diadenosine 5′,5′″-P1,P3-dithiotriphosphate, APsPPsA, should be an effective inhibitor for Fhit. However, the assumption that Fhit operates by water attack at P1 is insecure, not least because the lack of sequence homology between lupin and human Ap4A hydrolases and the Ap3A hydrolase16 from S. pombe makes it imperative to generate analogues of Ap3A that are able to resist water attack at either P1(P3) or P2. Moreover, bis-thiation at P1,P4 in Ap4A has been observed to drive the asymmetrical Ap4A hydrolase from the brine shrimp, Artemia, actually to cleave APsPPPsA symmetrically to ADPαS, albeit at 3.4 % of the regular Vmax rate.17
Fig. 1.

There are two acceptable design solutions to this problem. The first seeks to incorporate a bisphosphonomethylphosphinate, PCPCP, linker into an analogue of Ap3A.13 The second combines thiation at one end of the analogue with an isosteric carbon bridge at the other. The former design retains the simplicity of a C2-symmetry analogue, which may well prove to be important for either x-ray crystallographic studies or for NMR investigations. However, the introduction of two methylene bridges may affect the binding of the APCPCPA analogue too adversely for effective agonist/antagonist activity. By contrast, the dissymmetry of the analogues generated in the second approach has the potential benefit of offering alternative modes of binding for such APsPXPA analogues to the Fhit protein, and thereby optimising affinity and agonist/antagonist effects.
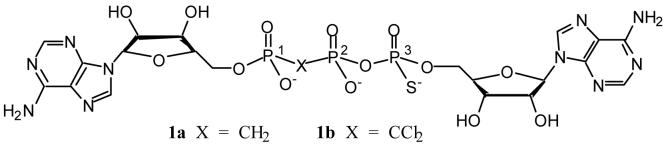
While our studies on the synthesis of species such as APCF2PCF2PA and APCCl2PCCl2PA will be described elsewhere, we here report the synthesis of the first two analogues of Ap3A of the second type, specifically diadenosine 5′,5′″-(P1,P2-methylene-P3-thio)-P1,P3-triphosphate 1a and diadenosine 5′,5′″-(P1,P2-dichloro-methylene-P3-thio)-P1,P3-triphosphate 1b.
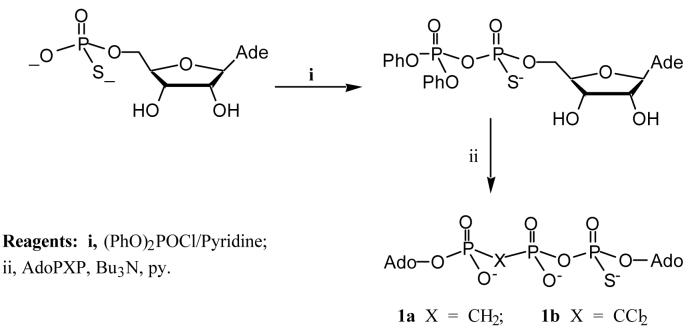
Syntheses were achieved by condensation of adenosine 5′-thiophosphate activated by diphenyl phosphorochloridate18 with an excess of adenosine 5′-P1,P2-methylenebisphosphonate, AMPPCP, or adenosine 5′-P1,P2-dichloromethylenebisphosphonate, AMPPCCl2P, respectively (FIG. 2). While other phosphorylating agents have been used for the large scale synthesis of Ap4A itself,19 diphenyl phosphorochloridate remains the most effective condensing agent in our hands for these thiated isosteric analogues with methylene bridges. Analogue 1b was obtained in a yield of 45% and 1a in 18% yield (with recovery of 61% starting material, AMPPCP). Both of the required P1,P2-methylene analogues of ADP, AMPPCP and AMPPCCl2P, were conveniently prepared by the method of Poulter.20,21
Fig. 2.
These condensation process necessarily generates a mixture of two diastereoisomers for 1a and 1b at the thiophosphate centre. Their existence is clearly manifest in the 31P NMR resonance for both species, most strongly in the signals for the thiophosphate residue. Compound 1a showed an AMX NMR spectrum with doublets for each of the two diastereoisomers at δP 43.0 ppm (J = 31.4) and 43.3 ppm (J = 31.4) while 1b showed two doublets for P3 at 43.77 ppm (J = 31.4), and 43.53 ppm (J = 34.5). Unfortunately, neither of these diastereoisomeric mixtures could be separated by anion exchange HPLC: 1a gave a single peak while 1b gave partial resolution with a shoulder peak. It may thus be necessary to seek partial resolution of these isomers by the use of stereoselective enzymatic hydrolysis. Positive ion FAB-MS spectra of the products showed a homologous series of peaks of mass increment 22 based on the protonated parent molecule: 1a ionises with 0-3 sodium ions and 1b with 0-2 sodium ions.
We have completed preliminary studies using cloned Fhit protein which show that the protein binds well to both of these analogues without cleaving them. Moreover, Fhit forms a crystalline complex with 1a, details of which will be published elsewhere.22
Experimental
Proton NMR were recorded on a Bruker AC-250 spectrometer at 250 MHz, unless otherwise stated. Chemical shifts (δH) are accurate to ± 0.01 ppm. Phosphorus nmr were recorded on a Bruker AM-250 spectrometer at 101.3 MHz. Chemical shifts were quoted in ppm downfield from 85% H3PO4 as the external reference. Fast atom bombardment (FAB) mass spectra were recorded on a Kratos MS80RF mass spectrometer. HPLC was performed using MA7Q anion exchange column (BIO-RAD) with eluent of a linear 0.05 M / 0.7 M aqueous NH4HCO3 gradient: from 100% 0.05 M NH4HCO3 to a mixture of 0.05 M aqueous NH4HCO3 and 0.7 M NH4HCO3 (4:1, v/v) in 10 min and then isocratic elution for another 2 min. The flow was 4 ml min-1 and the eluent was monitored at 254 nm. Ion-exchange medium pressure column chromatography (mplc) was performed using a DEAE A25 Sephadex (Aldrich) column (3 cm × 20 cm) and linear gradient of triethylammonium bicarbonate buffer (TEAB), pH 7.5. A flow of 6 ml min-1 was used and the eluent was monitored at 254 nm. TEAB (2 M) was prepared by adding triethylamine (557 ml, 4 mol) to distilled water (1.2 L) in a glass vessel cooled by ice, then CO2 was bubbled through until a pH of 7.5 was achieved. The buffer was diluted to a final volume of 2 L.
Diadenosine 5′,5′″-(P1,P2-dichloromethylene-P3-thio)-P1,P3-triphos-phate 1b
Adenosine 5′-thiophosphate (180 mg, 0.308 mmol) as the bis-(triethyl-ammonium salt) and tri-n-octylamine (0.141 ml, 0.323 mmol) were shaken in methanol (7 ml) until dissolution was achieved. The solution was evaporated under reduced pressure. The residue was then coevaporated with pyridine (3 × 10 ml) and further dried under vacuum over P2O5 for 12 h. The oily residue was then dissolved in dry dioxane (3 ml). Diphenyl phosphorochloridate (0.098 ml, 0.471 mmol) and tri-n-butylamine (0.176 ml, 0.739 mmol) was added. The mixture was stirred at rt. and the initial cloudy solution became clear gradually. After 3.5 h, the solvent was evaporated and oily residue was washed with dry diethyl ether (3 × 10 ml) and then coevaporated with dry pyridine (2 × 10 ml). Adenosine 5′-(P1,P2-dichloromethylene)diphosphate (165 mg, 0.237 mmol) as its tris-triethylammonium salt and tri-n-butylamine (0.113 ml, 0.474 mmol) were shaken in dry methanol (5 ml) until the dissolution was achieved, then the solution was evaporated under reduced pressure. The residue was coevaporated with pyridine (3 × 10 ml) and further dried over P2O5 overnight. The resulting oil was dissolved in dry pyridine (3.6 ml) and this solution was added to the activated nucleoside above. The reaction mixture was stirred at rt overnight and then evaporated under reduced pressure. The oily residue was partitioned between dichloromethane (2 × 15 ml) and water (50 ml), the aqueous layer evaporated under reduced pressure, and the residue chromatographied on a DEAE A-25 Sephadex column with a gradient eluent of aqueous (TEAB) from 0.05 M to 0.5 M in 4 L. The title compound as triethylammonium salt was eluted at a concentration of 0.37 M TEAB. The product-containing fractions were pooled and evaporated under reduced pressure. The residue was coevaporated with methanol (3 × 15 ml) and the title compound was obtained as its triethylammonium salt. To convert this salt into the sodium salt, the product was dissolved in 2 ml methanol and added dropwise to a stirred solution of NaI (1 M) in acetone (50 ml). The precipitate was collected by centrifugation and washed with acetone (4 × 50 ml). Yield 97 mg (45% as trisodium salt).
δP (D2O): 43.8 (d, J = 34.5) and 43.5 (d, J = 34.5) (P3, two diastereoisomers), 8.5 (d, J = 20.0, P1), -1.4 (dd, J = 20.0 and 34.5) and -1.5 (dd, J = 20.0 and 34.5) (P2, two diastereoisomers). δH (D2O): 8.45 (s), 8.42 (s), 8.40 (s) and 8.36 (s) [2H in total], 8.04 (m, 2H), 5.97 (m, 2H) and 4.56-4.25 (m, 10H). FAB-MS(positive): m/z 839 (M+H+), 861 (M+Na+) and 883 (M+2Na+-H+).
Diadenosine 5′,5′″-(P1,P2-methylene-P3-thio)-P1,P3-triphosphate 1a
This compound was prepared similarly to 1b. Starting from 221 mg adenosine 5′-(P1,P2-methylene)-diphosphate as its tris-triethylammonium salt, the sodium salt of the title compound was obtained as a white powder (58 mg, 18%) with recovery of 61% of starting material, adenosine 5′-(P1,P2-methylene)-diphosphate as its tris-triethyl-ammonium salt (136 mg, 0.25 mmol).
δp (D2O): 43.0 ( d, J = 31.4) and 43.3 (d, J = 31.4) (P3, two diastereoisomers), 17.9 (d J = 8.4) and 18.0 (d, J = 7.8) (P1, two diastereoisomers), 7.6 (dd, J = 31.2 and 7.9, P2). δH (D2O): 8.50 (s), 8.43 (s), 8.35 (s) and 8.31 (s) [2H in total], 8.02 (m, 2H), 5.91-6.01 (m, 2H), 4.05-4.74 (m, 10H), 3.28 (m, 2H, PCH2P). FAB-MS (positive): m/z 771 (M+H+), 793 (M+Na+), 815 (M+2Na+-H+), 837 (M+3Na+-2H+).
ACKNOWLEDGEMENTS
We gratefully acknowledge financial support from NIH for Grant CA75954 and Cancer Center Grant CA56336 (CB) and from BBSRC for Grants BO7047, BCI06200, and MOLO4558 (GMB).
Footnotes
Dedicated to the memory of Professor Tsujiaki Hata, friend and associate for many years, who himself held a close interest in diadenosine and diguanosine polyphosphates.
REFERENCES
- 1.McLennan AG.Ap4A and other dinucleoside polyphosphates 1992CRC Press; Boca Raton: FD 33431 [Google Scholar]
- 2.Finamore FJ, Warner AH. J. Biol. Chem. 1963;238:344–348. [PubMed] [Google Scholar]
- 3.Zamecnik PC, Stephenson ML, Janeway CM, Randerath K. Biochem. Biophys. Res. Commun. 1966;24:91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]
- 4.Kitzler JW, Farr SB, Ames BN. in Ref.1, 135–149.
- 5.Lüthje J, Ogilvie A. Biochem. Biophys. Res. Commun. 1983;115:253–260. doi: 10.1016/0006-291x(83)90997-x. [DOI] [PubMed] [Google Scholar]
- 6.Flodgaard H, Klenow H. Biochem. J. 1982;208:737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BK, Zamecnik PC, Taylor G, Guo M-J, Blackburn GM. Proc. Natl. Acad. Sci. USA. 1992;89:11056–11058. doi: 10.1073/pnas.89.22.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chan SW, Gallo SJ, Kim BK, Guo MJ, Blackburn GM, Zamecnik PC. Proc. Natl. Acad. Sci. USA. 1997;94:4034–4039. doi: 10.1073/pnas.94.8.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guranowski A, Sillero A. in Ref 1., 81–133.
- 9.Guranowski A, Brown P, Ashton PA, Blackburn GM. Biochemistry. 1994;33:235–240. doi: 10.1021/bi00167a031. [DOI] [PubMed] [Google Scholar]
- 10 (a).Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue CM, Druck T, Croce CM, Huebner K. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]; (b) Druck T, Hadaczek P, Fu TB, Ohta M, Siprashvili Z, Baffa R, Negrini M, Kastury K, Veronese ML, Rosen D, Rothstein J, McCue P, Cotticelli MG, Inoue H, Croce CM, Huebner K. Cancer Res. 1997;57:504–512. [PubMed] [Google Scholar]
- 11.Barnes LD, Garrison PN, Siprashvili Z, Guranowski A, Robinson AK, Ingram SW, Croce CM, Ohta M, Huebner K. Biochemistry. 1996;35:11529–11535. doi: 10.1021/bi961415t. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Garrison PN, Barnes LD. Biochem. J. 1995;312:925–932. doi: 10.1042/bj3120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackburn GM, Guo M-J, Langston SP, Taylor GE. Tetrahedron Lett. 1990;31:5637–5640. [Google Scholar]
- 14.Blackburn GM, Guo M-J. Tetrahedron Lett. 1990;31:4371–4374. [Google Scholar]
- 15.Blackburn GM, Ashton PR, Guo M-J, Guranowski A, Rogers M, Taylor GE, Watts D. Heteroatom Chem. 1991;2:163–170. [Google Scholar]
- 16.Maksel D, Guranowski A, Ilgoutz SC, Blackburn GM, Gayler KR. Biochem.J. 1997 doi: 10.1042/bj3290313., submitted for publication.
- 17.Blackburn GM, Taylor GE, Thatcher GRJ, Prescott M, McLennan AG. Nucleic Acids Res. 1987;15:1691–1704. doi: 10.1093/nar/15.17.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavis C, Haikal AF, Imbach J-L. Nucleosides Nucleotides. 19854:299. [Google Scholar]
- 19.Fukuoka K, Suda F, Ishikawa M, Hata T. Nucleosides Nucleotides. 1995;14:693–694. [Google Scholar]; Fukuoka K, Suda F, Suzuki R, Ishikawa M, Hata T. Chem. Lett. 1994;3:499–502. [Google Scholar]
- 20.Davisson VJ, Davis DR, Dixit VM, Poulter CD. J. Org. Chem. 1987;52:1794–1801. [Google Scholar]
- 21.Taylor GE. Ph.D.Thesis, Sheffield University. 1988. [Google Scholar]
- 22(a).Brenner C, Pace HC, Garrison PN, Robinson AK, Rösler A, Liu X, Blackburn GM, Croce CM, Huebner K, Barnes LD. Purification and Crystallization of Complexes Modeling the Active State of the Fragile Histidine Triad Protein. Protein Engineering. 1997;10:1461–1463. doi: 10.1093/protein/10.12.1461.Pace HC, Garrison PN, Robinson AK, Barnes LD, Draganescu A, Rösler A, Blackburn GM, Siprashvili Z, Croce CM, Huebner K, Brenner C.Genetic, Biochemical and Crystallographic Characterization of Fhit-Substrate Complexes as the Active Signaling Form of Fhit Proc. Natl. Acad. Sci., USA 1998. in press.