Abstract
Studies of the F344 rat have shown a variety of age-related auditory anatomy and physiology changes. The current study was undertaken to clarify the ARHL in the F344 rat, by examining the auditory pathway of the F344/NHsd substrain that is distributed by Harlan Laboratories for research in the United States. The F344/NHsd rat begins to lose its hearing at about 12 months, and by 24 months, there are 50–60 dB auditory brainstem response threshold shifts at 20 and 40 kHz and 20 dB losses at 5–10 kHz. Distortion product otoacoustic emissions (DPOAE) amplitudes at 1.8 to 12 kHz stimuli were depressed in the older (18–24 months) rats. Amplitude input-output functions of the compound action potential (CAP) were also depressed across frequency. The endocochlear potential (EP) was 90–100 mV in the 3 month old rats. All but one of the 24 month old rats’ EPs were in the +75–85 mV range. Tympanometry revealed no differences in middle ear function between the young and older rats. Collectively, these findings suggest damage to the outer hair cells, but anatomical examination of the outer hair cells revealed a relative lack of cell loss compared to the magnitude of the hearing and DPOAE loss.
Keywords: Presbycusis, Fischer 344 rat, endocochlear potential, compound action potential
1. Introduction
Age-related hearing loss (ARHL) is an ever-growing problem for the aging U.S. population. By age 65, estimates say, as much as 40% of the population has hearing impairment (Cruickshanks et al., 1998; Gates et al., 1990). The percentage continues to grow with increased age, peaking at 60–80% of people over the age of 85 (Gates et al. 1990; Desai et al., 2001). One of the challenges for researchers and clinicians is in detecting pure ARHL, since it is often combined with accumulated noise damage and other accumulated otologic challenges in patients whose lives have spanned five to eight decades.
The use of animal models to study ARHL is inherently complicated by the fact that it is an attempt to model a phenomenon that takes decades in humans with animal models whose life spans are under five years and whose loss of hearing occurs over a matter of months. Mouse models of ARHL have been used frequently in aging research, and appear to most closely model “sensory” ARHL, for which the underlying pathology is hair cell loss (Schuknecht, 1964). The CBA/Ca mouse retains most of its hearing sensitivity up to 18 months of age, after which hearing declines progressively beginning in the high frequencies and moving to the low frequencies (Li and Borg, 1991). Underlying the loss of hearing sensitivity late in its lifespan, the CBA/Ca mouse undergoes progressive loss of OHC and IHC (Li and Hultcrantz, 1994; Spongr et al., 1997). The C57/BL6 mouse has been used as a model for accelerated ARHL. The C57/BL6 mouse demonstrates a much more rapid onset of hearing loss and more rapid decline than the CBA/Ca mouse (Li and Borg, 1991). Hair cell degeneration also happens more rapidly, with potentially complete loss of hair cells by one year of age (Li and Hultcrantz, 1994; Spongr et al., 1997). In addition to their use as a model for sensory ARHL, mouse strains have also shown evidence of spiral ganglion cell degeneration (Saitoh et al., 1994; Dazert et al., 1996), although it is unknown if the pathology is indicative of neural ARHL, or if it is secondary to the loss of hair cells.
The Mongolian gerbil develops an ARHL that is consistent with a pattern of “metabolic” ARHL, for which the hallmark pathology is degeneration of the stria vascularis (Schuknecht, 1964). By 36 months of age, the Mongolian gerbil shows a 15–35 dB threshold shift, with greater damage in the high frequencies (Mills et al., 1990). The hearing loss is associated with damage to the stria vascularis (Thomopoulos et al., 1997; Gratton et al., 1997; Spicer and Schulte, 2005). The damage to stria vascularis is associated with a reduction in mitochondrial ATP production in the strial marginal cells (Spicer and Schulte, 2005), leading to a loss of Na, K-ATPase activity and a decrease in the EP (Schulte and Schmiedt, 1992; Schmiedt, 1996; Gratton et al., 1997). The loss of EPs in aged Mongolian gerbils is thought to be the causative factor in the Mongolian gerbils’ loss of hearing sensitivity with age.
The F344 is an inbred, albino rat strain with a median life span of 28–31 months (Rao and Boorman, 1990). Its limited inter-animal variability makes it a useful candidate for ARHL study. The F344 rat develops a progressive hearing loss that begins in the high frequencies and includes lower frequencies as the animal ages. The underlying pathology has been linked to a number of possible pathologies: combination of lost hair cells and spiral ganglion cells (Keithley et al., 1992). Recent studies on the F344/DuCrl substrain found that its ARHL is related to changes in middle ear impedance (Popelar et al., 2006), and strial degeneration leading to possible loss of EP and/or K+ cycling (Buckiova et al., 2006; Buckiova et al., 2007), as well as OHC loss.
While the F344 rat’s ARHL has been studied by a number of laboratories, the purpose of the current experiments was to comprehensively characterize the hearing loss in the F344/NHsd rat using a series of physiologic measures to assess the function of the cochlea, auditory nerve, and auditory brainstem in F344 rats of various ages. The F344/NHsd is distributed by Harlan Sprague Dawley, Inc. for research in the United States (Harlan Sprague Dawley, 2008). The F344/DuCrl substrain is bred and distributed by Charles River Laboratories Germany for research in Europe (Charles River Laboratories, 2007). The two substrains have ~60 years of genetic divergence, so the possibility exists that the middle ear (Popelar et al., 2006) and strial (Buckiova et al., 2006; Buckiova et al., 2007) pathologies that were identified as part of the F344/DuCrl rat’s ARHL may not be present in F344/NHsd rats exhibiting ARHL. The goal of the current study was to gain insight into the nature of ARHL modeled by the F344 rat by comprehensively examining the F344/NHsd substrain’s ARHL.
2. Materials and Methods
A total of fifty-nine F344/NHsd inbred male albino rats were used in the studies. They were obtained from Harlan Laboratories at various ages from 3 months to 24 months. The animals were housed in a quiet colony (<45 dBA). Animals that developed lesioned tumors or showed signs of liver failure (jaundiced ears or eyes) were rejected from the study. All procedures involving use and care of the animals were reviewed and approved by the State University of New York at Buffalo Institutional Animal Care and Use Committee.
2.1 Auditory Brainstem Response (ABR) testing
In order to assess the rate of hearing loss with age as well as the shape of the audiogram, groups of F344/NHsd rats at ages 3, 9, 12, 18 and 24 months were tested for hearing sensitivity using free-field ABR thresholds. The animals were anesthetized with inhalant isoflurane (4% for induction, 1.5% for maintenance, 1 L/min O2 flow rate). Needle recording electrodes were placed at the vertex (non-inverting), below the left pinna (inverting) and behind the shoulder blade (ground). During ABR recording, the rats were placed on a homeothermic blanket to maintain body temperature. Test stimuli consisted of alternating phase tone bursts at frequencies of 5, 10, 20, and 40 kHz, as well as a click. Signals were generated using Tucker Davis Technologies (TDT, Gainesville, FL) SigGen software. Each tone burst (1 msec duration) was gated through a Blackmann window, and had a 0.5 msec rise/fall time with no plateau. The click had a 25 µsec duration, and was presented with alternating polarity. Stimuli were presented at a rate of 21/sec. Signals were routed to a Leaf tweeter (model AS-TH400A) positioned at zero degrees azimuth, 17 cm from the vertex of each rat’s head. Acoustic stimuli were calibrated prior to each testing session, by recording the output of the speaker with a microphone placed at the animals’ head level. The rats’ evoked responses were amplified with a gain of 50,000, using a TDT Headstage-4 bioamplifier, and bandpass filtered from 100–3000 Hz. 250 sweeps were averaged at each stimulus level using TDT BioSig software. The level of the signal was decreased in 5 dB steps from 90 dB pSPL to a level 15 dB below that of the lowest level that evoked a detectable and repeatable response. Threshold was recorded as the lowest level at which a detectable response was elicited and could be repeated.
2.2 Compound Action Potential (CAP) Recording
CAPs were recorded from 2–40 kHz stimuli in a subset of 3-month-old (3 mo) and 24-month-old (24 mo) rats. Rats were deeply anesthetized during the CAP recording surgery with xylazine (6 mg/kg, i.m.) and ketamine (50 mg/kg, i.m.). The right cochlear round window was surgically exposed using a ventro-lateral approach and a Teflon-coat silver wire electrode (with a small, uninsulated ring at its tip) was carefully placed on the round window for recording cochlear potentials. A silver chloride reference electrode was placed in the neck muscles. Tone bursts (2, 6, 8, 12, 16, 20, 24, 30, 40 kHz) were generated in a real time processor (TDT RP2.1). The signals with 10-ms duration and 1-ms rise/fall time were attenuated by a TDT PA5 programmable attenuator and then amplified by an amplifier and delivered to a high frequency earphone (made from an ACO 1/2″. microphone, 7013, ACO Pacific, Belmont, CA) placed within a speculum that opened to the ear drum. Sound levels at all test frequencies were calibrated with a probe microphone located near the eardrum. The cochlear potentials were amplified with a preamplifier (WPI, Sarasota, FL). The gain of the preamplifier was set at 1000, and the band of the filter was from 0.1 Hz to 10 kHz. The cochlear responses were averaged 50 times using the TDT RP2.1 real time processor and stored in a computer. The CAP component of the cochlear response was obtained by low-pass filtering the cochlear potential at 3 kHz. Amplitudes of the N1 component of the CAP were measured and plotted as a function of the stimulation level, as input/output (I/O) functions. CAP threshold was defined as the stimulus level required to elicit a CAP amplitude of 5 µV.
2.3 Distortion Product Otoacoustic Emissions (DPOAE) Testing
In order to test the integrity of the OHC, cubic DPOAE were tested in the frequency range of 1.8 to 12 kHz in a subset of animals. With the animals anesthetized with inhalant Isoflurane (in the same procedure as described in section 2.1), 6 mm rubber probes were placed in the animals’ ear canals. DPOAEs were obtained using a Bio-logic Scout system (version 2.14). Stimuli were presented at 65/55 dB, with an f2/f1 ratio of 1.2. Eleven f2 frequencies were tested between 1.8 and 12 kHz. Noise floor measurements and two sets of responses were collected for each ear, and the 2f1-f2 DPOAE amplitudes of the two runs were averaged at each frequency.
2.4 Tympanometry
Because the F344/DuCrl rat showed age-related development of negative middle ear pressure that could account for some of their age-related hearing loss (Popelar et al., 2006), middle ear integrity was examined in the F344/NHsd rats in the current study. To assess the integrity of the middle ear, tympanometry was performed on a subset of rats. While the animals were anesthetized for DPOAE testing, tympanograms were obtained using a Grason-Stadler 38 tympanometer. Size 6 mm rubber probes were held in place by hand during the tympanogram acquisition. Peak pressure, peak compliance, and ear canal volume were measured. Peak pressure provides a measure of the pressure in the middle ear space. Middle ear infections often manifest with negative middle ear pressures, as seen in Popelar et al. (2006). Compliance provides an index of the tympanic membranes mobility. Very high, low, or absent mobility indicates a dysfunctional middle due to a variety of possible pathologies. Ear canal volume provides an indication of blockage of the external auditory meatus or occasions in which the probe was improperly inserted. A minimum 0.3 cm3 ear canal volume was required to obtain a curve on the tympanometer. Any flat tympanogram that registered 0.2 cm3 or below was rejected and the measurements were retaken until a sufficient ear canal volume was obtained.
2.5 Endocochlear potential recording
While still under anesthesia from the CAP recording described above, each of the F344/NHsd rats whose CAP was recorded underwent EP recording. A small hole was made in the lateral wall of the basal turn of each animal’s right cochlea, which had been surgically exposed using a ventro-lateral approach from the CAP recording. A glass micropipette electrode (filled with 157 mM KCl solution) was lowered into the scala media using a micromanipulator with a hydraulic drive. The voltage from the electrode was amplified 10X by a pre-amplifier (WPI) and recorded on a voltmeter and oscilloscope. The EP was recorded every 30 seconds over a 10-minute period to assure stability of the recording. Each subject then received an injection of KCN (2.5 mg/kg i.p.) and EP was recorded every thirty seconds until a stable EP was determined (up to 1.5 hours). The purpose of the KCN injection was to evaluate the effect of transient hypoxia-like blockade of ATP (Schoeplfe, 1963) production in the stria vascularis of the 3 mo versus the 24 mo rat. Combined with the standard EP recording, the KCN injections gave a perspective on the function of the stria vascularis under normal conditions and a stressed condition in both the young and aged rats. The procedure was adapted from a similar procedure used in the Long-Evans rat (Tawackoli et al., 2001). At the conclusion of the recording period, the electrode was withdrawn from the cochlea and the electrical potential assessed with the electrode just touching the outside of the cochlea to insure that there had been no drift from a 0-mV baseline setting established at the beginning of the experiment.
2.6 Quantification of dying and missing OHCs
In order to assess OHC damage in the young and aged F344/NHsd rats’ cochleae, the numbers of apoptotic, necrotic and missing OHCs in aging cochleae were counted in a subset of rats aged either 3 months (n=6) or 20–27 months (n=19). Following removal of the cochleae after decapitation, the organs of Corti were then dissected out from the cochleae, and each was double stained with propidium iodide and FITC-phalloidin. The propidium iodide stained the nuclei of the hair cells and the FITC-phalloidin permitted examination of the cuticular plate. The stained specimens were mounted on slides and examined under fluorescence microscopy. Based on the condition of the OHC nuclei, cells were labeled as viable, apoptotic, necrotic, or missing. Apoptotic OHCs showed condensed nuclei, which were smaller in size with increased PI fluorescence as compared with that of the neighboring nuclei of viable cells. Necrotic OHCs showed nuclear swelling, which included enlarged sizes, malformed shapes and loss of normal texture of nuclear staining. Missing OHCs were identified in the areas of the cuticular plates that lost F-actin labeling or loss of PI-stained nuclei. Apoptotic, necrotic, and missing cells were added together and counted as “deteriorated” in the assembly of the OHC cochleogram, as they collectively represented the group of OHC that were no longer viable.
2.7 Statistical Analysis
A two-factor ANOVA (Age × Frequency) was used to analyze differences between the mean ABR thresholds of the five age groups (3, 9, 12, 18, and 24 months) across the five different test stimuli (click, 5, 10, 20, and 40 kHz tone bursts). Age and Frequency were analyzed as between-subjects variables. If a significant main effect occurred for Age or Frequency, post hoc testing with Tukey A tests was performed to delineate the nature of the differences. Differences in CAP amplitudes were analyzed with a three-factor ANOVA (Age × Frequency × Stimulus level). Stimulus level was treated as a repeated measure. Differences in cubic DPOAE amplitudes were analyzed with a two-factor ANOVA (Age × Frequency). Significant main effects were tested post hoc with Tukey A tests. Differences between age groups on the two tympanometric measures were compared with one-factor ANOVAs and post hoc Tukey A testing as needed. EP differences were compared with an independent samples t-test. Correlations between CAP thresholds and EP values were analyzed with Pearson product moment correlations. Deteriorated OHC were compared between the young rats and the aged rats using a two-factor ANOVA.
3. Results
3.1 ABR thresholds
As a first measure of the progression of F344/NHsd rat ARHL, free field ABR thresholds were recorded for 5–40 kHz tone pip stimuli and a click stimulus in each of five age groups (3 mo, n=13; 9 mo, n=5; 12 mo, n=7; 18 mo, n=7; 24 mo, n=13). Results are displayed in Panel A of Figure 1. By 9 months, no significant threshold shift occurred. A 10–20 dB mean threshold shift across frequencies occurred between 9 and 12 months. At 18 and 24 months, the high frequencies (30–50 dB mean threshold shift re: 3 mo) were more strongly affected than the low frequencies and the click (10–25 dB shift re: 3 mo). A two-factor ANOVA (Age × Frequency) analyzed differences in threshold revealed a significant two-way interaction. To assess differences between age groups, a series of one-factor ANOVAs was run at each test frequency. Post-hoc Tukey A tests revealed that both the 18 and 24 mo rats had significantly higher thresholds than the 3 mo group at all frequencies tested (p<0.001). Thresholds from the 18 and 24 mo rats were also higher than those from the 9 mo rats at all (p<0.01) but 5 kHz (p>0.05). Thresholds from the 24 mo rats were higher than those from the 12 mo rats at 20 kHz (p<0.001), 40 kHz (p<0.001), and in response to the click (p<0.05),; and higher than those from the 18 mo rats at 20 kHz (p<0.05). Thresholds from the 18 mo rats were higher than those from 12 mo rats at 20 and 40 kHz (p<0.05). Thresholds from the 12 mo rats were higher than those from 3 mo rats at all frequencies but 20 kHz (p<0.05), and higher than those from the 9 mo rats at only 40 kHz (p<0.01).
Figure 1.
Mean ABR thresholds in response to 5, 10, 20, and 40 kHz tone burst stimuli, as well as a click stimulus for 3, 9, 12, 18, and 24 mo F344/NHsd rats. Error bars are +/− 1 standard deviation.
In order to assess the shape of the ABR threshold shift at 24 months, threshold shifts at each frequency were compared with one another. The shifts at 20 and 40 kHz were greater than the click, 5 kHz, and 10 kHz shifts (p<0.0001), but were not different from one another (p=.97). The shifts in response to the click, 5 kHz tone burst, and 10 kHz tone burst were not different from one another (p≥.86). The results demonstrated a sharply sloping high-frequency pattern of ARHL.
3.2 CAP amplitude changes
CAP I/O functions for 3 mo (n=7) and 24 mo (n=9) rats at 2 kHz (Panel A), 8 kHz (Panel B), 12 kHz (Panel C), 24 kHz (Panel D), 30 kHz (Panel E), and 40 kHz (Panel F) are plotted in Figure 2. Additionally, 6, 16, a 20 kHz were tested and included in the statistical analyses, but are not displayed. Three-factor ANOVA revealed a three-way interaction of Age, Frequency, and Stimulus level (p<0.001). Two-factor ANOVAs at each Frequency revealed significant interactions of Age and Stimulus level (p<0.001) at all frequencies, except 2 kHz, at which a trend toward the two-way interaction was revealed (p=0.066). Independent samples t-test comparison of Age at each Stimulus level revealed that 24 month CAP amplitudes were significantly lower than the 3 month amplitudes (p<0.05) at all but the lowest stimulus levels (0 and 10 dB) at each frequency, 6–40 kHz.
Figure 2.
CAP amplitude input-output functions for 2 kHz (A), 8 kHz (B), 12 kHz (C), 24 kHz (D), 30 kHz (E), and 40 kHz (F) for 3 and 24 mo F344/NHsd rats. Error bars are +/− 1 standard deviation.
3.3 DPOAE amplitude changes
Mean cubic DPOAE response amplitudes to the 65/55 dB SPL stimuli are plotted in Figure 3 for each of the four age groups tested (3 mo, n=6; 11 mo, n=7; 18 mo, n=5; and 24 mo, n=6). Two-factor ANOVA revealed a significant interaction of Age and Frequency (p<0.0001). A series of one-factor ANOVAs with Tukey A post hoc testing revealed that, with the exception of only f2 frequency 2343 Hz (p=0.001), mean amplitude was no different between 3 months and 11 months of age (p≥0.059). With the exception of the lowest f2 frequency (1968 Hz), amplitudes at all frequencies were significantly higher at 3 months than at 18 months or 24 months (p<0.05). Additionally, amplitudes at 11 months were higher across frequencies than the 18-month rats (p<0.05) (excepting at 1968, 2343, and 3983 Hz) and the 24-month rats (p<0.05) (excepting 1968 and 2343 Hz). Amplitudes did not differ between the 18 mo old rats and the 24 mo rats at any frequency (p≥0.058)
Figure 3.
DPOAE amplitudes at 1.8 to 12 kHz in response to 65/55 dB SPL stimuli for F344NHsd rats aged 3, 11, 18, and 24 months. Error bars are +/− 1 standard deviation.
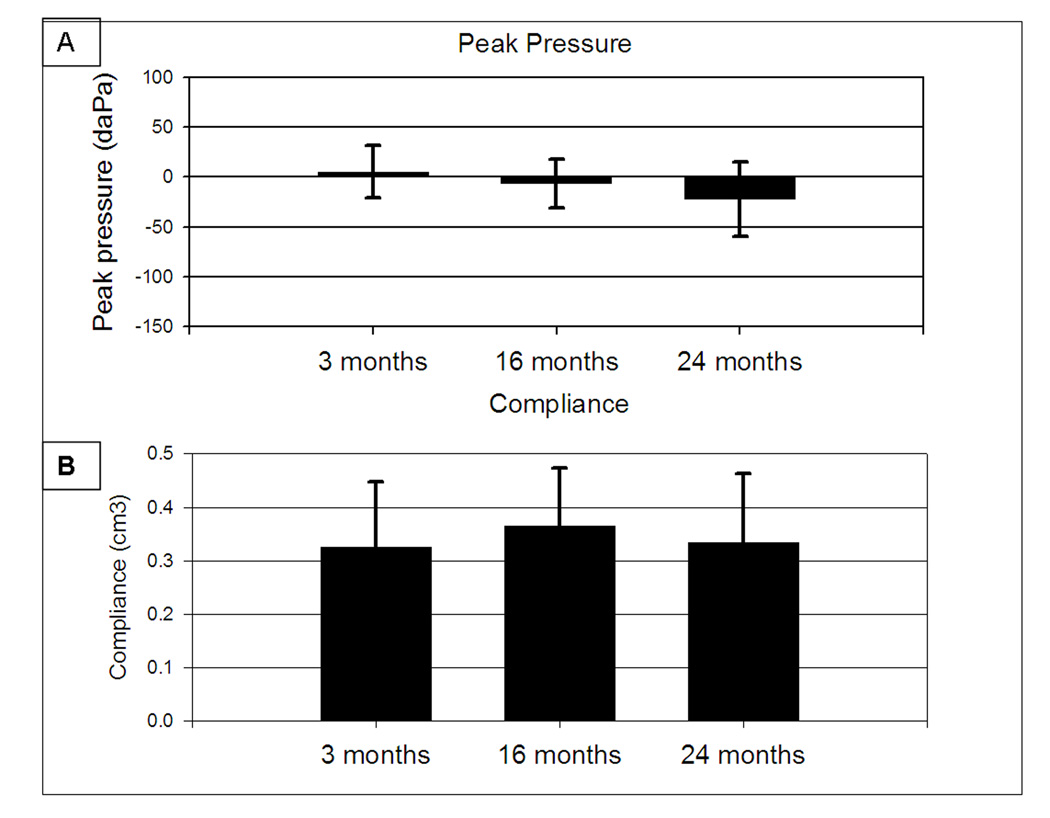
3.4 Tympanometry
Tympanometry was measured in a subset of aged F344/NHsd rats aged, 3 mo (n=12 ears), 16 mo (n=14 ears), and 24 mo (n=12 ears). Figure 4 displays tympanometry findings using two measures: Peak pressure in decaPascals (daPa) (Panel A) and Compliance in cm3 (Panel B). Single-factor ANOVAs revealed no statistically significant differences between the three age groups tested on either of the measurements, indicating normal middle ear function in the aged rats. A trend toward lower peak pressures was observed in the 24 mo rats (p=0.87), owing to larger negative pressures (−60 and −110 daPa) in two ears.
Figure 4.
Tympanometry for F344/NHsd rats aged 3, 16, and 24 months (A) Peak pressures with error bars +/−1 standard deviation. (B) Compliance with error bars +1 standard deviation.
3.5 EP
Figure 5 displays mean EP recordings for 3 mo rats (n=7) and 24 mo rats (n=9). Independent samples t-test comparison revealed no significant difference between old an young rats’ EPs, but a trend toward decreased EP in the older animals’ EPs relative to the younger rats (p=0.062). Additionally, as shown in Figure 6, the older animals’ EPs were much more sensitive to the KCN injections. All but one of the young animals showed a fairly small drop in EP that recovered quickly back to baseline, a finding consistent with a similar procedure performed in Long-Evans rats (Tawackoli et al., 2001) and guinea pigs using NaCN (Konishi and Kelsey, 1968). The EP in each of the six old rats injected with KCN declined over a period of 20–60 minutes, with varying degrees of severity of decline (Fig 6B). None recovered back to baseline within the 1.5 hour observation time following the KCN injections.
Figure 5.
Mean endocochlear potentials in mV for 3 and 24 mo F344/NHsd rats. Error bars are + 1 standard deviation.
Figure 6.
Individual rats’ EP values over time following KCN (2.5 mg/kg) injection. (A) 3 mo F344/NHsd rats (n=6). (B) 24 mo F344NHsd rats (n=8).
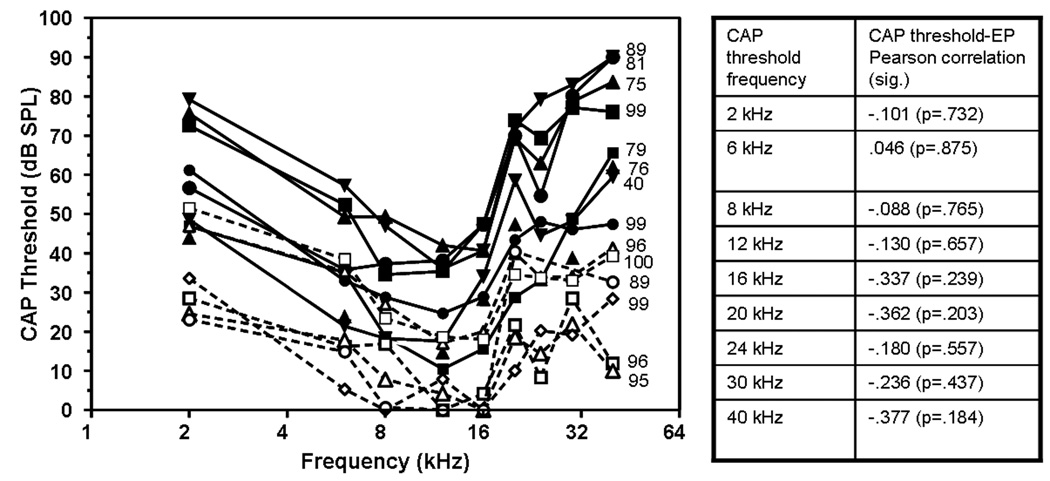
Figure 7 displays CAP threshold of each tested animal across frequency. Each animal’s data set is followed by notation of the ear’s EP. The young rats’ EPs ranged from 89.2 to 100.3 mV. The old rats were much more variable. Three rats had EPs in the same range as the young (88.9, 98.7, and 98.7 mV). Three were moderately depressed (75.1, 76.0, 79.1, 81.1 mV) and one was depressed severely (39.9 mV). Pearson’s Product Moment correlations were run to correlate EP changes to CAP threshold shift at each CAP frequency. As can be seen in Figure 7 with statistics in table alongside, the level of the EP did not correlate with the CAP thresholds (p≥ 0.184), suggesting that the rats’ CAP thresholds shifts are likely the result of an underlying pathology other than EP reductions.
Figure 7.
Individual F344/NHsd rats’ CAP thresholds. Dark symbols are 24 mo rats. Open symbols are 3 mo rats. Along the right side of each animal’s 40 kHz threshold is the animal’s EP value. No correlation between EP and CAP threshold was detected for young or old rats at any frequency. The table alongside shows the Pearson product moment correlations of EP and CAP thresholds at 9 CAP frequencies with significance values in parenthesis.
3.6 OHC damage
Figure 8 displays the patterns of OHC deterioration in the 3 mo and 20–27 mo F344/NHsd rats. OHC decayed significantly (p<0.01) in the aged rats in the 20% of the cochlea nearest to the apex and the 20% nearest to the base. The middle 60% showed less than 20% OHC deterioration and was not significantly different from the young rats. Most (>95%) of the cells included under the umbrella term “deteriorated” were indeed missing. In any given animal, ~10–25 apoptotic cells were identified, whereas ~0–5 necrotic cells were identified.
Figure 8.
Mean OHC cochleograms for F344/NHsd rats aged 3 and 20–27 months. Dying and missing OHC were grouped together under the label “deteriorated OHC” as presented on the y-axis.
Discussion
4.1 Age-related hearing loss
The F344/NHsd rats tested showed high-frequency loss at 12 months of age that progressed from a mild (mean < 20 dB threshold shift) at 20–40 kHz to a more severe (30–40 dB threshold shift) at 18 months and then more severe at 24 months (40–50 dB threshold shift). Between ages 12 and 18 months, the lower frequencies (5 and 10 kHz) also increased from ~10 dB to ~20 dB mean threshold shift. From age 3 months to 24 months, free-field ABR and pressure-field CAP findings showed consistent threshold shifts at age 24 months, with a considerable difference between the two measures only appearing at 20 kHz (mean ABR shift of 46.38 dB and mean CAP shift of 30.40 dB). The pattern of threshold shift: a progressive, sloping, high-frequency loss, is consistent with previous studies of F344/DuCrl rat ARHL (Popelar et al., 2006), and is comparable to ARHL in the screened human population (ISO1999, Annex A)
4.2 Endocochlear potential changes
A novel finding in the study of F344 rats was the relatively modest decrease in the EP from 3 to 24 months of age, a span in which the rats had 20–60 dB threshold shift of the CAP. Only one animal showed a greatly reduced EP (39.9 mV), and that animal did not appear as an outlier for CAP threshold shift. Three of the other 24 mo rats showed slightly depressed EP, but not enough to explain the substantial hearing loss they demonstrated. Like the current study, reports on the Mongolian gerbil (Schulte and Schmiedt, 1992; Schmiedt, 1996; Gratton et al., 1997) and the BALB/cJ mouse (Ohlemiller, 2006) have shown depressed EP in a portion of their aged populations. The wide range of measured EPs across aged animal populations suggests variability in the presence or expression of the genetic allele(s) for age-related strial degeneration, a variability that may exist in the human population as well. The poor correlations between EP changes and CAP thresholds imply that, although there may be a mild strial component to some F344/NHsd rats’ ARHLs, it is not the major driving force in F344/NHsd rat ARHL, unlike the Mongolian gerbil.
The KCN injections induced transient depressions of the EP in the young rats, all but one of which recovered to pre-injection levels within 15 minutes of the injection. This finding is consistent with similar procedures in the Long-Evans rat (Tawackoli et al., 2001) and the guinea pig (Konishi and Kelsey, 1968). In the aged rats, the KCN-induced EP depressions were more severe and did not recover during the 1.5 hour observation window. The implication is that, although the stria vascularis in most of the aged rats was able to produce an EP that was only slightly reduced under normal conditions, the stria in the aged rats was more sensitive to hypoxic conditions induced by the cyanide exposure. The resulting loss of ATP in the strial cells may have led to permanent cell damage and/or dysfunction of the Na+-K+-ATPase pump, with the result of a long-term reduction of the EP. Since the EP was only monitored for 90 minutes post KCN injection, the EPs may have recovered over a longer time period, possibly implying a longer time for the stria tissue to clear the KCN in the aged rats, compared to the young rats. Anatomical evaluations of the stria vascularis of young and aged F344/NHsd rats, both before and after exposure to KCN, are warranted in future studies.
4.3 Possible underlying pathologies
The pattern of the threshold shift is consistent with that which is reported as the standard audiogram for sensory presbycusis (Schuknecht and Gacek, 1993). Depressed DPOAE amplitudes at 12 kHz and below suggest OHC dysfunction or loss (Kemp, 1990) in the apical half of the basilar membrane (Müller et al., 1991). DPOAE from the basal half of the cochlea were not assessed. Loss of DPOAE amplitude occurred most precipitously between measures at 11 and 18 months (Fig. 3). ABR thresholds at 5 and 10 kHz shifted between 12 and 18 months, suggesting a temporal link between loss of DPOAE and threshold shift of ABR.
Patterns of CAP I/O amplitude changes also suggest damage or dysfunction primarily from the OHC, but with a secondary contribution, possibly from IHC or afferent auditory nerve dysfunction/damage. In the young, normal-functioning cochlea, CAP I/O functions are non-linear, reflecting contributions from OHC amplification. The 24 mo rats show CAP I/O functions that are consistent with loss of OHC or OHC function in rats (Chen and Liu, 2005; Chen, 2006). Additionally, overall CAP amplitudes are reduced, regardless of input stimulus level, suggesting that there is some contribution from damage/dysfunction in the IHC or afferent auditory nerve fibers.
Tympanometry findings suggest middle ear dysfunction is not pervasive across the F344 aged rat substrains, but the statistical trend toward lower negative pressures in the older animals suggests the possibility of some middle ear influence on ARHL in individual rats, and that middle ear screenings of older F344/NHsd rats may be warranted before analyzing hearing loss data obtained from those animals. Like humans, conductive middle ear pathology can not be ruled out for individual F344 rats’ ARHL, but conductive pathology does not appear to be an effect that is consistent across populations of F344 rats, and is not common pathology in the F344/NHsd rat.
Severe depression of the EP occurred in only one of the eight older animals in which the EP was recorded, and that animal’s CAP thresholds were not outside the range of thresholds for the other 24 mo rats that had much higher EPs. Similar to the middle ear findings, EP depression does not appear to be a consistent effect across the population of F344/NHsd rats, but it may occur in individual cases. Although there is no evidence that depressed EP contributes substantially to F344/NHsd rats’ ARHL, it can not be ruled out as a contributor for some individual F344/NHsd rats. F344 rats are albino, which results in altered sizes of the intermediate and marginal cell layers of the stria vascularis, relative to pigmented animals (Conlee et al., 1994). This altered pattern of strial cell layers may have an influence on the pattern of age-related strial degeneration in individual F344 rats or across populations of F344 rats. The severe effects of the KCN injections in the older rats did imply fragility of the stria in all of the older rats, even if the pre-KCN damage was not severe enough to reduce the EP significantly.
The EP and tympanometry findings suggest that the depressed CAP responses are not the result of lost voltage in the scala media or reduced input from the middle ear to the cochlea. Thus, the most likely anatomic contributors to the reduced CAP responses are the OHC, with contributions from IHC and/or spiral ganglion cells as well. Assessment of missing or dying OHC revealed significant age-related losses at the apex and the base, with the middle of the cochlea showing limited OHC loss in the aged rats. The findings are consistent with the pattern of deterioration previously found in F344 rats (Popelar et al., 2006) but the extent of OHC loss in the base and the apex was more severe in the current study than in previous studies. Of particular interest is the incompatibility of the extent of OHC loss with the ABR threshold shift and DPOAE losses in the regions corresponding to 6–24 kHz. OHC losses were less than 20% in those regions, yet mean ABRs shifted 20–50 dB in the 5–20 kHz frequency range (Fig. 1), and DPOAEs disappeared almost completely in the range of 6–12 kHz (Fig. 3). This finding combined with the finding of intact middle ear function and largely intact EP suggest that the OHC, while present in the cochlea, are not functioning properly, resulting in lost DPOAE and shifted hearing thresholds.
4.4 Similarities and differences in the F344/Nhsd and F344/DuCrl rat substrains
The F344 was first developed at Columbia University in 1920 after purchase from the local breeder, Fischer (Rao and Boorman, 1990). Charles River Laboratories purchased and began breeding F344 rats in 1960. The DuCrl substrain began when a population of the Charles River F344 rats was transferred to Germany for breeding in 1987 (Charles River Laboratories, 2007). The NHsd substrain is derived from an F344 population from the National Institutes of Health in Bethesda, MD colony that was derived from a population from Columbia in 1950 (Harlan Sprague Dawley, 2008). Thus, the F344/NHsd substrain and F344/DuCrl substrain have ~60 years of genetic drift to account for differences in ARHL that they may exhibit. Recent publications on the F344/DuCrl rat have implicated middle ear pathology (Popelar et al., 2006) and strial pathology (Buckiova et al., 2006; Buckiova et al., 2007) as contributors to the F344 rat’s ARHL, along with OHC loss. OHC loss in the F344/NHsd follows a pattern similar to the F344/DuCrl rat, but the severity of OHC loss at the base and apex is greater in the F344/NHsd. The current study does not find middle ear pathology in the F344/NHsd rat, but does show evidence of strial degeneration, in the form of reduced EP in some of the rats studied. The extent to which the strial deterioration shown in the F344/DuCrl rat (Buckiova et al., 2006; Buckiova et al., 2007) affects the EP in those rats is unknown, since the epithelium of the stria vascularis carries excess functional capacity at young age. Thus, it is unknown how much strial damage is required to affect the EP in F344 rats. Histopathological investigations of age-related strial changes in aging F344/NHsd rats are warranted in future studies, as are EP measurements in F344/DuCrl rats in order to correlate EP changes to strial pathology.
4.5 Conclusions
The F344/NHsd rat appears to be an intriguing and useful model for ARHL. The pattern of threshold shift is qualitatively similar to that seen in a screened population of aging humans (ISO1999, Annex A). There is evidence for multiple forms of underlying pathology, although the OHC appear to be the source of much of the hearing loss, with possible secondary contributions from damage to IHC and spiral ganglion cells (Keithley et al., 1992). Because of apparent inconsistencies between the auditory physiology changes t remains unclear from the current study if there is a unifying consistent pathology in the auditory periphery of the F344/NHsd rat that can be observed post mortem and can explain the pattern of ARHL observed physiologically. Further study of the underlying pathology of F344 rat ARHL may help to discriminate particular forms of ARHL in individual subjects, as well as providing insight into possible routes of intervention to prevent or treat ARHL.
Acknowledgements
The authors thank Dr. Robert Burkard his guidance and feedback on the planning and execution of the studies, as well as Ashley Gambino for her assistance with data collection. Research was supported by the NIH Grant: #1R01DC00686201A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buckiova D, Popelar J, Syka J. Collagen changes in the cochlea of aged Fischer 344 rats. Exp Gerontol. 2006;41(3):296–302. doi: 10.1016/j.exger.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Popelar J, Syka J. Aging cochleas in the F344 rat: morphological and functional changes. Exp Gerontol. 2007;42(7):629–638. doi: 10.1016/j.exger.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Charles River Laboratories. Charles River Laboratories Research Models and Services. 2007 Published online at www.criver.com, 14.
- Chen GD. Prestin gene expression in the rat cochlea following intense noise exposure. Hear Res. 2006;222(1–2):54–61. doi: 10.1016/j.heares.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Chen GD, Liu Y. Mechanisms of noise-induced hearing loss potentiation by hypoxia. Hear Res. 2005;200(1–2):1–9. doi: 10.1016/j.heares.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Gerity LC, Westenberg IS, Creel DJ. Pigment-dependent differences in the stria vascularis of albino and pigmented guinea pigs and rats. Hear Res. 1994;72(1–2):108–124. doi: 10.1016/0378-5955(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148(9):879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Dazert S, Feldman ML, Keithley EM. Cochlear spiral ganglion cell degeneration in wild-caught mice as a function of age. Hear Res. 1996;100(1–2):101–106. doi: 10.1016/0378-5955(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Desai M, Pratt LA, Lentzner H, Robinson KN. Trends in vision and hearing among older Americans. Aging Trends. 2001;(2):1–8. doi: 10.1037/e620682007-001. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cooper JC, Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11(4):247–256. [PubMed] [Google Scholar]
- Gratton MA, Smyth BJ, Lam CF, Boettcher FA, Schmiedt RA. Decline in the endocochlear potential corresponds to decreased Na,K-ATPase activity in the lateral wall of quiet-aged gerbils. Hear Res. 1997;108(1–2):9–16. doi: 10.1016/s0378-5955(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Harlan Sprague Dawley, Inc. F344 Fischer 344. 2008 Published online at www.harlaneurope.com.
- ISO1999. International Organization for Standardization; Acoustics–Determination of occupational noise exposure and estimation of noise-induced hearing impairment. 1990
- Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hear Res. 1992;59(2):171–178. doi: 10.1016/0378-5955(92)90113-2. [DOI] [PubMed] [Google Scholar]
- Kemp DT, Ryan S, Bray P. A guide to the effective use of otoacoustic emissions. Ear Hear. 1990;11(2):93–105. doi: 10.1097/00003446-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Konishi T, Kelsey E. Effect of cyanide on cochlear potentials. Acta Otolaryngol. 1968;65(4):381–390. doi: 10.3109/00016486809120979. [DOI] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111(5):827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Li HS, Hultcrantz M. Age-related degeneration of the organ of Corti in two genotypes of mice. ORL J Otorhinolaryngol Relat Spec. 1994;56(2):61–67. doi: 10.1159/000276611. [DOI] [PubMed] [Google Scholar]
- Mills JH, Schmiedt RA, Kulish LF. Age-related changes in auditory potentials of Mongolian gerbil. Hear Res. 1990;46(3):201–210. doi: 10.1016/0378-5955(90)90002-7. [DOI] [PubMed] [Google Scholar]
- Muller M. Frequency representation in the rat cochlea. Hear Res. 1991;51(2):247–254. doi: 10.1016/0378-5955(91)90041-7. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091(1):89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Popelar J, Groh D, Pelanova J, Canlon B, Syka J. Age-related changes in cochlear and brainstem auditory functions in Fischer 344 rats. Neurobiol Aging. 2006;27(3):490–500. doi: 10.1016/j.neurobiolaging.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Rao, Boorman GA. History of the Fischer 344 rat. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, editors. Pathology of the Fischer rat: Reference and Atlas. San Diego, CA: Academic Press, Inc.; 1990. pp. 5–8. [Google Scholar]
- Saitoh Y, Hosokawa M, Shimada A, Watanabe Y, Yasuda N, Takeda T, et al. Age-related hearing impairment in senescence-accelerated mouse (SAM) Hear Res. 1994;75(1–2):27–37. doi: 10.1016/0378-5955(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear Res. 1996;102(1–2):125–132. doi: 10.1016/s0378-5955(96)00154-2. [DOI] [PubMed] [Google Scholar]
- Schoepfle GM. Effects of cyanide at different concentrations on single nerve fibers. Am J Physiol. 1963;204:77–80. doi: 10.1152/ajplegacy.1963.204.1.77. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Further Observations on the Pathology of Presbycusis. Arch Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102(1 Pt 2):1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na,K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res. 1992;61(1–2):35–46. doi: 10.1016/0378-5955(92)90034-k. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Pathologic changes of presbycusis begin in secondary processes and spread to primary processes of strial marginal cells. Hear Res. 2005;205(1–2):225–240. doi: 10.1016/j.heares.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am. 1997;101(6):3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Tawackoli W, Chen GD, Fechter LD. Disruption of cochlear potentials by chemical asphyxiants. Cyanide and carbon monoxide. Neurotoxicol Teratol. 2001;23(2):157–165. doi: 10.1016/s0892-0362(01)00135-0. [DOI] [PubMed] [Google Scholar]
- Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA. Age-related thickening of basement membrane in stria vascularis capillaries. Hear Res. 1997;111(1–2):31–41. doi: 10.1016/s0378-5955(97)00080-4. [DOI] [PubMed] [Google Scholar]