Abstract
Hint is a universally conserved, dimeric AMP-lysine hydrolase encoded on the avian Z chromosome. Tandemly repeated on the female-specific W chromosome, Asw is a potentially sex-determining, dominant negative Hint dimerization partner whose substrate-interacting residues were specifically altered in evolution. To test the hypothesis that Gln127 of Asw is responsible for depression and/or alteration of Hint enzyme activity, a corresponding mutant was created in the chicken Hint homodimer and a novel substrate was developed that links reversal of lysine modification to aminomethylcoumarin release. Strikingly, the Hint-W123Q substitution reduced kcat/Km for AMP-lysine hydrolysis 17-fold, while it increased specificity for AMP-paranitroaniline hydrolysis by 160-fold. The resulting 2700-fold switch in enzyme specificity suggests that Gln127 could be the dominant component of Asw dominant negativity in avian feminization.
Keywords: AMP-lysine hydrolase, W chromosome, dominant negative, site-directed mutagenesis
Hint is a homodimer of ∼14 kDa subunits that functions as an AMP-lysine hydrolase and positive regulator of Kin28 in yeast, whose most conserved amino acids form the dimer interface and the substrate binding-site (2, 3, 8). The Hint active site consists of mostly nonpolar residues that contribute to adenosine binding (3), the histidine that forms a phosphoramide with the substrate α-phosphate (11), Ser107, which interacts with the leaving group amine (8), and Trp123, which interacts with the alkyl portion of the lysine leaving group across the dimer interface (8).
Though it is known that male birds are homogametic with a ZZ karyotype and females are heterogametic with a ZW karyotype, the molecular basis for sexual differentiation is unknown, though the existence of a ZZW female warbler strongly suggests that genetic information on the W chromosome is responsible for feminization (1). In birds other than ostriches and emus, the female-specific W chromosome carries ∼40 tandem repeats of an unusual Hint-related gene, ASW, whereas the Z chromosome carries a typical HINT gene (7, 12). In a striking departure from all previously isolated Hint homologous sequences, which conserve the AMP-lysine binding site more than other residues, the female-specific Asw protein has strong similarity to Hint except that 15 of 16 substrate-interacting residues are sexually dimorphic, i.e., altered in the W-encoded Asw with respect to the Z-encoded Hint (13). Thus, because the predicted dimerization interface (helix α2 and beta strand β4) is virtually unaltered in Asw (13), and a single His to Ala substitution can reduce the catalytic activity of Hint by over 100,000-fold (2), evolutionary pressures may have ablated the AMP-lysine binding site in Asw but allowed Asw to function as a Hint heterodimerization partner. In this regard, it is important to note that of the 16 substrate-interacting residues in the Hint dimer, only one interacts with the AMP-lysine substrate across the dimer interface (8). That residue, Trp123, in the C-terminal Trp-Pro-Pro-Gly motif of Hint, is conspicuously substituted by Gln in the repeated, female-specific Asw sequence (13).
As a candidate female sex-determining gene, we reasoned that dominance and negativity might be functionally separable components of Asw’s activities. Negativity would seem to be a function of loss of AMP-binding residues in the Asw sequence but, because the Hint dimer is not cooperative with respect to substrate hydrolysis (2), an inert dimerization partner would fail to depress Hint enzymatic activity by more than 50%. In fact, because there is no HINT gene on the W chromosome, HINT gene dosage is already reduced 50% by HINT gene absence. We therefore considered an explanation necessary for why a potential Hint dimerization partner lacking an active site is repeated 40 times on the W chromosome. By constructing a model of the putative Hint-Asw heterodimer based on crystal structures of rabbit Hint bound to products (3) and substrate analogs (8), we noted that Hint Trp123 is expected to be located on the Asw side of the dimer interface (13). More importantly, the model predicted that the residue in Asw that corresponds to Hint Trp123, namely Gln127, is physically located in the Hint half of the putative heterodimer. Thus, we proposed that Gln127 in place of Trp123 is responsible for dominant depression and/or dominant alteration of activity of the Hint active site (13). In this study, with site-directed mutagenesis of chicken Hint and synthesis of a novel fluorescent Hint substrate, we establish that the W123Q allele of Hint creates a 2,700-fold alteration of substrate specificity, supporting a mechanistic role for Asw’s C-terminal Gln in feminization of developing birds.
EXPERIMENTS AND RESULTS
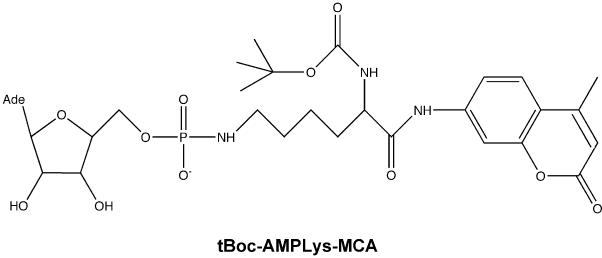
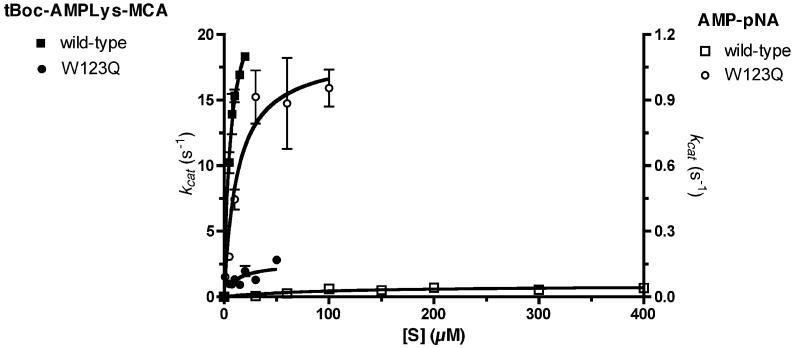
To study the AMP-lysine hydrolase activity of wild-type chicken Hint and the Hint-W123Q mutant, we modified a fluorigenic trypsin substrate (tert-butoxycarbonyl-(L)-lysine methylcoumarinamide, tBoc-Lys-MCA) by adenylylation of the ε amino group of lysine (tBoc-LysAMP-MCA, Fig. 1). Because this modification renders the lysinamide moeity resistant to trypsin, Hint incubations to liberate tBoc-Lys-MCA are coupled to tryptic digestion to quantitate Hint activity with aminomethylcoumarin (AMC) release from tBoc-Lys (see Supplementary Methods and Figures). As shown in Fig. 2 and Table 1, wild-type chicken Hint hydrolyzed tBoc-LysAMP-MCA with a kcat of 24 s-1 and a Km of 6.1 μM (specificity constant = 3,950,000 M-1 s-1). Consistent with a dominant negative role for the C-terminal Gln of Asw, the Hint-W123Q substitution depressed kcat nine-fold (to 2.6 s-1) and increased Km two-fold (to 11 μM) for an overall 17-fold decline in kcat/Km (229,000 M-1 s-1). Because an Asw-Hint heterodimer would have only a single Hint active site per dimer, the predicted depression in AMP-lysine hydrolytic activity is > 30-fold.
Fig. 1.
Structure and synthesis of tBoc-LysAMP-MCA. Because the ε adenylyl modification of Lys blocks trypsin cleavage, Hint enzymatic activity is limiting for production of tBoc-Lys-MCA, a trypsin substrate (see Supplementary Data). tBoc-LysAMP-MCA was made as a modification of the adenosine 5′phosphoramidate synthesis method of Fu (4). Under an argon atmosphere at room temperature, 0.25 mmol of tBoc-Lys-MCA (Bachem) and 0.12 mmol ADP (Sigma) were dissolved in 2 ml pyridine plus 0.6 ml trimethylsilyl chloride, added dropwise, and the resulting mixture was stirred for 2 days. The mixture was evaporated and 1 ml 2 M aqueous ammonia was added to hydrolyze the residue. Product was extracted with four 5 ml volumes of diethyl ether, evaporated to dryness, and dissolved in 1.5 ml isopropanol:2 M aqueous ammonia:methanol (7:1:2). tBoc-LysAMP-MCA (yield = 20.6%) was purified twice by silica gel column chromatography using a 10 × 1 cm column. Peak material was analyzed by MALDI-MS and NMR. MALDI was performed on an Applied Biosystems Voyager System 6235 in negative ion mode using 3-hydroxypicolinic acid (observed mass = 733.79; calculated mass = 734.26). 31P, 13C and 1H NMR data were collected using a 15 mm probe with the compound in DMSO. 1NMR (500mHz, DMSO-d6, 50°C) δ = 10.93 (s, 1H), 10.85 (s, 1H), 8.47 (s, 1H), 8.12 (s, 1H), 7.81 (d, J=2.0 Hz, 1H), 7.71 (d, J=8.5 Hz, 1H), 7.53 (d, J=8.8 Hz, 1H), 7.35-7.44 (m, 2H), 7.27 (d, J=7.6 Hz, 1H), 7.10-7.16 (m, 1H), 6.25 (d, J=1.2 Hz, 1H), 5.89 (d, J=5.4 Hz, 1H), 5.57-5.62 (m, 1H), 4.55-4.59 (m, 1H), 4.18-4.21 (m, 1H), 4.07-4.21 (m, 1H), 4.02-4.05 (m, 1H), 3.83-3.88 (m, 1H), 3.74-3.80 (m, 1H), 2.72-2.77 (m, 1H), 2.66-2.70 (m, 1H), 2.36 (d, J= 4.2 Hz, 3H), 1.64-1.70 (m, 1H), 1.57-1.61 (m, 1H), 1.33 (d, J=13.9 Hz, 9H), 1.23-1.25 (m, 1H), 1.00 (d, J= 6.1 Hz, 3H); 13C NMR (hmqc, DMSO-d6, 50°C, partial) δ = 153.3, 126.5, 116.0, 112.7, 106.2, 87.5, 84.4, 74.5, 71.3, 64.3, 41.5, 40.3, 31.6, 28.8, 28.6, 28.1, 26.0, 18.6; 31P NMR (300mHz, DMSO-d6) δ = 2.66.
Fig. 2.
2,700-fold alteration of substrate specificity by W123Q alteration. The chicken Hint cDNA was amplified from EST clone pat.pk0069.a10 from the University of Delaware using primers 7038 (5′ GATCGTCCATATGGCTGACGAGATCCGCAAGG) and 7039 (5′ CACTCTCGAGTTAGCCAGGAGGCCAGCCCAACTG) and cloned into pSGA02 as an NdeI-XhoI fragment. The W123Q substitution was introduced (9) with mutagenic primer 7046 (5′ GGTCGTCAGTTGGGCCAGCCTCCTGGCTAA). Enzymes were purified as described (2, 8). tBoc-LysAMP-MCA assays, at 5 - 50 μM, described and validated in detail in Supplementary Materials, were performed with 1.8 fmol of wild-type or 19 fmol Hint-W123Q at pH 5.5 with 1 mM EDTA. Reactions (10-20 min) were stopped by adjusting the pH to 9.5, addition of trypsin to a final concentration of 60 μg/μl to liberate AMC, and quantitated by fluorescence. AMP-pNA assays with 3.6 pmol wild-type or 0.36 pmol Hint-W123Q were as described (8). Filled symbols are on the left Y-axis. Open symbols are on the right Y-axis.
Table 1.
Kinetic constants for wild-type and W123Q chicken Hint
| tBoc-AMPLys-MCA | AMP-pNA | |||||
|---|---|---|---|---|---|---|
| kcat (s-1) | Km (μM) | kcat/ Km (M-1s-1) | kcat (s-1) | Km (μM) | kcat/ Km (M-1s-1) | |
| Wild-type | 24.1 +/- 1.56 | 6.14 +/- 1.11 | 3,950,000 | 0.051 +/- 0.011 | 93.0 +/- 57.6 | 549 |
| W123Q | 2.57 +/- 0.473 | 11.2 +/- 5.11 | 229,000 | 1.12 +/- 0.168 | 12.7 +/- 7.27 | 88,200 |
Conversion of Hint dimers to Asw-Hint heterodimers could either depress AMP-lysine hydrolytic activity, promote another activity, or both. To test whether substitution of Trp123, which makes a hydrophobic interaction with the Lys leaving group of AMP-lysine in the substrate across the dimer interface (8), would promote hydrolysis of a bulkier adenylylated phosphoramide substrate, we tested wild-type and W123Q forms of chicken Hint on AMP-paranitroaniline (AMP-pNA) (8). This molecule, which is followed spectroscopically, is a poor substrate for wild-type rabbit Hint (8). In the chicken system, the wild-type kcat was depressed 470-fold (to 0.051 s-1) and Km was elevated 15-fold (to 93 μM) for an overall specificity constant of 550 M-1 s-1 with AMP-pNA. Remarkably, Hint-W123Q is 160-fold superior AMP-pNA hydrolase compared to wild-type Hint (kcat = 1.1 s-1; Km = 13 μM; kcat/K = 88,000 M-1 s-1m). The 17-fold depressed AMP-lysine hydrolase activity coupled with 160-fold superior AMP-pNA hydrolase activity represents a 2,700-fold alteration of specificity, which is remarkable for a single amino acid substitution.
DISCUSSION
Though sex-specific body plans are fundamentally similar in all vertebrate phyla, birds and mammals make use of unique chromosomal sex determination systems while crocodiles, many turtles and some lizards lack genetically encoded switches and make use of environmental sex determination (5). Thus, it has been argued that genetic switches have evolved independently in birds and mammals that take the place of temperature-dependent switches in organisms without sex chromosomes. Because Asw is repeated 40-times on the female specific W chromosome and is highly expressed at the urogenital ridge precisely at the time of feminization (7, 12), we have considered Asw to be a strong candidate gene for female sex determination (13). The ability of Asw residue 127 to repress and alter Hint enzymatic activity is a plausible mechanism for function of Asw in this process—either of these activities might be sufficient for Asw function. Because functions of Hint that depend on protein-protein interactions have been proposed (10), one could hypothesize that Asw functions by altering putative Hint complexes or promoting a female-specific complex. However, as a Hint active site mutant is a functional null (2), it was interesting to determine whether an Asw residue could repress Hint enzymatic activity in trans.
This study is also instructive in dissection of how nature constructs a dominant negative allele. As described by Ira Herskowitz, a dominant negative allele ought to have two characteristics: simple loss of function and dominant interference (6). In the case of Asw, loss of function can be attributed to mutation of the active-site residues (13), which is sufficient to produce an inactive allele in vitro and in vivo (2). Because overexpression of an inert molecule would be pointless, however, we searched for the source of dominant interference in Asw. Transplantation of the dimer-crossing Trp to Gln substitution of Asw into the Hint sequence both depressed and altered specificity, consistent with a dominant-negative mechanism for Asw function in avian feminization.
ACKNOWLEDGMENTS
We thank Dr. Joan Burnside of the University of Delaware for the kind gift of EST clone pat.pk0069.a10. This work is dedicated to the memory of Ira Herskowitz. Work was supported by National Cancer Institute grant CA75954 to C.B.
REFERENCES
- 1.Arlt D, Bensch S, Hansson B, Hasselquist D, Westerdahl H. Observation of a ZZW female in a natural population: implications for avian sex determination. Proc Royal Soc London Ser B. 2004;271:S249–S251. doi: 10.1098/rsbl.2003.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieganowski P, Garrison PN, Hodawadekar SC, Faye G, Barnes LD, Brenner C. Adenosine Monophosphoramidase Activity of Hint and Hnt1 Supports Function of Kin28, Ccl1 and Tfb3. J Biol Chem. 2002;277:10852–10860. doi: 10.1074/jbc.M111480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner C, Garrison P, Gilmour J, Peisach D, Ringe D, Petsko GA, Lowenstein JM. Crystal structures of Hint demonstrate that histidine triad proteins are GalT-related nucleotide-binding proteins. Nature Struct Biol. 1997;4:231–238. doi: 10.1038/nsb0397-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu H, Han B, Zhao Y-F. Reaction of ADP with amino acid methyl esters mediated by trimethylsilyl chloride. Chemistry Communications. 2003;33:134–135. doi: 10.1039/b207992e. [DOI] [PubMed] [Google Scholar]
- 5.Graves JAM, Shetty S. Sex from W to Z: evolution of vertebrate sex chromosomes and sex determining genes. J Exp Zool. 2001;290:449–462. doi: 10.1002/jez.1088. [DOI] [PubMed] [Google Scholar]
- 6.Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- 7.Hori T, Asakawa S, Itoh Y, Shimizu N, Mizuno S. Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination. Mol Biol Cell. 2000;11:3645–3660. doi: 10.1091/mbc.11.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakowiak A, Pace HC, Blackburn GM, Adams M, Mekhalfia A, Kaczmarek R, Baraniak J, Stec WJ, Brenner C. Biochemical, crystallographic, and mutagenic characterization of Hint, the AMP-lysine hydrolase, with novel substrates and inhibitors. J Biol Chem. 2004;279:18711–18716. doi: 10.1074/jbc.M314271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee YN, Nechushtan H, Figov N, Razin E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity. 2004;20:145–151. doi: 10.1016/s1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]
- 11.Lima CD, Klein MG, Hendrickson WA. Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science. 1997;278:286–290. doi: 10.1126/science.278.5336.286. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill M, Binder M, Smith C, Andrews J, Reed K, Smith M, Miller C, Lambert D, Sinclair A. ASW: a gene with conserved avian W-linkage and female-specific expression in chick embryonic gonad. Dev Genes Evol. 2000;210:243–249. doi: 10.1007/s004270050310. [DOI] [PubMed] [Google Scholar]
- 13.Pace HC, Brenner C. Feminizing Chicks: A Model for Avian Sex Determination Based on Titration of Hint Enzyme Activity and the Predicted Structure of an Asw-Hint Heterodimer. Genome Biol. 2003;4:R18. doi: 10.1186/gb-2003-4-3-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]