Abstract
The estrogen-related receptor alpha (ERRα) is a potential target for activation in the treatment of metabolic disease. To date, no small molecule agonists of ERRα have been identified despite several high-throughput screening campaigns. We describe the synthesis and profiling of a small array of compounds designed based on a previously reported agonist-bound crystal structure of closely-related receptor ERRγ. The results suggest that ERRα may be intractable as a direct target for pharmacologic activation.
The estrogen-related receptor alpha (ERRα, NR3B1) is an orphan member of the nuclear receptor superfamily that is broadly expressed in adult tissues.1;2 Genetic and functional analyses have implicated the receptor as a critical regulator of oxidative metabolism in muscle, a pathway that contributes to the pathology of type 2 diabetes.3 As such, small molecule ERRα agonists have the potential to increase expression of mitochondrial OXPHOS genes that would ameliorate metabolic diseases of poor energy balance. Despite its evolutionary relationship to the classical estrogen receptor alpha (ERα), the orphan ERRα does not bind to any of the known steroidal hormones.4 Instead, the activity of ERRα has been shown to be regulated by coactivator proteins, such as the peroxisome proliferator-activated receptor gamma coactivators (PGC-1α and PGC-1β) and the nuclear receptor corepressor-interacting protein 140 (RIP-140).5–8 X-ray crystallography has shown that the ligand binding domain (LBD) of ERRα contains a small hydrophobic pocket, suggesting that the receptor may be able to accommodate a synthetic small molecule ligand.9 Although several ERRα inverse agonists have been identified,10;11 the tractability of the receptor for small molecule activation remains an open question. Having failed to identify ERRα agonists through random screening of the GSK compound collection, we utilized structure-guided design to address the issue of the chemical tractability of this orphan receptor as a molecular target for metabolic diseases.
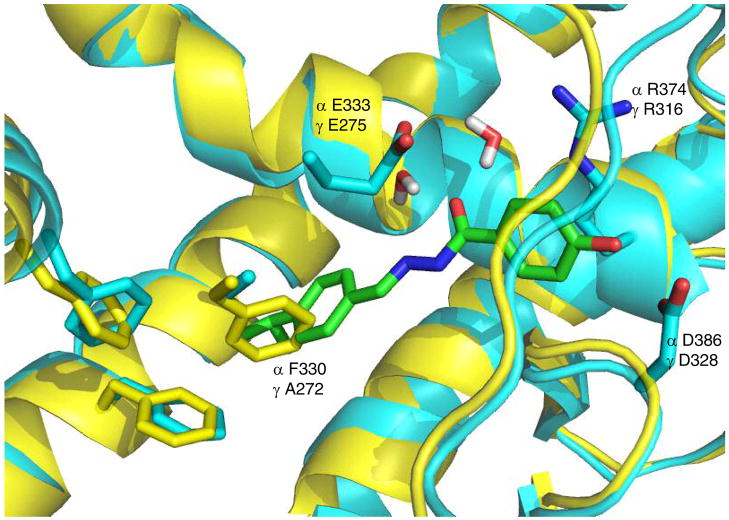
We have previously identified and characterized a phenolic acyl hydrazone, 1 (GSK4716) as a small molecule agonist of the closely related ERRβ (NR3B2) and ERRγ (NR3B3).12 X-ray crystallography of 1 bound to the ERRγ LBD revealed a rearrangement of a loop between helices 1 and 3, which allowed the agonist ligand to access a lipophilic pocket that was not available in the unliganded receptor. (Fig. 1).13 Due to this rearrangement, the phenol of 1 did not interact with E275 and R316 that are analogous to the classical phenol-binding residues in ERα. Instead, the phenolic hydroxyl formed a hydrogen bond with D328 near the surface of the receptor. E275 was rotated into a conformation to make contact with E247, while R316 interacted with the acyl hydrazone carbonyl of 1 through a bridging water molecule. The agonist profile of 1 was ascribed to global stabilization of the receptor rather than direct interaction with the C-terminal activation helix, AF-2.
Figure 1.
Overlay of the X-ray crystal structures of the 1-ERRγ complex and apo-ERRα. 1 is shown in stick representation colored by atom type with carbon in green, nitrogen in blue and oxygen in red. The protein backbone is shown in ribbon view with ERRγ in cyan and ERRα in yellow. The helix 3 backbones have been removed from the view. The sidechains of key residues in the ligand binding pocket are shown as sticks with carbon atoms colored to match their respective proteins. Two water molecules that contact GW4716 are also shown. A272 in ERRγ is replaced by F330 in ERRα, which occludes the binding pocket.
Although 1 and close analogs showed no measurable binding affinity to ERRα, an analysis of the apo-crystal structure suggested that a parallel ligand-induced conformational change might be possible, since all of the critical polar residues present in ERRγ are conserved (Fig. 1). However, the comparison also showed that F272 in ERRα occludes the region of the pocket where the 4-isopropylphenyl group of 1 sits in ERRγ. The alanine to phenylalanine switch explained the selectivity profile of 1 across the ERR subtypes, but also suggested that new analogs could be designed to fit the smaller ERRα pocket. Specifically, molecular docking studies predicted that secondary phenolic acyl hydrazones and amides with small N-alkyl groups would be accommodated within the volume of the ERRα LBD.
Two small arrays of compounds prepared as potential ERRα agonists (Table 1). Phenolic acyl hydrazones (2) were formed in a single step by condensation of 4-hydroxybenzoic hydrazide and alkyl aldehydes followed by reagent scavenging with PS-NHNH2 and PS-CHO. Secondary phenolic amides (3) were synthesized from 4-acetoxybenzoic acid through formation of the acid chloride with SOCl2 followed by exposure to an amine and subsequent cleavage of the aryl acetate. The resulting compounds were screened in biochemical and cell-based reporter assays for activation of ERRα. Using a fluorescence resonance energy transfer (FRET)-based assay to measure the interaction of the ERRα LBD with a peptide fragment of the cofactor RIP-140, none of the compounds showed activity up to a concentration of 30 μM, and select compounds were assayed up to 100 μM concentrations with no observable effect on RIP-140 interaction. In addition to being inactive in the ERRγ FRET assay, the compounds also failed to show activity in the ERRγ FRET assay, consistent with the previously observed structure-activity. A heterologous reporter assay was also developed in which HeLa cells were transfected with an expression vector for the human ERRα and a reporter construct engineered from a triple repeat of an estrogen response element (ERE) fused to luciferase. Coexpression of ERRα and the reporter led to an increase in normalized luciferase signal. The luciferase activity could be fully ablated by exposure to the ERRα inverse agonist 414 or further increased by coexpression of PCG-1α, but the activity was unaffected by any of the phenolic hydrazones (2a – 2h) or amides (3a – 3k) at concentrations up to 50 μM.
Table 1.
a. RCHO, MeOH then PS-NHNH2, PS-CHO; b. i. SOCl2, Δ, ii. RNH2, Et3N, CH2Cl2
 | |
|---|---|
| Compound | R |
| 2a | ethyl |
| 2b | isopropyl |
| 2c | 2-butyl |
| 2d | 3-buten-1-yl |
| 2e | 3-methyl-2-pentyl |
| 2f | 3-buten-3-yl |
| 2g | n-pentyl |
| 3a | H |
| 3b | methyl |
| 3c | ethyl |
| 3d | 1-propyl |
| 3e | 2-propyl |
| 3f | methallyl |
| 3g | 1-butyl |
| 3h | isobutyl |
| 3i | neopentyl |
| 3j | 1-pentyl |
| 3k | 3-methyl-1-butyl |
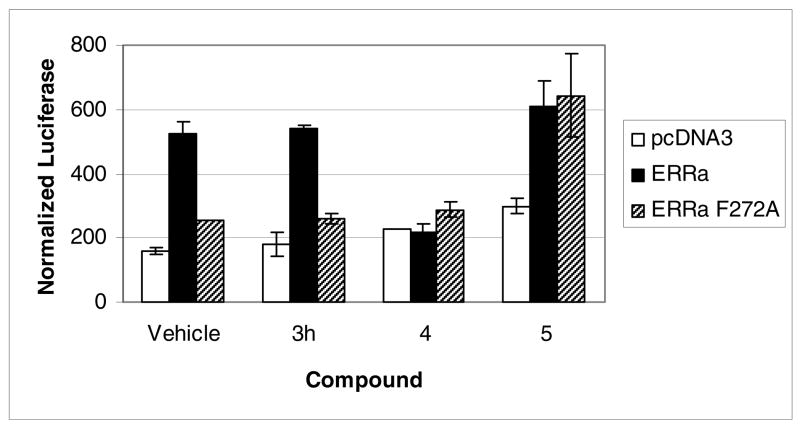
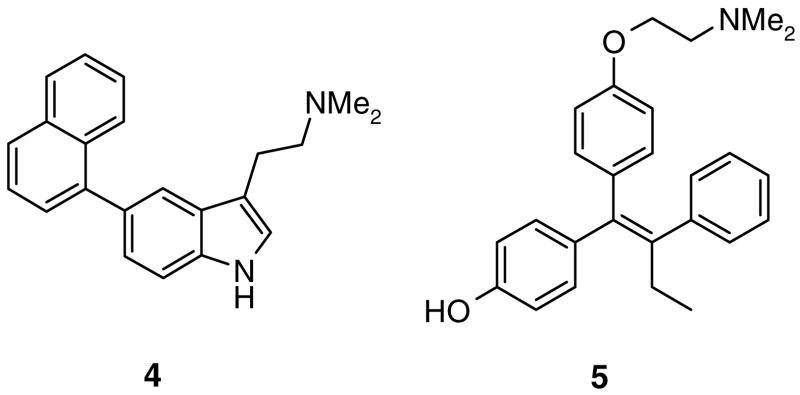
The lack of observed activity in biochemical and cellular assays called into question the hypothesis that ERRα could undergo a conformational rearrangement similar to that observed with in ERRγ. To examine the validity of the structure-based design, we prepared a point mutant of ERRα where F272 was converted to the alanine found in ERRγ. Consistent with previous reports, the level of constitutive activity in cells of the ERRα F272A mutant was greatly attenuated relative to wild type ERRα.15 Unexpectedly, there was no observed increase in luciferase activity upon exposure of 1 relative to vehicle treated cells. Moreover, none of the truncated analogs of 1 showed any effect on the activity of the point mutant receptor; representative data is shown for 3h (Fig. 2). In contrast to the inability of the phenolic acyl hydrazones and amides to activate the mutant receptor, we were surprised to find that 4-hydroxytamoxifen (4-OHT, 5) profiled as an agonist. It has been previously reported that 4-OHT could bind the point mutant receptor.12 Our observation that 4-OHT not only binds, but is also an agonist of the ERRα F272A mutant, demonstrates that the mutant receptor is not inert to activation by small molecule ligands. Although 4-OHT has been shown to function as an inverse agonist of ERRβ and ERRγ, the compound has no activity on wild type ERRα.16
Figure 2.
We have utilized a cocrystal structure of 1 bound to ERRγ to design potential ERRα agonists. However, the compounds neither increase nor decrease the activity of ERRα, raising doubt as to the ability of small molecules to induce a conformational change that would lead to activation of the receptor. The prospects for ERRα agonists are further diminished by the inability of 1 or its analogs to activate the ERRα F272A point mutant. Whether the poor tractability of ERRα is due to occlusion of a critical part of the ligand binding pocket by F232 or an inability to expand the apo-receptor to accommodate synthetic activating molecules remains unclear. However, the accumulated evidence from extensive high throughput screening combined with the failure of structure-guided design demonstrates that ERRα is a low tractability molecular target for metabolic diseases.
Experimental
General procedure for the preparation of phenolic acyl hydrazones 2. N′-[(1E)-hexylidene]-4-hydroxybenzohydrazide (2g)
To a solution of 4-hydroxybenzoic hydrazide (152 mg, 1.0 mmol) in absolute ethanol (2 mL) containing a catalytic amount of acetic acid, was added hexanal (100 mg, 1.0 mmol). The mixture was stirred at room temperature for 2 h and precipitation was observed. The mixture was then neutralized by pouring 10% aqueous sodium bicarbonate solution. The precipitate formed was filtered and dried, furnishing 2g (149 mg, 64%): 1H NMR (MeOD-d4) δ0.92 (t, J = 7.0 Hz, 3H), 1.34–1.39 (m, 4H), 1.53–1.60 (m 2H), 2.34 (q, J = 6.9 Hz, 2H), 6.84 (d, J = 8.9 Hz, 2H), 7.64 (t, J = 5.8 Hz, 1H), 7.75 (d, J = 8.9 Hz, 2H); 13C NMR (MeOD-d4) δ 13.12, 22.31, 26.21, 31.37, 32.22, 115.03, 123.54, 129.49, 153.36, 161.40, 165.37; MS (ESI): m/z 217 (M − H)−, 219 (M+H)+.
General procedure for the preparation of phenolic amides 3. 4-hydroxy-N-(2-methylpropyl)benzamide (3h)
To 4-acetoxybenzoic acid (0.5 mmol) was added SOCl2 (2 mmol). The reaction mixture was stirred at 80°C for 30 min. The volatiles were then removed under reduced pressure and isobutylamine (1 mmol) and Et3N (1 mmol) in CH2Cl2 (2 ml) were added. The reaction mixture was allowed to stir at room temperature overnight, after which time amide formation and acetate cleavage were checked for completion. Following removal of volatiles and dissolution in MeOH, the phenolic amide product 3h was purified by reverse phase HPLC. 1H NMR (DMSO-d6) δ 0.84 (d, J = 6.7 Hz, 6H), 1.79 (m, 1H), 3.01 (m, 2H), 6.76 (d, J = 8.8 Hz, 2H), 7.68 (d, J = 8.9 Hz), 8.17 (m, 1H), 9.89 (m, 1H); 13C NMR (DMSO- d6) δ 20.92, 28.84, 47.28, 115.34, 126.15, 129.70, 160.56, 166.56; MS (ESI): m/z 192 (M − H)−, 194 (M+H)+.
Supplementary Material
General experimental procedures, FRET and cell-based assay protocols, and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
Chart 1.
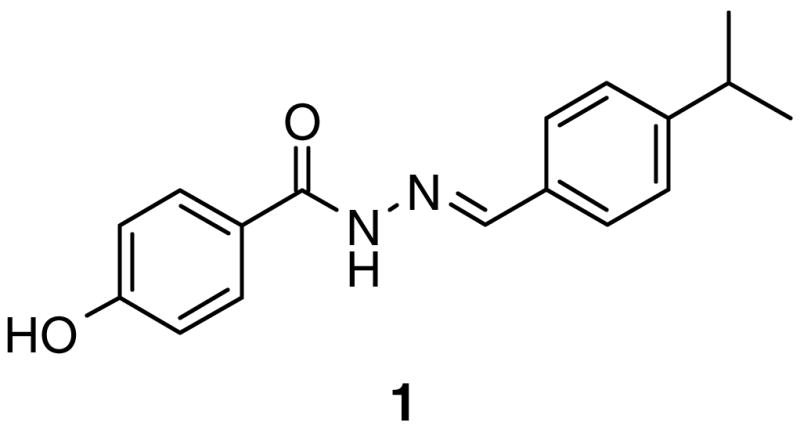
ERRγ agonist acyl hydrazone 1
Chart 2.
ERRα inverse agonist indole 4 and ERα antagonist/ERRγ inverse agonist 5 (4-OHT).
Acknowledgments
The authors thank Carrow Wells for post-synthesis compound processing and Matt Lochansky for HRMS data collection. R.A.S. and D.P.M. acknowledge the support of the NIH (DK074652).
Abbreviations
- ERR
estrogen receptor-related receptor
- PGC-1
peroxisome proliferator-activated receptor gamma coactivator
- RIP-140
nuclear receptor corepressor-interacting protein 140
- LBD
ligand-binding domain
- FRET
fluorescence resonance energy transfer
- ERE
estrogen response element
- 4-OHT
4-hydroxytamoxifen
Reference List
- 1.Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 2.Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- 3.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horard B, Vanacker JM. Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol. 2003;31:349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- 5.Hentschke M, Susens U, Borgmeyer U. PGC-1 and PERC, coactivators of the estrogen receptor-related receptor gamma. Biochem Biophys Res Commun. 2002;299:872–879. doi: 10.1016/s0006-291x(02)02753-5. [DOI] [PubMed] [Google Scholar]
- 6.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 7.Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci USA. 2003;100:12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debevec D, Christian M, Morganstein D, Seth A, Herzog B, Parker M, White R. Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor alpha. Mol Endocrinol. 2007;21:1581–1592. doi: 10.1210/me.2007-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor alpha (ERRalpha): crystal structure of ERRalpha ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1alpha. J Biol Chem. 2004;279:49330–49337. doi: 10.1074/jbc.M407999200. [DOI] [PubMed] [Google Scholar]
- 10.Busch BB, Stevens WC, Jr, Martin R, Ordentlich P, Zhou S, Sapp DW, Horlick RA, Mohan R. Identification of a selective inverse agonist for the orphan nuclear receptor estrogen-related receptor alpha. J Med Chem. 2004;47:5593–5596. doi: 10.1021/jm049334f. [DOI] [PubMed] [Google Scholar]
- 11.Kallen J, Lattmann R, Beerli R, Blechschmidt A, Blommers MJ, Geiser M, Ottl J, Schlaeppi JM, Strauss A, Fournier B. Crystal structure of human estrogen-related receptor alpha in complex with a synthetic inverse agonist reveals its novel molecular mechanism. J Biol Chem. 2007;282:23231–23239. doi: 10.1074/jbc.M703337200. [DOI] [PubMed] [Google Scholar]
- 12.Zuercher WJ, Gaillard S, Orband-Miller LA, Chao EY, Shearer BG, Jones DG, Miller AB, Collins JL, McDonnell DP, Willson TM. Identification and structure-activity relationship of phenolic acyl hydrazones as selective agonists for the estrogen-related orphan nuclear receptors ERRbeta and ERRgamma. J Med Chem. 2005;48:3107–3109. doi: 10.1021/jm050161j. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Zuercher WJ, Consler TG, Lambert MH, Miller AB, Orband-Miller LA, McKee DD, Willson TM, Nolte RT. X-ray crystal structures of the estrogen-related receptor-gamma ligand binding domain in three functional states reveal the molecular basis of small molecule regulation. J Biol Chem. 2006;281:37773–37781. doi: 10.1074/jbc.M608410200. [DOI] [PubMed] [Google Scholar]
- 14.Nolte RT, Wang L, Orband-Miller LA, Way JM, Martin JJ, Roa AM, Vela L, Miller AB, Willson TM, Zuercher WJ. Identification and X-ray crystal structure of an ERR-alpha inverse agonist reveals a new mechanism of nuclear receptor antagonism. Abstracts of Papers, 230th ACS National Meeting; Washington, DC, United States. Aug. 28-Sept. 1, 2005; MEDI-474. [Google Scholar]
- 15.Suetsugi M, Su L, Karlsberg K, Yuan YC, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1:981–991. [PubMed] [Google Scholar]
- 16.Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci USA. 2001;98:8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General experimental procedures, FRET and cell-based assay protocols, and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.