Abstract
Ovarian hormone elevations are associated with enhanced learning/memory. During behavioral estrus or pregnancy, progestins, such as progesterone (P4) and its metabolite 5α-pregnan-3α-ol-20-one (3α,5α-THP), are elevated due, in part, to corpora luteal and placental secretion. During ‘pseudopregnancy’, the induction of corpora luteal functioning results in a hormonal milieu analogous to pregnancy, which ceases after about 12 days, due to the lack of placental formation. Multiparity is also associated with enhanced learning/memory, perhaps due to prior steroid exposure during pregnancy. Given evidence that progestins and/or parity may influence cognition, we investigated how natural alterations in the progestin milieu influence cognitive performance. In Experiment 1, virgin rats (nulliparous) or rats with two prior pregnancies (multiparous) were assessed on the object placement and recognition tasks, when in high-estrogen/P4 (behavioral estrus) or low-estrogen/P4 (diestrus) phases of the estrous cycle. In Experiment 2, primiparous or multiparous rats were tested in the object placement and recognition tasks when not pregnant, pseudopregnant, or pregnant (between gestational days (GDs) 6 and 12). In Experiment 3, pregnant primiparous or multiparous rats were assessed daily in the object placement or recognition tasks. Females in natural states associated with higher endogenous progestins (behavioral estrus, pregnancy, multiparity) outperformed rats in low progestin states (diestrus, non-pregnancy, nulliparity) on the object placement and recognition tasks. In earlier pregnancy, multiparous, compared with primiparous, rats had a lower corticosterone, but higher estrogen levels, concomitant with better object placement performance. From GD 13 until post partum, primiparous rats had higher 3α,5α-THP levels and improved object placement performance compared with multiparous rats.
Introduction
In the natural environment, sexually receptive female rodents will traverse complicated and potentially dangerous terrain in order to access mating partners. Among rats, the cyclical enhancement of estrogen (E2) and progestins facilitate these reproductive behaviors in a number of ways. Actions of E2, progesterone (P4), and the P4 metabolite 5α-pregnan-3α-ol-20-one (3α,5α-THP) are important not only for copulation but also for an entire suite of behaviors relevant for successful reproduction, including cognition. When compared with non-receptive rats, naturally receptive females demonstrate increased exploratory and anti-anxiety behavior, as well as attenuated social neophobia and intersex aggression (Frye 2001, Frye et al. 2006). The systemic administration or central infusions of 3α,5α-THP to sexually non-receptive females can enhance the instatement of these behaviors (Frye & Rhodes 2006a, 2008). As well, performance in cognitive tasks can be better during behavioral estrus. Corticolimbic tasks, such as eye-blink-conditioning, passive avoidance, radial arm maze, and object recognition are enhanced among receptive, compared with non-receptive, female rodents (Shors et al. 1998, Bowman et al. 2001, Rhodes & Frye 2004, Walf et al. 2006, Frye et al. 2007, Wood et al. 2001). Such enhancements may be important, considering the spatial and mnemonic requirements associated with finding mating partners and successfully returning to safe locations to rear pups.
Among both people and rodents, aging is associated with a decline in ovarian hormones, reproductive viability, and cognition. Reproductively senescent rats have decreased ovarian hormone levels, poorer reference, and working memory on both spatial and non-spatial tasks, and take longer to learn associations in a conditioning paradigm compared with reproductively viable rats (Pitsikas & Algeri 1992, Leblanc et al. 1996, Walf & Frye 2006). Among postmenopausal women, there are reports of age-associated decline in working memory (Zelinski et al. 1993, Small et al. 1999, Sherwin & Henry 2008). Thus, a natural decline in ovarian hormones is associated with poorer cognitive performance and decrements in learning.
Models of hormonal decline, such as removal of the ovaries, the primary source of endogenous hormones, can produce behavioral and/or cognitive deficits among rodents akin to those observed when cyclical hormone levels are low. Ovariectomy (OVX) attenuates mating, increases anxiety, and decreases social behavior (Frye 2001, Frye & Rhodes 2006b, Frye et al. 2006). The administration of E2 alone or in conjunction with P4 or 3α,5α-THP reinstates these behaviors and can enhance sexual motivation in a mate-finding task (Frye 2001, Frye et al. 2006, Pazol et al. 2006, López et al. 2007). Further, E2 alone or with P4 can produce mnemonic effects in OVX rats (Gibbs 2000, Sandstrom & Williams 2001, Markham et al. 2002, Markowska & Savonenko 2002, Sato et al. 2004, Frye & Rhodes 2006a, Frye et al. 2006). In particular, oral or systemic E2 administration can reinstate object recognition performance in OVX females to levels commensurate with that of sexually receptive rats (Luine et al. 2003, Fernandez & Frick 2004, Walf et al. 2006). Thus, behavioral and cognitive deficits associated with hormonal depletion can be overcome by E2 and/or P4 administration.
In contrast to the reduction in ovarian hormones, pregnancy is associated with increases in steroids for longer periods than are seen over the estrous cycle. In particular, circulating and central levels of progestins, including 3α,5α-THP, are greatly elevated during pregnancy (Majewska et al. 1989, Concas et al. 1998, Frye & Walf 2004). This is due, in part, to the additional steroid secretion by the corpora lutea and placenta. These levels increase steadily throughout gestation and do not decline until parturition is imminent (Concas et al. 1998). The resulting endocrine milieu, as well as limbic morphology and function, are altered following pregnancy, parturition, and/or pup rearing (Featherstone et al. 2000, Tomizawa et al. 2003, Pawluski & Galea 2006). First, oxytocin associated with motherhood enhances long-term potentiation (LTP) of hippocampal tissue slices in culture and central infusions of oxytocin enhance long-term spatial memory in virgin mice (Tomizawa et al. 2003). Second, hippocampal spinal density is increased in pregnant compared with non-pregnant rats and is also altered by postpartum mothering (Kinsley et al. 2006, Pawluski & Galea 2006). Third, neurogenesis in the subventricular zone of mice and rats is enhanced during pregnancy (Shingo et al. 2003, Furuta & Bridges 2005). Thus, plastic changes in substrates and/or regions relevant for cognition are associated with pregnancy.
Motherhood is associated with increased cognitive performance among rodents. For instance, rats that have had one or more litters outperform virgin rats in either probe or competitive trials of a dry land maze (Love et al. 2005) and make fewer errors in a radial arm maze than do virgins (Kinsley et al. 1999, Pawluski et al. 2006a, 2006b). These data are of particular interest because they suggest that a prolonged exposure to steroids can have long-lasting (even life-long) effects on cognition. Some of these effects may be due to trophic actions and/or morphological factors associated with hormonal milieu. 3α,5α-THP is one such steroidogenic factor that is enhanced throughout pregnancy and has demonstrated neuroprotective and neuroproliferative effects (Rhodes et al. 2004, Djebaili et al. 2005, Wang et al. 2005, VanLandingham et al. 2006, Leonelli et al. 2007, Wang et al. 2008). Of note, parity has been associated with reductions in age-related cognitive decrement and markers of neurodegeneration, as well as the increased expression of brain-derived neurotrophic factor (Gatewood et al. 2005, Pawluski & Galea 2007, Macbeth et al. 2008). Whether 3α,5α-THP may underlie some of the cognitive and/or trophic effects associated with reproductive experience has not been systematically investigated.
In order to further address the role that hormonal factors, such as E2 and/or 3α,5α-THP, may play in cognitive enhancement associated with parity, we conducted three experiments utilizing natural variations of hormonal milieu. We hypothesized that rats with more reproductive experience would demonstrate greater cognitive performance compared with rats with less reproductive experience and that this enhancement may be related to an increased production of 3α,5α-THP. To test this, the following experiments were conducted: (1) cycling rats that either had two prior pregnancies (multiparous) or were virgin (nulliparous) were tested in the object placement (OP) and object recognition (OR) tasks when in the low-hormone phase of their cycle (diestrus) and in the high-hormone phase of their cycle (behavioral estrus); (2) multiparous and nulliparous rats were mated resulting in some rats becoming pregnant, some becoming pseudopregnant, and others not becoming pregnant. First-time (primiparous) or third-time (multiparous) dams that were pregnant, pseudopregnant, or not pregnant were assessed on the object placement and object recognition tasks between gestational days (GDs) 6–12 and (3) virgin and multiparous rats were mated and assessed on the object placement and object recognition tasks daily across every day of gestation until 2 days post partum. The plasma hormone levels of E2, 3α, 5α-THP, and the stress hormone, corticosterone (CORT), were also assessed daily.
Results
Experiment 1: endogenous hormonal variations in virgin versus multiparous rats
Object placement
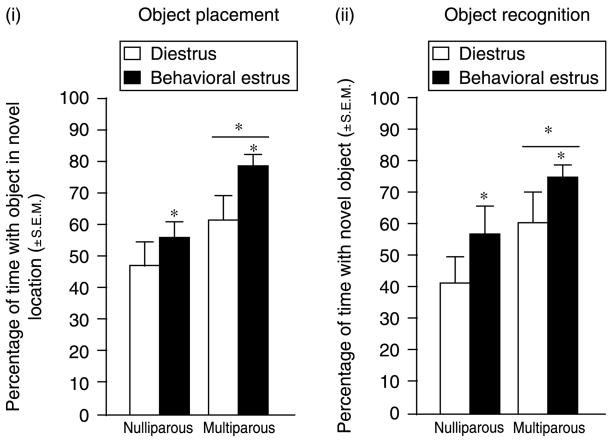
Parity (F(1,44) = 8.95, P<0.05) and estrous cycle phase (F(1,44) = 4.42, P<0.05) influenced performance in the object placement task. Multiparous rats spent a greater percentage of time with the object in the novel location (n = 24; 3.6±0.5 s with object in novel location versus 1.6±0.3 s with object in familiar location) compared with nulliparous rats (n = 24; 3.4±0.5 s with object in novel location versus 2.8±0.4 s with object in familiar location). As well, rats in behavioral estrus spent a greater percentage of time with the object in the novel location (4.1±0.5 s with object in novel location versus 2.0±0.4 s with object in familiar location) compared to diestrous rats (n = 24; 2.9±0.5 s with object in novel location versus 2.4±0.4 s with object in familiar location) (Fig. 1(i)).
Figure 1.
(i) Multiparous rats (n = 12/grp) significantly outperform nulliparous rats (n = 12/grp) and rats in behavioral estrus significantly outperform diestrous rats on the OP task. (ii) Multiparous rats (n = 12/grp) significantly outperform nulliparous rats (n = 12/grp) and rats in behavioral estrus significantly outperform diestrous rats on the OR task. *Indicates significant difference, P<0.05.
Object recognition
Parity (F(1,44) = 5.38, P<0.05) and estrous cycle phase (F(1,44) = 4.11, P<0.05) influenced performance in the object recognition task. Multiparous rats spent a greater percentage of time spent with the novel object (n = 24; 5.5±1.0 s with novel object versus 2.0±0.3 s with the familiar object) compared with nulliparous rats (n = 24; 3.5±0.7 s with the novel versus 2.2±0.4 s with the familiar object). In addition, rats in behavioral estrus spent a greater percentage of time with the novel object (5.7±0.8 s with the novel object versus 2.3±0.4 s with the familiar object) compared with diestrous rats (n = 24; 3.4±0.8 s with the novel object versus 2.0±0.3 s with the familiar object) (Fig. 1(ii)).
Experiment 2: variations in pregnancy status of primiparous versus multiparous rats
Object placement
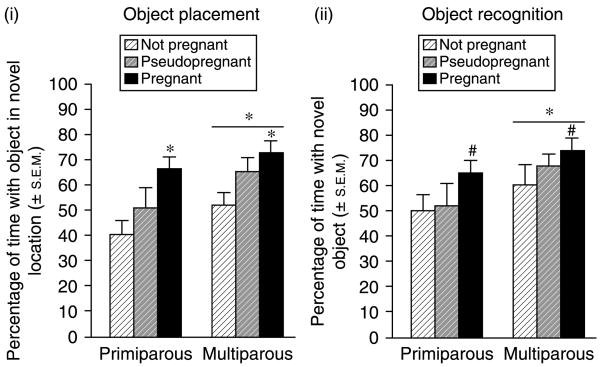
Parity (F(1,66) = 5.14, P<0.05) and pregnancy status (F(2,66) = 7.70, P<0.05) influenced performance in the object placement task. Multiparous rats spent a greater percentage of time with the object in the novel location (n = 36; 4.3±0.7 s with object in novel location versus 2.3±0.4 s with object in familiar location) than did primiparous rats (n = 36; 3.6±0.4 s with object in novel location versus 2.5±0.5 s with object in familiar location). As well, pregnant rats spent a greater percentage of time with the object in the novel location (4.1±0.6 s with object in novel location versus 2.0±0.4 s with object in familiar location) than did pseudopregnant rats (n = 24; 3.7±0.9 s with object in novel location versus 2.4±0.6 s with object in familiar location) and a significantly greater percentage of time than non-pregnant rats (n = 24; 3.9±0.6 s with object in novel location versus 2.9±0.5 s with object in familiar location) (Fig. 2(i)).
Figure 2.
(i) Multiparous rats (n = 12/grp) significantly outperform nulliparous rats (n = 12/grp) and pregnant rats significantly outperform non-pregnant rats on the OP task. (ii) Multiparous rats (n = 12/grp) significantly outperform nulliparous rats (n = 12/grp) on the OR task and pregnant rats tend to outperform non-pregnant rats on the OR task. *Indicates significant difference, P<0.05. #Indicates trend towards difference, P<0.10.
Object recognition
Parity influenced (F(1,66) = 5.08, P<0.05), and pregnancy status tended to influence (F(2,66) = 2.61, P<0.10), performance in the object recognition task. Multiparous rats spent a greater percentage of time with the novel object (n = 36; 6.5±0.8 s with novel object versus 2.7±0.3 s with familiar object) than did primiparous rats (n = 36; 4.0±0.5 s with novel object versus 2.9±0.7 s with familiar object). Additionally, pregnant rats (n = 24) spent a greater percentage of time with the novel object (6.4±0.7 s with novel object versus 2.4±0.4 s with familiar object) than did pseudopregnant rats (n = 24; 4.2±0.8 s with novel object versus 3.3±1.0 s with familiar object) or non-pregnant rats (n = 24; 5.2±0.9 s with novel object versus 2.6±0.3 s with familiar object) but this difference did not reach statistical significance (Fig. 2(ii)).
Experiment 3: hormonal variations across gestation in primiparous versus multiparous rats
Object placement
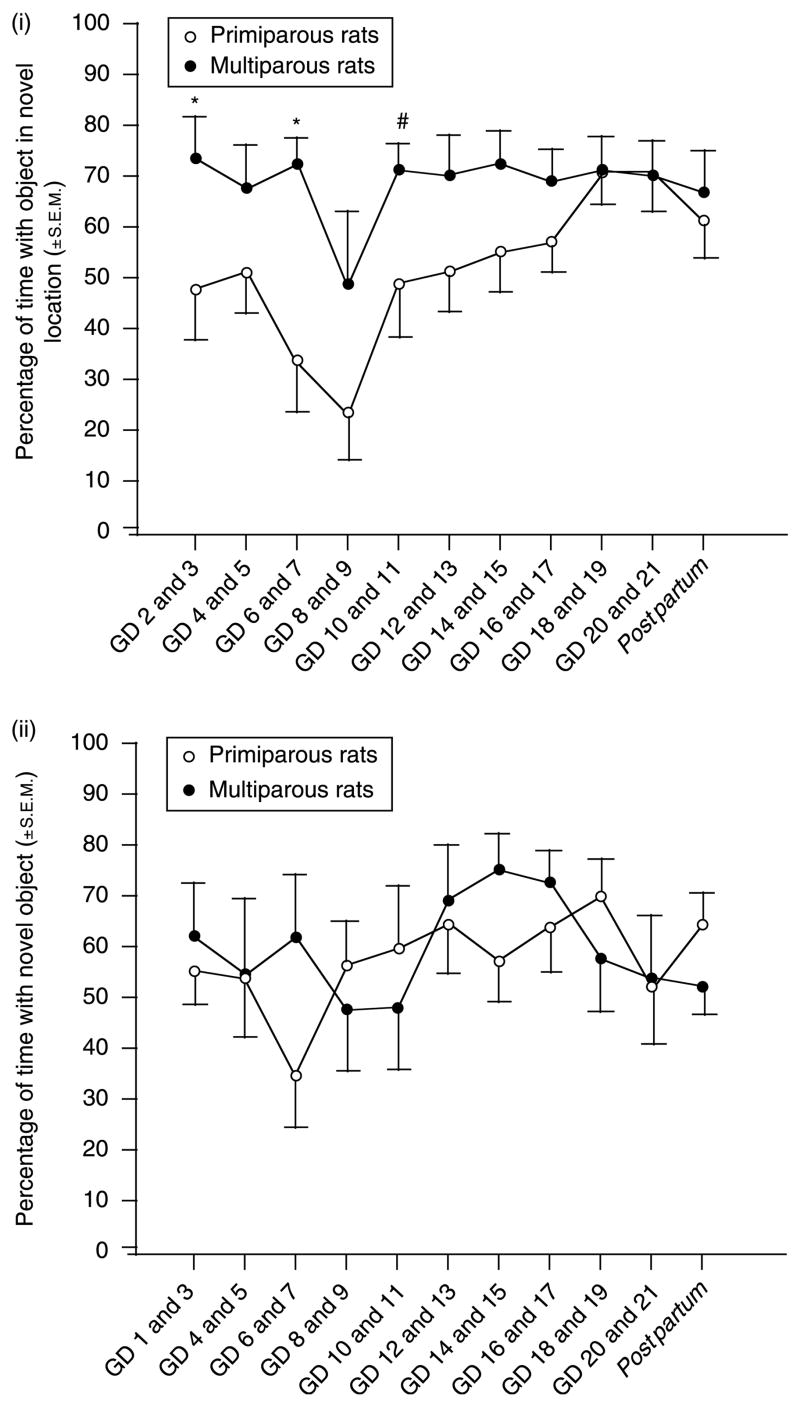
Parity (F(1,180) = 21.30, P<0.05) and day of gestation (F(10,180) = 2.69, P = 0.05) influenced performance across pregnancy in the object placement task. The percentage of time spent with the object in the novel location was greater among multiparous rats compared with primiparous rats and significantly varied as a function of GD due to an increase in the performance of primiparous rats in mid- to late gestation (Fig. 3(i)).
Figure 3.
(i) Multiparous rats (n = 10) significantly outperform nulliparous rats (n = 10) on the OP task in a repeated measures analysis across gestation. (ii) Multiparous (n = 10) and nulliparous rats (n = 10) do not significantly differ on the OR task in a repeated measures analysis across gestation. *Indicates significant difference, P<0.05. #Indicates trend towards difference, P<0.10.
Object recognition
Significant changes in performance that were dependent upon parity or the day of gestation were not observed when object recognition was examined across gestation of primiparous or multiparous dams (Fig. 3(ii)).
Endocrine measures
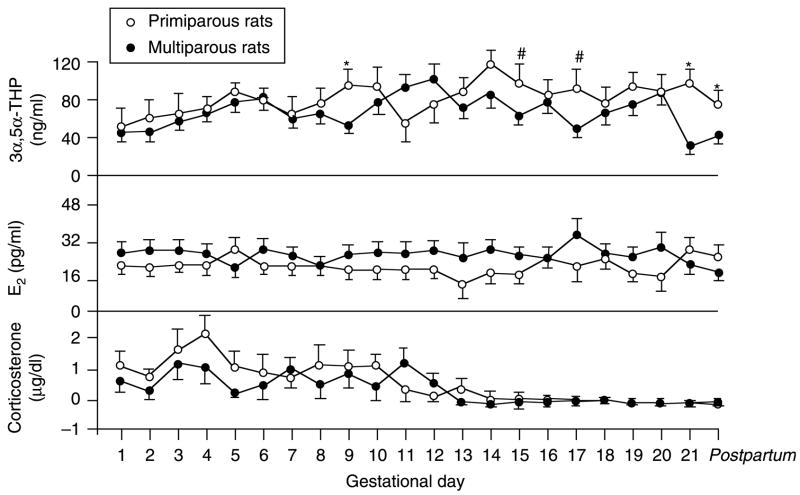
Overall, primiparous rats had significantly higher CORT levels than did multiparous rats (F(1,378) = 4.50, P<0.05). Among rats in either condition, CORT levels varied significantly by the day of gestation with much lower concentrations on GD 14–21 (F(21,378) = 5.00, P<0.05). Circulating E2 levels were significantly higher, overall, among multiparous compared with primiparous rats (F(1,378) = 4.65, P<0.05). Parity and day of gestation interacted to influence circulating 3α,5α-THP concentrations (F(21,328) = 1.68, P<0.05) with primiparous rats having greater levels of plasma 3α,5α-THP on GD 13 of pregnancy until post partum compared with multiparous rats (Fig. 4).
Figure 4.
Depicts circulating concentrations of 3α,5α-THP (top), estrogen (E2, middle), and corticosterone (bottom) in tail blood of primiparous (n = 10) and multiparous (n = 10) rats across gestation. *Indicates significant difference, P<0.05. #Indicates trend towards difference, P<0.10.
Discussion
The present study examined the role of endogenous hormones on cognitive performance associated with reproductive experience. The hypothesis that reproductive experience would be indicative of greater cognitive performance in rats, in part, due to contributions of endogenous 3α,5α-THP, was upheld in the following ways. In the first experiment, female rats in the high endogenous E2/progestin phase of their estrous cycle outperformed females in the low E2/progestin phase of their cycle on both hippocampal- (object placement) and corticolimbic- (object recognition) mediated tasks. In the second experiment, pregnant rats, which were expected to have high endogenous E2 and progestin levels, tended to outperform non-pregnant rats expected to have lower E2/progestin levels on both tasks. The performance of pseudopregnant rats fell between that of non-pregnant and pregnant rats. In both the experiments, multiparous rats significantly outperformed rats with less reproductive experience. In the third experiment, performance on object placement was significantly altered across gestation with multiparous dams maintaining a high performance throughout pregnancy, and primiparous dams incrementally increasing to multiparous levels of performance. The object recognition performance did not significantly vary as a function of gestation. An examination of circulating steroids revealed that 3α,5α-THP levels were commensurate between groups until late gestation when first-time mothers were found to have a later and greater peak, in addition to a lesser decline, in circulating 3α,5α-THP compared with multiparous rats. As well, the largest increases in object placement performance among primiparous dams (GD 10–11 and GD 18–19) occurred following peak 3α,5α-THP increases and just after days when 3α,5α-THP was enhanced (GD 9 and GD 17) compared with multiparous dams. E2 levels were relatively stable and consistently higher among multiparous compared with primiparous dams. When elevated, CORT levels were higher in first-time mothers compared with multiparous dams, but both groups fell to nadir concomitantly prior to parturition. These data support the hypothesis that 3α,5α-THP may underlie, in part, some of the cognitive-enhancing effects associated with reproductive experience.
The present investigation confirms prior reports that endogenous increases in E2 and/or progestins are associated with enhanced cognitive task performance on hippocampally mediated tasks (Shors et al. 1998, Galea et al. 2000, Frick & Berger-Sweeney 2001, Wood et al. 2001, Rhodes & Frye 2004, Walf et al. 2006, Frye et al. 2007). This report extends these findings by assessing performance in object placement and object recognition among pseudopregnant dams, which are unique in that they have enhanced E2 and progestin production but do not have the greatly exacerbated levels that are associated with placental secretion. Stepwise effects were observed with pregnant>pseudopregnant>non-pregnant in task performance, supporting the notion that ovarian hormones play an important role. This may be due, in part, to hippocampal remodeling. In support of this, natural enhancements of E2 and P4 that occur during behavioral estrus can modulate hippocampal dendritic spine density, such that density is greatest when rats are sexually receptive (Woolley et al. 1990, Woolley & McEwen 1993, McEwen & Woolley 1994, Kinsley et al. 2006). Further, enhancements in E2 have also been shown to increase the amount of synaptic connections made to pyramidal cells in the CA1 region of the hippocampus (Woolley & McEwen 1992) as well as increase the number of cells to which synaptic connections are made (Yankova et al. 2001). While increased cell–cell signaling in hippocampus may, in part, underlie enhancements in learning, concomitant rapid actions of progestins at membrane receptors and downstream signal transduction factors may also play a role. E2 and 3α,5α-THP have been shown to modulate the activity at glutamatergic N-methyl-D-aspartate receptors in culture (Weiland 1992, Park-Chung et al. 1994), which are important in hormone-facilitated hippocampal learning (Morris et al. 1986, El-Bakri et al. 2004, Frye et al. 2006, Wang et al. 2007). P4 administration enhances cAMP in brain (Collado et al. 1985) and antagonism of second messenger systems, including G-proteins, protein kinase C, or cAMP-dependent protein kinase A, in vitro, attenuates effects of 3α,5α-THP to facilitate GABAA activation (Fáncsik et al. 2000). Further, the MAPK pathway has been demonstrated to be integral to progestins’ functional effects; blocking MAPK attenuates the intracellular actions of P4 or membrane actions of 3α,5α-THP to enhance sexual behavior among rats (González-Flores et al. 2004, Acosta-Martínez et al. 2006). Tomizawa et al. (2003) have found that MAPK inhibition also attenuates LTP in multiparous mouse hippocampal slices. These data suggest that signal transduction factors are important in steroid actions and may, in part, underlie some functional cognitive performance changes associated with endogenous E2 and progestin elevations.
Apart from cyclical changes, these factors may also play a role in parity-related cognitive enhancement. Dams having had at least one litter typically outperform virgins on tasks of cognitive performance (Kinsley et al. 1999, Love et al. 2005, Bodensteiner et al. 2006, Pawluski et al. 2006a, 2006b, Macbeth et al. 2008). Primiparous or multiparous dams are found to have a greater spinal density on hippocampal dendrites compared with nulliparous dams (Pawluski & Galea 2006). Dendritic remodeling has also been detected in late-pregnant and postpartum rats (Kinsley et al. 2006) and may have functional effects due to actions of steroids. In support, Morris water maze performance was found to be associated with alterations in endogenous E2 and progestins, with higher levels of P4 indicating better performance among pregnant rats (Galea et al. 2000). The present study supports these findings, demonstrating a greater cognitive performance in multiparous, compared with nulliparous, dams. It cannot be ignored, however, that many studies to date have either found no cognitive and/or morphological differences between primiparous and multiparous dams, or have found differences that favor primiparous over multiparous dams (Wartella et al. 2003, Love et al. 2005, Pawluski & Galea 2006, Pawluski et al. 2006a, 2006b). One explanation may be that the beneficial effects of multiple pregnancies do not summate in a long-term manner. While parity appears to clearly be beneficial compared with no reproductive experience, benefits of more reproductive experience may not extend beyond the postpartum period. Another explanation is that the cognitive skills and associated neurobiology which are enhanced by multiple pregnancies may be more region-and task-specific. In the present study, performance in the object placement task was more divergent among primiparous and multiparous dams than object recognition performance. It may be the case that object recognition was more challenging since there were many different objects used as novel stimuli, whereas, in object placement, only two corners of the arena can be considered novel locations. As such, object placement may better lend itself to discriminating spatial differences than object recognition, which may be a primary difference between multiparous and primiparous rats. As well, there can be differences between studies utilizing virgin versus mated nulliparous rats as controls. However, we did not see large differences between virgin controls (Experiment 1) and mated nulliparous controls (Experiment 2) in these tasks.
In order to elucidate endocrine factors that may underlie performance differences and extend the present literature, circulating hormone levels of 3α,5α-THP, E2, and CORT were assessed in tail blood daily across gestation. The circulating levels of 3α,5α-THP were higher in first-time mothers than those in multiparous dams in late gestation (GD 13 until post partum), coincident with enhanced primiparous performance. These data support the notion that elevated 3α,5α-THP levels may alter the hippocampal plasticity to enhance cognitive performance. Past reports find that administration of P4 with E2 to OVX rats, increases hippocampal spine density (Woolley et al. 1996, Miranda et al. 1999) and some of these effects are likely due to actions through its metabolism to 3α,5α-THP. In support, 3α,5α-THP has trophic effects to enhance central and peripheral myelination, it is neuroprotective in traumatic brain injury and ischemic stroke, and improves functional outcome after trauma (Magnaghi et al. 2001, Schumacher et al. 2001, 2007, Ghoumari et al. 2003, Sayeed et al. 2006, VanLandingham et al. 2007). As such, trophic effects of 3α,5α-THP may underlie some of the neuroprotective aspects that have been reported with motherhood in aging (Gatewood et al. 2005, Pawluski & Galea 2007). As well, we have previously found that the administration of 3α,5α-THP to OVX rats increases cognitive performance on the object placement task commensurate to P4 administration alone (Frye et al. 2007). Thus, 3α,5α-THP elevations in pregnancy may promote hippocampal remodeling in primiparous rats to enhance cognitive performance.
In the brain, E2 can enhance the expression of the enzymes that convert P4 to 3α,5α-THP and can enhance 3α,5α-THP production (Cheng & Karavolas 1973, Micevych et al. 2003, Frye & Rhodes 2005). As such, circulating levels of E2 were assessed and were higher among multiparous compared with primiparous dams. In the present study, E2 levels were largely stable in both groups and did not greatly fluctuate across gestation as did cognitive performance. While the trophic effects of E2 and/or 3α,5α-THP (such as morphological changes in dendritic spine density and neuroproliferation) were not assessed, alterations in circulating E2 concentrations did not appear to adequately account for cognitive improvement among primiparous dams when considered alone. The contribution of central 3α,5α-THP biosynthesis should be considered in future investigations.
Tail blood collection is a mild stressor that was utilized in the present investigation to assess the function of the hypothalamic–pituitary–adrenal axis (HPA) in multiparous and primiparous rats. Overall, the concentrations of CORT among rats in both groups were scarcely elevated beyond what is normal during pregnancy. Commensurate with past reports, we observed an initial increase in circulating CORT levels followed by a gradual attenuation of the HPA response in later gestation (Neumann et al. 1998, Russell & Brunton 2006, Brunton & Russell 2007). Of interest, primiparous dams had significantly higher CORT levels than multiparous dams across early- to mid-gestation and the attenuation of primiparous CORT levels was somewhat delayed compared with multiparous dams in late-gestation. Despite this, it is not likely that stress accounts for differences in cognitive performance of primiparous and multiparous dams. On the contrary, investigations of interactions between stress and cognition reveal that stressed female rats typically perform better on radial arm maze, object recognition, and object placement tasks (Bowman et al. 2001, Beck & Luine 2002, Luine 2002), even when the stress is extended for 21 days, commensurate with the time course of the present investigation (Bowman et al. 2001, Beck & Luine 2002). In fact, alterations in circulating CORT levels are an indication of central 3α,5α-THP function. In rats, 3α,5α-THP plays a homeostatic role, increasing in the brain in response to enhanced glucocorticoid secretion to reinstate parasympathetic tone and dampen the HPA-stress response (Patchev et al. 1996, Bitran et al. 2000). The blockade of 3α,5α-THP formation or actions at its GABAergic substrates prevents anti-anxiety behavior and stress-induced glucocorticoid secretion (Rhodes & Frye 2001, Reddy 2002). As such, one intriguing possibility is that central 3α,5α-THP enhancement may underlie some of the cognitive effects of parity. These data support this notion, demonstrating that cognitive performance is best among all rats when CORT levels are attenuated, possibly due to the enhancement of central progestins. Thus, HPA attenuation may be indicative of 3α,5α-THP enhancement to levels that can improve cognitive performance.
The present investigation brings up several clinically relevant points. First, among premenopausal women, differences in cognitive performance have been observed across the menstrual cycle (Rupp & Wallen 2008). In support of this, Wisconsin Card-Sorting performance is better during high-progestin, compared with low E2/progestin, phases of the cycle (Solis-Ortiz et al. 2004). As well, among pregnant women, circulating 3α,5α-THP levels are observed to mimic the steady elevation and sharp decline across gestation that is observed among rodents (Gilbert-Evans et al. 2005), although the endocrine effects of parity among women have not been systematically investigated. Lastly, among natural or surgery-related postmenopausal women, the cognitive decline associated with hormonal depletion is marked (Zelinski et al. 1993, Small et al. 1999, Sherwin & Henry 2008). The findings of the present study add support to an overwhelming literature that attests to the beneficial effects hormones can have upon cognitive performance (Hays et al. 2003, Smoller et al. 2003, Brunner et al. 2005).
Although the results of this study are intriguing, it had several limitations. First, multiparous rats were typically a little more than one month older than other groups at the time of testing. However, all rats experienced their first mating at the same approximate age and the data do not suggest that this influenced our results since multiparous animals outperformed primiparous and virgin rats. If age were a confounding factor, it would be expected that multiparous animals would demonstrate a decreased cognitive performance compared with other groups. Second, Experiment 2 utilized rats that were mated and became pregnant, pseudopregnant, or did not become pregnant. Although all multiparous rats had successful pregnancies prior to this experiment, we cannot rule out the possibility that there may have been a genetic or physiological advantage for those that did become pregnant over those that did not become pregnant. However, this seems less likely considering evidence that pseudopregnant rats also increased performance over non-pregnant rats, despite not reproducing. Third, the contribution of other factors, such as alterations in anxiety and exploratory behavior, which are affected by parity and hormonal status (Byrnes & Bridges 2006, Frye et al. 2006), may underlie some of the cognitive performance effects and/or hormonal alterations indicated on these tasks. Future studies may aim to utilize a battery of anxiety measures in addition to object placement and recognition in order to assess this possibility.
These experiments demonstrate that utilizing natural hormone states associated with elevations in progestins (via cyclical- and pregnancy-related enhancement) yield differences in cognitive performance favoring high-progestin states. These data confirm past reports and suggest that some of the cognitive enhancement that is associated with reproductive experience may be related to elevations in circulating 3α,5α-THP. Future studies should aim to elucidate the trophic effects of 3α,5α-THP on hippocampal plasticity as well as its behavioral correlates throughout the estrous cycle and the reproductive experience.
Materials and Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at the University at Albany, SUNY.
Animals
Adult, female Long-Evans rats (n = 140), which were experimentally naïve, weighing between 250 and 325 g, were obtained from the breeding colony at The University at Albany, SUNY (stock originally purchased from Charles River, Raleigh, NC, USA). The rats were ear-tagged for identification and housed four per cage in polycarbonate cages (45×24×21 cm) in a temperature-controlled room (21±1 °C) in the Laboratory Animal Care Facility in The Life Sciences Research Building at SUNY, Albany. At the time of testing, multiparous (two pregnancies) rats were between 4 and 5 months of age and primiparous (one pregnancy) and nulliparous rats (no pregnancies) were over 3 months of age, but all rats had their first mating at approximately 50 days of age, irrespective of the experimental condition. All rats were maintained on a 12 h:12 h reversed-light cycle (lights off at 0800 h) with continuous access to Purina Rat Chow and tap water.
Object placement
The object placement task is a spatial memory task that primarily relies on hippocampal functioning (Ennaceur et al. 1997, Wallace et al. 2006). During training, the rats were placed in a white open field (76×57 cm with 35 cm high walls) in a brightly lit testing room. Two identical objects were used in this task. The rats were given 3 min to explore the open field which contained the two objects that were always placed in adjacent corners farthest from the rat starting location. These objects were colored and spherical in shape (plastic toys in shapes such as oranges, lemons, apples, hamburgers). After training, the rats were placed in a dark, sound-dampened room for 4 h. Following this interval, they were returned to the open field for testing. During testing, one of the spherical objects was moved to the opposing corner of the open field box. The rats were allowed to explore these objects for 3 min. They spent equal amounts of time exploring objects in this task (typically 4.7±1.0 s). Although they did not show a bias towards exploring objects on the right versus left side of the open field, placement of the novel object was counterbalanced such that, across all subjects, every corner of the open field was used. In this manner, the novel object location was counterbalanced across treatment groups and testing sessions to eliminate any possible effects of side preference. As well, the open field box and objects were cleaned with Quatricide (15 g/l) between subjects to control for confounding olfactory stimuli between testing sessions. A greater percentage of the time spent exploring the object in the novel location as a function of the total amount of the time spent exploring both objects during testing (duration spent with object in novel location/(duration spent with object in novel location+duration spent with object in familiar location)×100) is considered an index of enhanced cognitive performance in this task. Rats were not required to investigate the objects for any minimum amount of time during the training phase to be considered trained in this task for the following two reasons: (1) the lack of criterion for investigation allows this test to be suitable for repeated testing without test-decay effects (Luine et al. 2003) and (2) the objective of the present investigation was to assess the endocrine status that is changing over time. Thus, a rigorous maintenance of the test-timing was required in order to avoid confounds that could have shifted the time of day at which assessment occurred during gestation.
Object recognition
The object recognition task is a working memory task that primarily relies on cortical functioning and, to a lesser extent, hippocampal functioning (Ennaceur et al. 1997, Broadbent et al. 2004, Akirav & Maroun 2006). This task was used as modified from previously published methods (Ennaceur & Delacour 1988, Frye & Lacey 2001, Luine et al. 2003). Three objects were used in this task. The rats were trained as described above; however, during the testing trial, one of the spherical objects was replaced by a third (novel), cone-shaped object (a plastic toy in the shape of a buoy, a cartoon mouse, a soda bottle, or a pear) of a similar size. A greater percentage of time spent exploring the novel object as a function of the total amount of time spent exploring both objects during testing (duration spent with novel object/(duration spent with novel object+duration spent with familiar object)×100) is considered an index of enhanced cognitive performance in this task.
Procedure
Determination of estrous cycle phase
Estrous cycle phase was determined by daily examination of vaginal epithelium (between 0900 and 1000 h), as per previous methods (Long & Evans 1922, Frye et al. 2000). The rats were cycled through two normal estrous cycles (4–5 day cycle) prior to testing. All rats were tested on the morning of behavioral estrus, which is characterized by the presence of many nucleated epithelial cells, when E2 levels are declining, but progestin levels are high (Feder 1981, Frye & Bayon 1999).
Experiment 1: endogenous hormonal variations in nulliparous versus multiparous rats
Rats that were randomly assigned to be mated as multiparous (having had two prior births) or not mated as nulliparous (virgin) rats (n = 12/grp) were each assessed once in the object placement and once in the object recognition tasks on separate testing occasions when in behavioral estrus and diestrus.
Experiment 2: variations in pregnancy status of multiparous versus primiparous rats
Multiparous or nulliparous rats were mated and determined by daily examination of vaginal epithelium (between 0800 and 0900 h), to be pregnant (in anestrous by GD 6 continuing until parturition, n = 12 multiparous, n = 12 primiparous), pseudopregnant (in anestrous by GD 6 continuing at least until GD 12 without parturition, n = 12 multiparous, n = 12 primiparous), or not pregnant (regularly cycling without parturition, n = 12 multiparous, n = 12 primiparous). The rats were trained and tested between GDs 6–12 and each rat was assessed once on the object placement and object recognition task.
Experiment 3: hormonal variations across gestation in multiparous versus primiparous rats
Virgin or multiparous rats were mated and determined to be pregnant by daily examination of vaginal epithelium, consistent weight gain and other observable criteria (presence of teats, palpable pups). Rats (n = 10/grp) were tested on each day of pregnancy from GD 1 (approximately 24 h after mating) to GD 21 on either the object placement orthe object recognition task. After testing on GD 21, dams were single-housed in a polycarbonate cage (45×24×21 cm) for birthing. One postpartum test was performed in both tasks with object placement 2 days after parturition and object recognition 3 days after parturition or vice versa. Throughout pregnancy, the task-type was counterbalanced such that some rats in each group performed object placement on odd GDs and object recognition on even GDs, while others were tested on the opposite schedule. Since gestation lasts an odd amount of days in the rat (approximately 21 days) there were two days (GDs 1 and 2) wherein all rats performed in the same task (object recognition on GD 1 and object placement on GD 2) in order to maintain a counterbalanced design and the number of observations in each group throughout testing. Likewise, stimuli were counterbalanced across gestation such that no stimuli were used more than once within 7 GDs. Tail blood was collected at the end of each testing occasion.
Plasma collection
The rats were gently restrained by hand and blood was collected from a small nick at the tip of the tail. They were allowed limited ambulation while tail was massaged and blood was collected (~1 ml) in order to minimize stress and/or discomfort. Following collection, blood remained on ice until refrigerated centrifugation (4 °C at 3000 g for 10 min). Serum was immediately stored in a −80 °C freezer until analysis.
Radioimmunoassays
RIA for E2, 3α,5α-THP, and CORT was conducted on plasma by previously reported methods (Choi & Dallman 1999, Frye & Bayon 1999, Frye et al. 2007).
Radioactive probes
Tritiated [3H] 3α,5α-THP (NET 1047, specific activity = 65.0 Ci/mmol), [3H] E2 (NET 317, specific activity = 51.3 Ci/mmol), and [3H] CORT (NET 182, specific activity = 48.2 Ci/mmol) used for RIAs were purchased from Perkin–Elmer (Boston, MA, USA).
Antibodies
The 3α,5α-THP antibody (purchased from Dr Robert Purdy, Veterans Medical Affairs, La Jolla, CA, USA) was used in a dilution of 1:5000, which binds between 40 and 60% of [3H] 3α,5α-THP. The E2 antibody (E#244, Dr G D Niswender, Colorado State University, Fort Collins, CO, USA) was used in a dilution of 1:40 000 and bound between 40 and 60% of [3H] E2. The CORT antibody (Endocrine Sciences, Calabasas Hills, CA, USA: #B3-163) was used in a dilution of 1:20 000 and bound between 40 and 60% of [3H] CORT.
Analyses
In Experiments 1 and 2, behavioral data were analyzed using two-way ANOVAs to determine the effects of parity (Experiment 1, nulliparous or multiparous and Experiment 2, primiparous or multiparous) and hormone condition (Experiment 1, diestrus or behavioral estrus and Experiment 2, not pregnant, pseudopregnant, or pregnant) on performance in the object placement and recognition tasks. In Experiment 3, repeated measures ANOVAs were utilized to determine effects of parity and GD differences with parity condition (primiparous or multiparous) as the between-subjects factor and GD (1–21 and post partum) as the within-subjects factor. Planned comparison t-tests were used to determine significant differences between primiparous and multiparous rats on each GD. An α-level of P<0.05 was used to determine statistical significance in all analyses. An α-level of P<0.10 was determined to indicate a trend towards significance. When appropriate, significant analyses were assessed using Fisher’s protected least significant difference comparisons to determine group differences.
Acknowledgments
This research was supported by funding from the National Institute of Mental Health (MH06769801) and the National Science Foundation (IBN 95-14463, 98-96263, 03-16083). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Acosta-Martínez M, Gonzalez-Flores O, Etgen AM. The role of progestin receptors and the mitogen-activated protein kinase pathway in delta opioid receptor facilitation of female reproductive behaviors. Hormones and Behavior. 2006;49:458–462. doi: 10.1016/j.yhbeh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cerebral Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiology and Behavior. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Bodensteiner KJ, Cain P, Ray AS, Hamula LA. Effects of pregnancy on spatial cognition in female Hooded Long-Evans rats. Hormones and Behavior. 2006;49:303–314. doi: 10.1016/j.yhbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Broadbent N, Squire L, Clark R. Spatial memory, recognition memory, and the hippocampus. Neuroscience. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner RL, Gass M, Aragaki A, Hays J, Granek I, Woods N, Mason E, Brzyski R, Ockene J, Assaf A, et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Clinical Trial. Archives of Internal Medicine. 2005;165:1976–1986. doi: 10.1001/archinte.165.17.1976. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Attenuated hypothalamo-pituitary–adrenal axis response to immune challenge during pregnancy: the neurosteroidopioid connection. Journal of Physiology. 2007;586:369–375. doi: 10.1113/jphysiol.2007.146233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM, Bridges RS. Reproductive experience alters anxiety-like behavior in the female rat. Hormones and Behavior. 2006;50:70–76. doi: 10.1016/j.yhbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medial basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology. 1999;140:4081–4088. doi: 10.1210/endo.140.9.6964. [DOI] [PubMed] [Google Scholar]
- Collado ML, Rodriguez-Manzo G, Cruz ML. Effect of progesterone upon adenylate cyclase activity and cAMP levels on brain areas. Pharmacology Biochemistry and Behavior. 1985;23:501–504. doi: 10.1016/0091-3057(85)90408-3. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. PNAS. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SM, Vanlandingham JW, Stein DG. Tapered progesterone withdrawal promotes long-term recovery following brain trauma. Experimental Neurology. 2006;200:78–85. doi: 10.1016/j.expneurol.2006.02.137. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. Journal of Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. Journal of Cellular and Molecular Medicine. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioral Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. Journal of Neuroscience. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS, Ivy GO. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behavioral Neuroscience. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- Feder HH. Hormones and sexual behavior. Annual Review of Psychology. 1984;35:165–200. doi: 10.1146/annurev.ps.35.020184.001121. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behavioral Neuroscience. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Research Reviews. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THPand 3α-Diol. Journal of Neuroendocrinology. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 2001;28:550–563. [Google Scholar]
- Frye CA, Rhodes ME. Estrogen-priming can enhance progesterone’s anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacology Biochemistry and Behavior. 2005;81:907–916. doi: 10.1016/j.pbb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006a;83:336–347. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. Journal of Neuroendocrinology. 2006b;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behavioral Brain Research. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacology Biochemistry and Behavior. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacology Biochemistry and Behavior. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-Hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Bridges BS. Gestation-induced cell proliferation in the rat brain. Developmental Brain Research. 2005;156:61–66. doi: 10.1016/j.devbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Hormones and Behavior. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, et al. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Research Bulletin. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. Journal of Neurochemistry. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gilbert-Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-Reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecological Endocrinology. 2005;21:268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- González-Flores O, Shu J, Camacho-Arroyo I, Etgen AM. Regulation of lordosis by cyclic 3′,5′-guanosine monophosphate, progesterone, and its 5α-reduced metabolites involves mitogen-activated protein kinase. Endocrinology. 2004;145:5560–5567. doi: 10.1210/en.2004-0823. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women’s Health Initiative recruitment methods and results. Annals of Epidemiology. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Hormones and Behavior. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Leblanc P, Weyers MH, Soffié M. Age-related differences for duration discrimination in rats. Physiology and Behavior. 1996;60:555–558. doi: 10.1016/s0031-9384(96)80031-x. [DOI] [PubMed] [Google Scholar]
- Leonelli E, Bianchi R, Cavaletti G, Caruso D, Crippa D, Garcia-Segura LM, Lauria G, Magnaghi V, Roglio I, Melcangi RC. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience. 2007;144:1293–1304. doi: 10.1016/j.neuroscience.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. In: Leuschner AO, editor. Memoirs of the University of California. Vol. 6. Berkeley: University of California Press; 1922. pp. 1–146. [Google Scholar]
- López HH, Wurzel G, Ragen B. The effect of acute bupropion on sexual motivation and behavior in the female rat. Pharmacology Biochemistry and Behavior. 2007;87:369–379. doi: 10.1016/j.pbb.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. Maternal experience produces long-lasting behavioral modifications in the rat. Behavioral Neuroscience. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Hormones and Behavior. 2008 doi: 10.1016/j.yhbeh.2007.08.011. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnaghi V, Cavarretta I, Galbiati M, Martini L, Melcangi RC. Neuroactive steroids and peripheral myelin proteins. Brain Research Reviews. 2001;37:360–371. doi: 10.1016/s0165-0173(01)00140-0. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Falkay G, Baulieu EE. Modulation of uterine GABAA receptors during gestation and by tetrahydroprogesterone. European Journal of Pharmacology. 1989;174:43–47. doi: 10.1016/0014-2999(89)90871-6. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Hormones and Behavior. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. Journal of Neuroscience. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Experimental Gerontology. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. Journal of Neuroscience. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. Journal of Physiology. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Farb DH. 3α-Hydroxy-5α-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Molecular Pharmacology. 1994;46:146–150. [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. Journal of Neurobiology. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Hormones and Behavior. 2006a;49:43–49. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behavioral Brain Research. 2006b;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Pazol K, Northcutt KV, Wilson ME, Wallen K. Medroxyprogesterone acetate acutely facilitates and sequentially inhibits sexual behavior in female rats. Hormones and Behavior. 2006;49:105–113. doi: 10.1016/j.yhbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiology of Aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The clinical potentials of endogenous neurosteroids. Drugs of Today. 2002;38:465–485. doi: 10.1358/dot.2002.38.7.820115. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of proestrous rats decreases anxiolytic, and enhances exploratory and motor behaviors. Cognitive, Affective & Behavioral Neuroscience. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic enhancing effects in the passive avoidance task. Pharmacology Biochemistry and Behavior. 2004;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3α,5α-THP mediates progestins’ effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacology Biochemistry and Behavior. 2004;78:505–512. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex differences in response to visual sexual stimuli: a review. Archives of Sexual Behavior. 2008;37:206–218. doi: 10.1007/s10508-007-9217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Brunton PJ. Neuroactive steroids attenuate oxytocin stress responses in late pregnancy. Neuroscience. 2006;138:879–889. doi: 10.1016/j.neuroscience.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115:384–393. [PubMed] [Google Scholar]
- Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behavioural Brain Research. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Annals of Emergency Medicine. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Mercier G, Desarnaud F, Lacor P, Benavides J, Ferzaz B, Robert F, Baulieu EE. Progesterone synthesis and myelin formation in peripheral nerves. Brain Research Reviews. 2001;37:343–359. doi: 10.1016/s0165-0173(01)00139-4. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacology & Therapeutics. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Frontiers in Neuroendocrinology. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Small SA, Stern Y, Tang M, Mayeux R. Selective decline in memory function among heathy elderly. Neurology. 1999;52:1392–1396. doi: 10.1212/wnl.52.7.1392. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Pollack MH, Wassertheil-Smoller S, Barton B, Hendrix SL, Jackson RD, Dicken T, Oberman A, Sheps DS. Prevalence and correlates of panic attacks in postmenopausal women: results from an ancillary study to the Women’s Health Initiative. Archives of Internal Medicine. 2003;163:2041–2050. doi: 10.1001/archinte.163.17.2041. [DOI] [PubMed] [Google Scholar]
- Solis-Ortiz S, Guevara MA, Corsi-Cabrera M. Performance in a test demanding prefrontal functions is favored by early luteal phase pro-gesterone: an electroencephalographic study. Psychoneuroendocrinology. 2004;29:1047–1057. doi: 10.1016/j.psyneuen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nature Neuroscience. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, Hammes SR, Jamnongjit M, Stein DG. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cekic M, Cutler S, Hoffman SW, Stein DG. Neurosteroids reduce inflammation after TBI through CD55 induction. Neuroscience Letters. 2007;425:94–98. doi: 10.1016/j.neulet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Research. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. Journal of Neuroscience. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Marx CE, Morrow AL, Wilson WA, Moore SD. Neurosteroid modulation of GABAergic neurotransmission in the central amygdala: a role for NMDA receptors. Neuroscience Letters. 2007;415:118–123. doi: 10.1016/j.neulet.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Liu L, Irwin RW, Chen S, Brinton RD. Regenerative potential of allopregnanolone. Brain Research Reviews. 2008;57:398–409. doi: 10.1016/j.brainresrev.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiology and Behavior. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. Journal of Neuroscience. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. Journal of Comparative Neurology. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic CA1 pyramidal cells: a serial electron-microscopic study. PNAS. 2001;98:3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging. 1993;8:176–186. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]