Abstract
Introduction
Recombinant members of the vitamin K-dependent protein family (factors IX and VII and protein C) have become important pharmaceuticals in treatment of bleeding disorders and sepsis. However, because the in vivo γ-carboxylation system in stable cell lines used for transfection has a limited capacity of post translational γ carboxylation, the recovery of fully γ-carboxylated and functional proteins is low.
Materials and Methods
In this work we have engineered recombinant factor VII producing HEK 293 cells to stably overexpress VKORC1, the reduced vitamin K γ-carboxylase cofactor and in addition stably silenced the γ-carboxylase inhibitory protein calumenin.
Results and Conclusions
Stable cell lines transfected with only a factor VII cDNA had a 9% production of functional recombinant factor VII. On the other hand, these recombinant factor VII producing cells when engineered to overexpress VKORC1 and having calumenin stably suppressed more than 80% by shRNA expression, produced 68% functional factor VII. The technology presented should be applicable to all vertebrae members of the vitamin K-dependent protein family and should lower the production cost of the clinically used factors VII, IX and protein C.
Keywords: Recombinant Factor VII, Vitamin K, Gamma-Carboxylation, Vitamin K-dependent Coagulation Factors
Recombinant protein technology has set the stage for production of clinically used proteins of the vitamin K-dependent protein family (1). These include recombinant factor IX used to treat hemophilia B (2), activated protein C (APC) to treat sepsis (3) and recombinant factor VIIa to treat a variety of bleeding complications (4). A major problem with production of recombinant vitamin K-dependent coagulation factors for use as pharmaceuticals has been poor recovery of functional proteins from the cell medium (5,6). It has been shown that poor recovery results from, 1) incomplete γ-carboxylation of the secreted proteins (6) and 2) incomplete removal of the propeptide by the protease furin in the Golgi apparatus(5). Incomplete γ-carboxylation is a significant problem as only <10% of the secreted recombinant vitamin K-dependent proteins have been reported to be fully γ-carboxylated and functional (6). It is believed that incomplete γ-carboxylation occurs when an excess of newly synthesized precursors of vitamin K-dependent proteins appear in the endoplasmic reticulum (ER) and exceed the capacity of the cell’s γ-carboxylation system to fully modify all of the precursors (5,6).
The vitamin K-dependent γ-carboxylation system is a multicomponent system of proteins embedded in the ER membrane (7). The system consists of 1) the vitamin K-dependent γ-carboxylase which requires the reduced hydroquinone form of vitamin K (vit.KH2) as cofactor and 2) the warfarin sensitive enzyme vitamin K 2,3-epoxide reductase (VKOR), which produces the cofactor. Concomitant with γ-carboxylation, the hydroquinone is converted to the metabolite vitamin K 2,3 epoxide (VitK>O), which is reduced back to the vit.KH2 cofactor by VKOR. Since VKOR has been shown to be the rate limiting step in γ-carboxylation of vitamin K-dependent proteins (8,9), the recent discovery of the gene for the warfarin sensitive VKORC1 enzyme, which appears to be a subunit in a complex formed with protein disulfide isomerase (PDI) in the ER (10), have made it possible to generate cell lines with increased VKOR activity and capacity to γ-carboxylate recombinant vitamin K-dependent proteins (11). Previously we have demonstrated that stable transfection of recombinant human factor IX (r-hFIX) producing BHK cells with a VKORC1 construct increased production of fully γ-carboxylated and functional r-hFIX from 18% in cells that only overexpressed r-hFIX to 50% in the same cells after a VKORC1 construct was stably inserted into the BHK genome (12). By using siRNA technology to silence the γ-carboxylase inhibitor calumenin (13) we were able to increase this yield of active factor IX to 80% (14). Since the siRNA technology that was used in that work was based on transient transfection with oligonucleotides, this prevented generation of stable cell lines with the high production capacity. Since the pharmaceutical industry would be dependent upon stable suppression of calumenin, we sought to engineer cell lines that stably suppressed calumenin. Since HuSH 29mer shRNA constructs against human calumenin were found to be commercially available as a kit, we acquired the kit in order to make cell lines that would stably suppress human calumenin. Our r-hFIX producing cells were hamster BHK cells and since the available HusH 29mer shRNA constructs were prepared against human calumenin, we selected HEK293 cells for transfection. In this paper we present our biotechnology that was used to overproduce recombinant human factor VII (r-hFVII) in HEK 293 cells stably transfected with cDNAs of h-rFVII and rat VKORC1 in addition to being stably transfected with different Hush 29mer shRNA human calumenin constructs respectively. The results from these experiments demonstrate an increase in functional r-hFVII yield from 9% in cells only over expressing r-hFVII to 68% in cells over expressing r-hFVII and rat VKORC1 where calumenin was silenced stably with a calumenin HuSH 29mer vector with the construct AAGCCTGAGATCAATAAGAATGTTCAGG. The results demonstrate a new technology that can be used in enhanced production of functional recombinant important vitamin K-dependent proteins used as pharmaceuticals.
MATERIALS AND METHODS
Cloning of human FVII
Normal human liver cDNA was purchased from Biochain, Hayward, CA and used as template for the generation of human FVII. The following primers were used to clone the full length coding sequence of human FVII. Sense primer 5’-CATG GTC TCC CAG GCC CTC AGG CT-3’ and antisense primer 5’- GCTA GGG AAA TGG GGC TCG CAG GA-3’ were used in PCR and 35 cycles of PCR were performed under the following conditions; 1) denaturizing at 94° C for 30 sec, 2) annealing for 1 min at 58° C which was extended for 3 min at 72° C. At the end of 35 cycles the reaction was extended for an additional 7 min at 72° C. The PCR products were purified using QIAGEN’s PCR purification kit (QIAGEN, Valencia, CA) and separated on a 1% ETBr-stained agarose gel. The purified PCR product was then ligated into the TA cloning vector pCR 2.1 (Invitrogen, Carlsbad, CA). Positive clones were identified by EcoR I digestion and sequenced on both strands using M13 reverse and forward primers. Not I and Xho I restriction sites were then generated at the 5’ and 3’ ends of the human FVII cDNA respectively in order to clone the cDNA into the pBUDCE4.1 vector (Invitrogen, Carlsbad, CA) for expression in mammalian cell lines. A Kozak sequence was generated at the 5’ end of human FVII cDNA. The oligonucleotides used for generation of the Not I and Xho I cloning sites were: Sense primer 5’- TT GCG GCC GC GCC ACC ATG GTC TCC CAG GCC CTC AGG CTC CT-3’ (Not I site bases are underlined, bases in italics were used to generate a Kozak sequence) and antisense primer 5’ – GG CTC GAG CTA GGG AAA TGG GGC TCG CAG GA – 3’ (underlined bases were used to generate Xho I site). Thirty-five cycles of PCR were performed at the following conditions; 1) Denaturing at 94° C for 30 sec, 2) annealing for 1 min at 62° C which was extended for 3 min at 72° C. At the end of 35 cycles, the reaction was extended for an additional 7 min at 72° C. The PCR product of human FVII was purified using QIAGEN’s PCR purification kit and separated on a 1% ETBr-stained agarose gel. The purified human factor VII PCR product was than ligated into the TA cloning vector pCR 2.1 (Invitrogen, Carlsbad, CA). Positive clones were identified by Not I and Xho I digestion and the full length human FVII insert was sequenced from both the strands to eliminate any PCR generated errors. The modified cDNA for human FVII was then subcloned into the Not I and Xho I site of the pBUDCE4.1 vector under the control of EF-1 promoter. The new plasmid with human FVII cDNA was named HFVII pBUDCE4.1.
Construction of plasmid containing human FVII and Rat VKORC1 cDNA
Plasmid pBUDCE4.1 (Invitrogen, Carlsbad, CA) is a dual promoter vector capable of expressing two independent recombinant proteins. It has two multiple cloning sites and each under the control of two independent promoters. The promoters are CMV and EF-1. The human FVII insert was excised from plasmid HFVII pBUDCE4.1 using Not I and Xho I restriction digestion. Excised human FVII insert was gel purified and sub cloned into Not I and Xho I restriction sites of the plasmid RatVKORpBUDCE4.1 as described previously by our laboratory (11). The resulting plasmid contains both the human FVII and rat VKOR of cDNAs. The new plasmid was named HFVIIRVKOR pBUDCE4.1.
Stable cell lines expressing human FVII
Plasmid HFVII pBUDCE4.1 containing the human FVII cDNA was used to transfect HEK 293 cells purchased from ATCC (Manassas, VA). HFVII pBUDCE4.1 was linearized by digesting with the Nhe I restriction enzyme. The linearized vector was purified and transfected into HEK 293 cells using a FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis) according to the manufacture’s recommendations. After 24 hours of transfection cells were passaged at 1:20 dilution into fresh growth medium (DMEM containing 10% fetal bovine serum) with 200 µg/ml Zeocin antibiotic (InvivoGen, San Diego, CA) for selective pressure. Medium containing 200µg/ml Zeocin was changed after every 2–3 days for 6 weeks. After selection of stable cell lines expressing h-rFVII, cells were maintained at 100µg/ml of Zeocin in DMEM containing 10% fetal bovine serum.
Stable cell lines co-expressing human FVII and rat VKORC1
Plasmid HFVIIRVKOR pBUDCE4.1 containing human FVII and rat VKORC1 cDNAs was used to transfect HEK 293 cells. The plasmid was linearized by digesting with a Nhe I restriction enzyme. The linearized vector was purified and used for transfection of HEK 293 cells with a FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) according to the manufacture’s recommendations. After 24 hours of transfection, the cells were passaged at 1:20 dilution into fresh growth medium (DMEM containing 10% fetal bovine serum) with 200 µg/ml Zeocin antibiotic for selective pressure. Medium containing 200 µg/ml Zeocin was changed after every 2–3 days for 6 weeks. After selection of stable cell lines expressing r-hFVII and rat VKOR, cells were maintained at 100 µg/ml of Zeocin in DMEM containing 10% fetal bovine serum.
Packaging of human calumenin shRNA into replication deficient retrovirus
The kit HuSH 29mer shRNA Construct against CALU (TR314215) against human calumenin was purchased from ORIGEN (Rockville, MD). The pRS shRNA plasmids in the kit expressed 29 bases of human calumenin shRNA under the control of the U6 promoter and the SV40 early promoter for expression of the puromycin-N-acetyl transferase gene respectively. The following constructs against human calumenin were provided in the kit: 1) CALU1: AAGCCTGAGATCAATAAGAAATGTTCAGG, 2) CALU2: CACCAGAAGAGAGCAAGGAAAGGCTTGGA, 3) CALU3: GACAAGGATGGAGACCTCATTGCCACCAA, 4) CALU4: TGGACCTGCGACAGTTTCTTATGTGCCTG. The naming CALU 1, 2, 3 and 4 are used in the result section to identify the various plasmids used for stable suppression of calumenin in HEK 293 cells. Each plasmid was packaged into the retroviral packaging cell line PT67 purchased from ATCC (Manassas, VA) according to the manufacturer’s recommendations. Briefly, the retroviral packaging PT67 cells were grown in DMEM plus 10% FBS in 10 cm cell culture dishes. Cells were transfected separately with the four different constructs (CALU1, 2, 3 and 4) of the human calumenin shRNA plasmids using a FuGene transfection reagent. Cells were incubated in 5% CO2 incubator for 48 hours. After 48 hours, the cells were harvested and filtered through 0.45 µm filters. The filtered medium was used as the viral stock.
Stable cell lines coexpressing human calumenin shRNA, human FVII and rat VKOR
Replication deficient retro viruses expressing the different human calumenin shRNAs were used to infect HEK 293 cells co-expressing human FVII and rat VKOR. After 48 hours of infection 1µg/ml puromycin was added for cell selection. After stable selection of HEK 293 cells, cells were maintained at 1µg/ml puromycin and 100ug/ml of Zeocin. Stable cell lines were used for determination of; 1) expression of r-h FVII, 2) rat VKOR activity and 3) silencing of cellular human calumenin. One control was HEK 293 cells only overexpressing r-hFVII.
SDS-PAGE and Western blotting
SDS-PAGE was carried out on 8–16% CRITERION Gels (Bio-Rad, Richmond CA). Western Blotting was carried out as described previously (11).
Human factor VII blood clotting activity assay and factor VII quantification
Human Factor VII activity was measured with the Hemosil PT-Fibrinogen kit from Instrumentation Laboratory Company, Lexington, MA. Factor VII deficient plasma was from the same company. Standard curves of dilutions of pooled human plasma and human factor VII purified from human blood was made. Purified human factor VII was purchased from Enzyme Research Laboratories, South Bend, IN. The purchased purified protein had a concentration of 1.87 mg/ml and a specific activity of 2109.00 PEU/mg. Clotting times of recombinant factor VII harvested from cells cultured for 24 hours in 10% serum containing medium followed by 24 hours in serum free medium were compared to the standard curve and the specific activity was used to calculate the concentration of active r-hFVII per ml of serum free medium. Total concentration of r-hFVII per ml was determined by chemiluminescence of standard curves of various concentrations of factor VII and r-hFVII in the media harvested from the cultured cells after Western blotting. All measurements were in the linear range of the standard curve. Western blots were carried with a human factor VII monoclonal antibody purchased from Sigma, St.Louis, MO.
VKOR Activity
VKOR activity was measured as described (15) by estimating the percent conversion of vitamin K1 2,3-epoxide (VitK>O) to vitamin K1.
Materials
Vitamin K1 2,3-epoxide was made as described by our laboratory (15). Affinity purified rabbit calumenin antibodies were prepared by our laboratory (13). Beta-Actin antibodies were purchased from Abcam Inc., Cambridge, MA. All other purchased chemicals were of the highest quality that could be supplied by the various companies.
RESULTS
Stable silencing of calumenin in HEK 293 cells with the pRS shRNA CALU plasmids
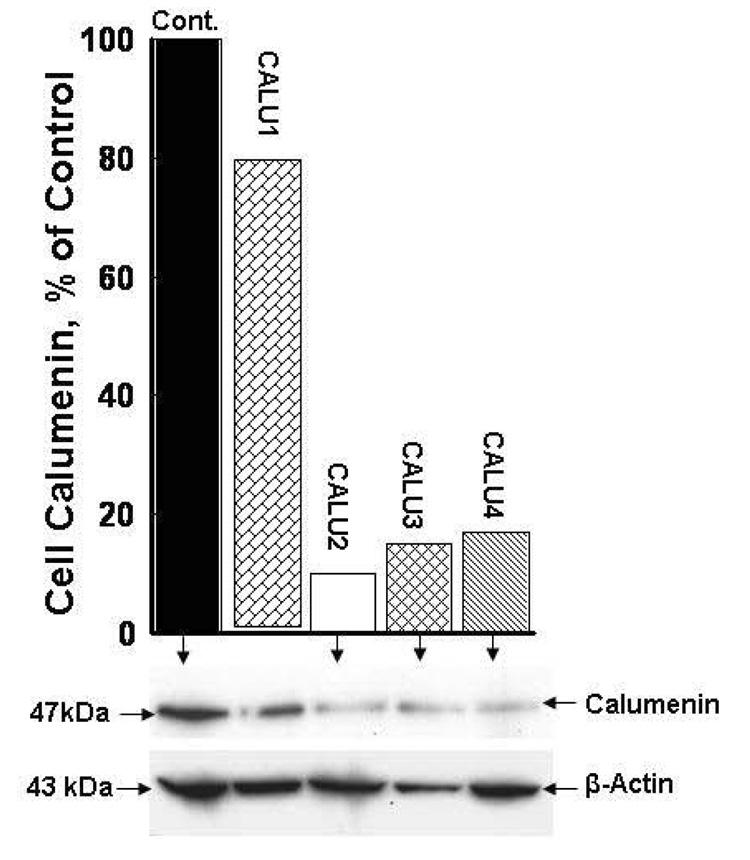
All HEK 293 cell lines engineered to stably overexpress r-hFVII and r-hFVII + rat VKOR from the dual promoter vector pBUDCE4.1 were found to secrete the same amount of r-hFVII into the serum free medium. This finding was equivalent to our finding with r-hFIX expressed by this system in BHK cells (12). As shown in Figure 2, panel B, cell lines stably transfected with the CALU1, 2, 3 and 4 pRS shRNA plasmids respectively showed only small variations in the total amount of r-hFVII secreted into the medium which further demonstrate the stability of recombinant protein production by the expression system used in our experiments. All individual cell lines were viable and showed no difference in proliferation rate. These findings demonstrated that stable silencing of calumenin could be used to generate stable HEK 293 cell lines with high capacity for production of fully γ-carboxylated and functional r-hFVII. Furthermore it showed that suppression of calumenin did not cause cell death which has been shown by eliminating another member of the CREC family of ER proteins to which calumenin belongs (16). The effect of silencing calumenin in r-hFVII + VKORC1 overexpressing HEK 293 cells stably transfected with the pRS shRNA CALU 1, 2, 3 and 4 constructs respectively is shown in Figure 1. The cell lysates were Western blotted with calumenin antibodies (47 kDa) and band intensities in each lane quantified by chemiluminescence. The blot was stripped and reprobed with a β-Actin antibody (43 kDa) to correct for protein loading in each lane. The bar graph shows the band intensities of calumenin in % of the band intensity of calumenin in cells transfected with the pRS vector only (100 %). The CALU 2, 3 and 4 pRS shRNA constructs significantly reduced the intracellular calumenin content more so than the CALU 1 construct with the nucleotide sequence AAGCCTGAGATCAATAAGAAATGTTCAGG. Therefore we eliminated the cell line stably transfected with the CALU 1 pRS construct form the next set of experiments aimed at finding the most effective cell line engineered to stably overproduce functional r-hFVII.
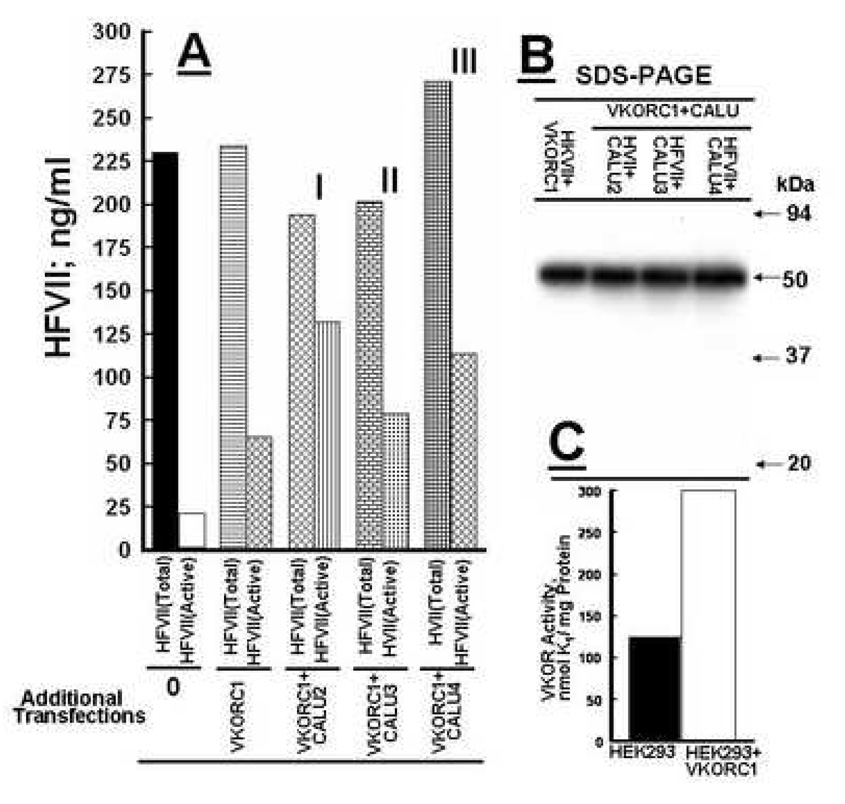
Figure 2.
Production of active recombinant factor VII by variously engineered HEK 293 cell lines. Panel A; Total factor VII (HFVII)and Active (HFVII) were measured in aliquots from HEK 293 cell media harvested from cells stably overexpressing 1) only recombinant human (HFVII) (Additional Transfections 0), 2) HFVII + VKORC1 (Additional Transfections VKORC1), 3) HFVII + VKOC1 + CALU 2 (Additional Transfections VKORC1+CALU 2 I), 3) HFVII + VKORC1 + CALU 3 (Additional Transfections VKORC1 + CALU3 II) and 4) HFVII + VKORC1 + CALU 4 (Additional Transfections VKORC1 + CALU 4 III ). Estimation in ng/ml of total HFVII protein by chemiluminescence and active HFVII by clotting assay is described in MATERIALS AND METHODS. Bars representing total HFVII protein concentration and active HVII protein concentration are labeled in panel A as Total and Active respectively. Panel B is a Western blot of HVII in aliquots from HEK 293 cells stably transfected with VKORC1 and VKORC1 + CALU 2, 3, and 4 respectively. A monoclonal factor VII antibody identified the 50 kDa recombinant HFVII. Each bar represents the average concentration calculated from 3 individual experiments which varied < 5%. Panel C shows that HEK 293 cell acquire a higher VKOR activity after stable transfection with a human factor VII cDNA.
Figure 1.
Stable silencing of calumenin in HEK 293 cells with HuSH 29mer shRNA constructs against human calumenin. Four pRS shRNA vectors with different 29mer oligonucleotides called CALU1, 2, 3 and 4 were used individually to stably transfect HEK 293 cells stably overexpressing r-hFVII and VKORC1 (See MATERIALS AND METHODS). The cellular concentration of calumenin in the various CALU transfected cells was measured after Western blotting and compared to calumenin concentration in cells transfected with the vector (Control, 100 %). Aliquots from the media harvested from the different cell cultures were loaded in each lane. Affinity purified antibodies against the 47 kDa calumenin were used to identify the protein. The same blot were stripped and reprobed with a β-Actin antibody to correct for equal protein loading in each lane. Quantification of the various immunoreactive bands was made by chemiluminescence (see MATERIALS AND METHODS).
Production yield of functional recombinant human factor VII by the stably engineered HEK 293 cell lines
Having determined to focus on the CALU 2, 3, and 4 stably transfected cell lines we measured total r-FVII and functional r-hFVII secreted into the serum free medium. In this set of experiments we used two controls. One control was their content in medium from HEK 293 cells only stably transfected with the factor VII cDNA construct in the pBUDCE4.1 vector. The concentration of total r-hFVII (228 ng/ml) and functional r--hFVII (20 ng/ml) resulted in a production yield of 9% of the functional enzyme (Figure 2, panel A, Additional Transfections 0). The second control was HEK 293 cells stably transfected with the pBUDCE4.1 vector expressing both r-hFVII and VKORC1. The medium concentration of total r-hFVII (230 ng/ml) and functional r-hFVII (67 ng/ml) resulted in a production yield of 30% of the active enzyme (Figure 2, panel A, Additional Transfections VKORC1). The remaining bar graphs in Figure 2 represent the results obtained from cells stably transfected with the pBUDCE4.1 vector expressing both r-hFVII and VKORC1 but in addition were transfected stably with the CALU 2, 3 and 4 pRS shRNA constructs respectively. The concentrations of total r-hFVII and functional r-hFVII in these engineered cells (Figure 2, Additional Transfections, VKORC1+CALU 2(I), VKORC1+CALU 3 (II) and VKORC1+CALU 4 (III) were: I; Total193 ng/ml, Active132 ng/ml: II; Total 200 ng/ml, Active 82 ng/ml and III; Total 273 ng/ml, Active 114 ng/ml. The production yield of active r-hFVII in I, II and III were 68%, 41% and 42% respectively. As seen by the bars representing total r-hFVII there was some variation in total factor FVII secreted from the cell lines. However CALU 2 and CALU 4 was the highest producer of active r-hFVII. If calculated in % of total r-hFVII, cells engineered with the AAGCCTGAGATCAATAAGAAATGTTCAGG nucleotide vector CALU 2 was the most effective cell line in secretion of active r-hFVII (68%). Figure 2, panel B shows Western blots of r-hFVII in aliquots from the serum free media harvested from the various engineered r-hFVII producing HEK 293 cell lines. The human factor VII monoclonal antibody shows only the 50 kDa single chain factor VII protein and no factor VIIa. The blot also demonstrates that all of the engineered cell lines used in these experiments produces similar quantities of r-hFVII. Figure 2, panel C, show that VKOR activity in HEK 293 cells is significantly enhanced following transfection with a VKORC1 cDNA as was demonstrated by our earlier work with BHK cells (12).
DISCUSSION
The main objective behind this work was to expand our earlier work on calumenin (13) as an inhibitor of the γ-carboxylase by stably silencing the inhibitor to create cell lines with increased capacity to produce fully γ-carboxylated and functional recombinant vitamin K-dependent proteins. Our earlier work (14) with transient transfection experiments using siRNA oligonucleotides against hamster calumenin in BHK cells indicated that this goal could be accomplished. Only cell lines engineered to stably silence calumenin would be important for pharmaceutical mass production of clinically used functional vitamin K-dependent proteins as factor IX, protein C and factor VII. The common commercial systems used to produce recombinant factor VII, factor IX and protein C are cell lines which only stably overexpress these proteins. Since the in vivo γ-carboxylation system in these cell lines used for transfection does not have the capacity to fully γ-carboxylate an excess of newly synthesized precursors of the overproduced vitamin K-dependent protein, it is obvious that a significant amount of the protein will exit the ER as a none or partly γ-carboxylated non functional protein. Thus engineering of the γ-carboxylation system to increase its γ-carboxylation capacity is essential for enhanced production of functional recombinant proteins. As demonstrated in this work, the yield of functional r-hFVII increased from 9% in HEK 293 cells only overexpressing factor VII to 68% in stable cell lines where VKORC1 also was overexpressed and the γ-carboxylase inhibitor calumenin was silenced more than 80%. Although the total amount of r-hFVII produced by the cell lines engineered in this work is less than reported by some published work on production of recombinant vitamin K-dependent proteins (6), culturing conditions may have caused these differences. Our newly designed cell system carried out under improved culturing conditions is likely to further improve the production yield of functional vitamin K-dependent proteins.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (NIH) grant RO1HL69331 (R.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Furie B, Furie B. Molecular and Cellular Biology of Blood Coagulation. N Engl J Med. 1992;326:800–806. doi: 10.1056/NEJM199203193261205. [DOI] [PubMed] [Google Scholar]
- 2.Pipe SW, Saint-Remy J-M, Walsh CE. New high-technology products for treatment of hemophilia. Haemophilia. 2004;10:55–63. doi: 10.1111/j.1365-2516.2004.00996.x. [DOI] [PubMed] [Google Scholar]
- 3.Russell JA. Management of Sepsis. N Eng J Med. 2006;355:699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 4.Hedner U. Mechanism of Action of Recombinant Activated Factor VII: An Update. Semin Hematol. 2006;3 suppl 1:S105–S107. doi: 10.1053/j.seminhematol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Wasley LC, Rehemtulla A, Bristol JA, Kaufman RJ. PACE/furin can process the vitamin K-dependent pro-factor IX precursor within the secretory pathway. J Biol Chem. 1993;268:8458–8465. [PubMed] [Google Scholar]
- 6.Kaufman RJ, Wasley LC, Furie BC, Furie B, Shoemaker BC. Expression, purification and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986;261:9622–9628. [PubMed] [Google Scholar]
- 7.Wallin R, Hutson SM. Warfarin and the Vitamin K-Dependent Gamma-Carboxylation System. Trends Mol Med. 2004;10:299–302. doi: 10.1016/j.molmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Wallin R, Sane DC, Hutson SM. Vitamin K 2,3-epoxide reductase and the vitamin K-dependent gamma-carboxylation system. Thromb Res. 2002;8:221–226. doi: 10.1016/s0049-3848(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 9.Hallgren KW, Qian W, Yakubenko AV, Runge KV, Berkner KL. R-VKOR expression in factor IX BHK cells increases the extent of factor IX carboxylation but is limited by saturation of another carboxylation component or by a shift in the rate-limiting step. Biochemistry. 2006;45:5587–5598. doi: 10.1021/bi051986y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajih N, Hutson SM, Wallin R. Disulfide-dependent protein folding is linked to operation of the vitamin K cycle in the endoplasmic reticulum. J Biol Chem. 2007;282:2626–2635. doi: 10.1074/jbc.M608954200. [DOI] [PubMed] [Google Scholar]
- 11.Wajih N, Sane DC, Hutson DC, Wallin R. Engineering of a recombinant vitamin K-dependent gamma-carboxylation system with enhanced gamma-carboxyglutamic acid forming capacity: evidence for a functional CXXC redox center in the system. J Biol Chem. 2005;280:10540–10547. doi: 10.1074/jbc.M413982200. [DOI] [PubMed] [Google Scholar]
- 12.Wajih N, Hutson SM, Owen J, Wallin R. Increased production of functional recombinant human clotting factor IX by baby hamster kidney cells engineered to overexpress VKORC1, the vitamin K 2,3-epoxide-reducing enzyme of the vitamin K cycle. J Biol Chem. 2005;280:31603–31607. doi: 10.1074/jbc.M505373200. [DOI] [PubMed] [Google Scholar]
- 13.Wajih N, Sane DC, Hutson SM, Wallin R. The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system. Characterization of the system in normal and warfarin resistant rats. J Biol Chem. 2004;279:25276–25283. doi: 10.1074/jbc.M401645200. [DOI] [PubMed] [Google Scholar]
- 14.Wajih N, Hutson SM, Wallin R. siRNA silencing of calumenin enhances functional factor IX production. BLOOD. 2006;108:3657–3660. doi: 10.1182/blood-2006-02-004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallin R, Martin LF. Vitamin K-dependent gamma-carboxylation and vitamin K metabolism in liver: Effects of warfarin. J Clin Invest. 1985;76:1879–1884. doi: 10.1172/JCI112182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honore B, Vorum H. The CREC family, a novel family of multiple EF-hand, low-affinity Ca(2+)-binding proteins localised to the secretory pathway of mammalian cells. FEBS Lett. 2000;466:11–18. doi: 10.1016/s0014-5793(99)01780-9. [DOI] [PubMed] [Google Scholar]