Abstract
Rationale
3,4-Methylenedioxymethamphetamine (MDMA or “ecstasy”) causes serotonin neuron damage in laboratory animals. The serotonin system is known to be important in the regulation of mood. Previous research has shown that MDMA users score higher on self-report ratings of depression than controls. However, MDMA users commonly take other illicit substances and many studies do not fully control for poly-drug use.
Objectives
The aim of this study was to examine the relationship between MDMA use and affective disturbance, while fully controlling for poly-drug use.
Methods
Participants were 30 current MDMA users, 30 poly-drug controls who had never used MDMA, 30 drug-naïve controls with no history of illicit drug use and 20 ex-MDMA users. The current MDMA users and poly-drug controls were well matched on all indices of non-MDMA drug use. All participants were administered the Beck Depression Inventory (BDI) and the Affective Go/No-go task, which has been shown to be sensitive to depression.
Results
The current and ex-MDMA users scored significantly higher on the BDI than the drug-naive controls, but were not significantly different from the poly-drug controls. There were no differences between the groups in terms of affective bias scores on the Affective Go/No-go task.
Conclusions
Increased scores on self-report depression scales in MDMA users are not entirely attributable to MDMA use. MDMA users do not show the same attentional bias towards negatively toned material as depressed patients.
Keywords: 3,4-Methylenedioxymethamphetamine (MDMA); Ecstasy; Depression; Poly-drug use; Beck Depression Inventory (BDI); Affective Go/No-go
Introduction
3,4-Methyldioxymethamphetamine (MDMA), or “ecstasy”, has been shown to cause permanent reductions in serotonin axons and receptors in rats (Ricaurte et al. 1987; McKenna and Peroutka 1990), non-human primates (Hatzidimitriou et al. 1999) and humans (Semple et al. 1999; Kish et al. 2000; Reneman et al. 2001). Due to the important role of serotonin in modulating mood, it might be expected that such long-lasting depletions may cause vulnerability to depression in chronic MDMA users. Consistent with this hypothesis, a number of studies have reported that MDMA users report higher levels of depression than controls, both in the days following MDMA use (Curran and Travill 1997; Parrott and Lasky 1998; Verheyden et al. 2002) and following a period of abstinence (Gerra et al. 2000; Gamma et al. 2001; MacInnes et al. 2001; Thomasius et al. 2003). However, not all studies have found evidence to support a relationship between depression and MDMA use (Parrott et al. 2000, 2001; Verkes et al. 2001). In addition, many studies have suffered from design issues that limit the inferences that can be drawn about the relationship between MDMA use and depression.
Firstly, almost all chronic MDMA users also use other drugs, in particular cannabis and amphetamine (Parrott et al. 2002). Some studies have failed to include a drug-using control group in order to control for the effects of these other drugs (Gerra et al. 2000; MacInnes et al. 2001), while others have included such a control group but have failed to adequately match drug-use to that of the MDMA-using group (Gamma et al. 2001; Verkes et al. 2001; Thomasius et al. 2003). Therefore, the elevated levels of depression reported in such studies cannot unequivocally be attributed to ecstasy use.
Secondly, although previous studies have found that MDMA-using groups report significantly higher scores on self-report depression inventories than control groups, the mean levels of depression reported in currently abstinent MDMA users have been in the mild or non-clinical range (Gamma et al. 2001; MacInnes et al. 2001; Verkes et al. 2001; Verheyden et al. 2002). MDMA is known to cause somatic complaints such as appetite and sleep disturbance, as well as sexual dysfunction, and these symptoms appear on the scales commonly used in research, such as the Beck Depression Inventory (BDI) (Beck et al. 1961) and the Hamilton Depression Rating Scale (HDRS) (Hamilton 1960). Therefore it is possible that these slightly elevated, though sub-clinical depression rating scale scores, could be accounted for by somatic problems related to MDMA use. Previous studies have found that both cognitive and somatic complaints arise in the days following MDMA use (Curran and Travill 1997; Verheyden et al. 2002), though no study has yet differentiated between the two following a period of abstinence.
A number of studies have shown that patients suffering from depression show involuntary cognitive biases towards negatively toned material. In tests involving memory, depressed patients show enhanced recall for negative words or stories (e.g. Matt et al. 1992), while in tests involving attention, depressed patients show slowed reaction times to positive words (Murphy et al. 1999) (see Tavares et al. 2003 for a review)
Depressed patients show slowed responding to happy words compared to controls on the Affective Go/No-go task, which has been termed a negative “affective bias” (Murphy et al. 1999). The same effect has been observed in healthy controls under conditions of acute tryptophan (Murphy et al. 2002) or tyrosine depletion (McLean et al. 2003). If MDMA use does cause depression, then MDMA users would be expected to show the same involuntary cognitive biases towards negative material as seen in clinically depressed patient groups.
In this study, we examined the relationship between MDMA use and self-reported depression, while accurately controlling for other drug use. We also examined the relationship between MDMA use and emotional attentional bias using the Affective Go/No-go task (Murphy et al. 1999).
Materials and methods
Participants
Seventy-three current MDMA users and 20 ex-MDMA users were recruited by means of advertisement in magazines and newspapers. Inclusion criteria for the two MDMA-using groups were use of ecstasy on a minimum of 30 separate occasions. Current MDMA users were required to abstain from MDMA use for 3 weeks before the study. The participants classified as ex-MDMA users had not used the drug for at least 1 year before the study. Thirty controls with a history of poly-drug use, but who had never taken ecstasy, and 30 controls with little or no experience of illicit drug use were also recruited in a similar manner. Thirty current MDMA users were selected from the original sample of 73 to match the control groups on demographic and drug use variables for direct comparison with the other groups.
Participants with current or past diagnosed axis I psychiatric disorders were excluded, as were any who reported any drug use on the day of testing. All participants gave written informed consent. The study was approved by the Cambridge Local Research Ethics Committee.
Measures
All participants were administered the BDI and a substance use questionnaire. For analysis, BDI scores were divided into cognitive and somatic subscales (based on the first 15 questions and last six questions of the questionnaire respectively). All participants performed the Affective Go/No-go task, which has previously been shown to be sensitive to depression (Murphy et al. 1999).
In the Affective Go/No-go task, words are presented in the middle of the screen sequentially. Half the words are targets, to which the subject must respond, while half are distractors, which the subject must ignore. Responses are made by pressing the space bar as quickly as possible. Words are presented for 300 ms with an inter-stimulus interval of 900 ms. For each error, a 500 ms/450 Hz tone sounds for each commission error, but not for omission errors. The task consists of ten blocks of 18 stimuli, nine positive or “happy” (H) and nine negative or “sad” (S) words per block. The first two blocks are treated as practice blocks. In each block, either positive or negative words are specified as targets, with targets for the ten blocks presented in a HHSSHHSSHHSSHH or SSHHSSHHSSHHSS order. Four blocks can therefore be considered “shift” blocks, where participants must start responding to stimuli that were previously distractors, while withholding response to stimuli that were previously targets. On each block, mean correct reaction time, number of commission errors (false alarms) and number of omission errors (misses) were recorded. “Affective bias” scores were calculated by subtracting the mean happy reaction time from the mean sad reaction time.
Blood samples were taken to corroborate information provided by participants about drug use. To check for abstention from MDMA, plasma was screened for amphetamines using enzyme assay—minimum detection threshold 5 ng/ml, sensitive to use of MDMA within 72 h. Positive results were confirmed using gas chromatography/mass spectroscopy. Analysis was carried out by Tricho-Tech Ltd (www.tricho-tech.co.uk).
Statistical analysis
Data were analysed using SPSS 10 (SPSS Inc, Chicago, Ill., USA). Group differences were analysed using one-way analysis of variance (ANOVA) where test assumptions were met. Where appropriate, data were transformed prior to analysis as appropriate to reduce skew and stabilise variances. Post-hoc comparisons were conducted using the Tukey Honest Significant Difference (HSD) tests if variances were equivalent, or Tamhane T2 (T) tests if variances were significantly different. Where assumptions of parametric analysis could not be met, appropriate non-parametric analyses were employed.
Data from the Affective Go/No-go task were analysed using a repeated measures ANOVA: the analysis for reaction times included valence of word (happy/sad) and shift (shift/non-shift) as within subjects measures and group as the between subjects measure; the analysis for errors was similar to that for reaction time. Omission errors and commission errors were analysed separately.
Results
Demographic factors
Demographic and illicit drug use characteristics are reported in Tables 1, 2 and 3. The selected current MDMA-using group (n=30) did not differ from the poly-drug using control group on any demographic drug-use variable. Both the MDMA-using group and poly-drug control group had relatively low use of illicit drugs other than cannabis and MDMA. No subject showed a positive plasma screen.
Table 1.
Demographic characteristics, mean (SD)
| Current MDMA (CM) | Poly-drug control (PC) | Drug naive control (NC) | Ex-MDMA (EM) | Significant difference | |
|---|---|---|---|---|---|
| n (M:F) | 30 (15:15) | 30 (15:15) | 30 (15:15) | 20 (10:10) | - |
| Age | 22.4 (6.0) | 25.7 (8.9) | 24.0 (3.6) | 27.5 (6.3) | EM vs CM |
| NART IQ | 115.2 (7.1) | 111.7 (8.8) | 113.9 (5.8) | 111.9 (8.2) | - |
| Alcohol (units last month) | 67.5 (48.4) | 41.4 (37.3) | 32.6 (28.6) | 43.2 (57.9) | CM vs NC |
| Cigarettes (last month) | 186 (189) | 173 (252) | - | 186 (223) | - |
Table 2.
Self reported use of ecstasy, mean (SD)
| Current MDMA | Ex-MDMA | Significant difference | |
|---|---|---|---|
| Life exposure (tablets) | 273.4 (411.0) | 792.6 (1525.8) | - |
| Highest regular dose (tablets) | 3.8 (2.8) | 4.0 (2.6) | - |
| Highest regular frequency (times per month) | 4.4 (3.2) | 9.9 (7.2) | P<0.01 |
| Highest amount in 12-h period (peak) (tablets) | 7.0 (4.1) | 5.2 (2.6) | P<0.05 |
| Time since last taken (days) | 75 (79) | 1021 (1018) | P<0.001 |
Table 3.
Self reported use of illicit drug use other than ecstasy, mean (SD)a
| Current MDMA (CM) | Poly-drug control (PC) | Ex-MDMA (EM) | Significant comparisons | |
|---|---|---|---|---|
| Cannabis (n) | 30 | 30 | 20 | - |
| Joints last month | 35.1 (63.4) | 53.0 (87.0) | 41.1 (104.0) | - |
| Years of use | 3.7 (2.9) | 3.6 (3.5) | 5.1 (4.5) | - |
| Psilocybin (n) | 24 | 6 | 14 | - |
| Times life | 5.8 (6.9) | 4.9 (3.3) | 14.3 (21.9) | - |
| LSD (n) | 18 | 5 | 15 | - |
| Trips life | 8.9 (9.4) | 4.8 (5.8) | 44.8 (99.1) | - |
| Amphetamine (n) | 26 | 10 | 17 | - |
| Grams life | 21.7 (32.8) | 86.1 (148.0) | 231.0 (330.9) | EM vs CM |
| Amyl nitrate (n) | 20 | 4 | 13 | - |
| Times life | 11.5 (12.3) | 57.3 (67.9) | 9.7 (8.6) | - |
| Ketamine (n) | 14 | 0 | 5 | - |
| Grams life | 6.7 (7.0) | - | 6.2 (6.9) | - |
| Cocaine (n) | 24 | 8 | 19 | - |
| Grams life | 12.1 (14.9) | 6.4 (8.3) | 161.0 (591.3) | EM vs PC |
| Opiates (n) | 5 | 0 | 9 | - |
| Grams life | 0.2 (0.1) | - | 68.2 (83.6) | EM vs CM |
Statistics reported include only those subjects reporting use of the substance
As expected, the groups differed in terms of alcohol consumption [F(3,106)=3.6, P=0.016]; the current MDMA-using group had drunk more alcohol in the last month than the drug naive control group (P<0.05). The groups also differed in terms of age [F(3,106)=2.9, P=0.038], the ex-MDMA-using group being significantly older than the current MDMA-using group (P<0.05). Although neither age nor alcohol consumption correlated with any of the behavioural measures in this study, these measures were nevertheless controlled for in future analyses.
The ex-MDMA-using group also had a more extensive drug use history than the other two drug^-using groups. The ex-MDMA-using group had taken more ecstasy tablets than the current MDMA-using group, but this difference failed to reach statistical significance [see Table 2, t(30.8)=1.8, P=0.08]. The ex-MDMA-using group had taken ecstasy more frequently than the current MDMA-using group [t(25.4)=2.9, P=0.007], but had a lower peak exposure to MDMA [t(47.8)=2.2, P=0.03] and, as expected, had a longer abstinence period than the current MDMA-using group [t(19.1)=4.1, P<0.001]. In addition, the ex-MDMA-using group had a greater lifetime exposure to amphetamine (U=76.5, P<0.01) and opiates (U=4, P=0.012) than the current MDMA-using group and greater lifetime exposure to cocaine (U=34.5, P=0.025) than the poly-drug control group.
Beck Depression Inventory
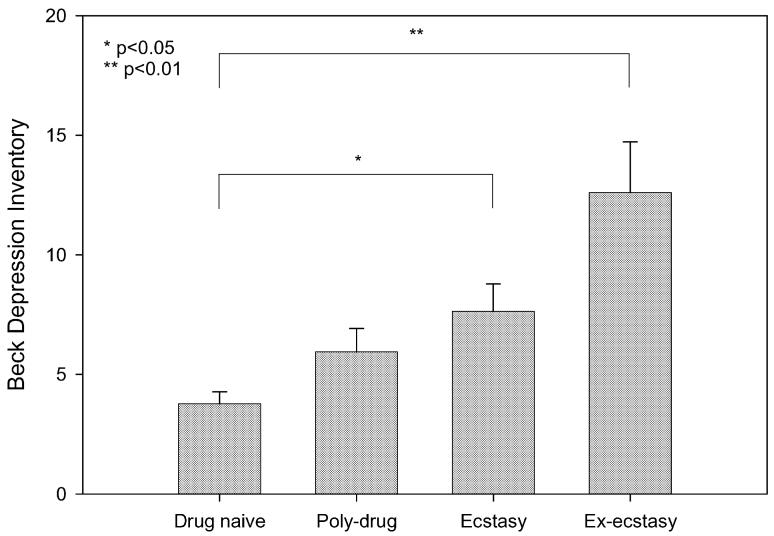
Measures of affective and cognitive function are presented in Table 4 and Figs 1 and 2. The groups differed on BDI score [F(3,106)=5.88, P<0.001]. Post-hoc analysis revealed that the ex-MDMA-using group reported significantly higher scores on the BDI than the drug naïve control group (P<0.01), and non-significantly higher scores than the poly-drug control group (P=0.09). The current MDMA-using group reported significantly higher scores on the BDI than the drug naive control group (P<0.05), but did not differ from the poly-drug control group (P=0.74) (see Fig. 1). Combining the two MDMA-using groups, there was no sex difference on the BDI (male mean=9.9, SD=7.9, female mean=9.7, SD=8.4, t(48)=0.08, P=0.94).
Table 4.
BDI scores and affective bias, mean (SD)
| Current MDMA | Poly-drug control | Drug-naive control | Ex-MDMA | |
|---|---|---|---|---|
| BDI (total) | 7.9 (6.5) | 6.0 (5.4) | 3.8 (2.8) | 12.6 (9.5) |
| BDI (cognitive) | 5.8 (4.9) | 4.3 (4.8) | 2.8 (2.1) | 9.4 (7.8) |
| BDI (somatic) | 2.1 (1.9) | 1.7 (1.8) | 1.0 (1.0) | 3.3 (2.6) |
| Affective bias (ms) | +11.0 (33.7) | -0.7 (33.0) | +2.67 (32.2) | +0.66 (32.6) |
| Commission errors | 10.1 (7.2) | 11.1 (7.0) | 13.0 (9.6) | 10.0 (5.1) |
| Omission errors | 3.0 (2.7) | 5.2 (5.1) | 4.8 (4.7) | 4.8 (4.6) |
Fig. 1.
Comparison of the four groups on Beck Depression Inventory Scores: bars represent the mean, error bars 1 SEM
Fig. 2.
Comparison of the four groups on commission errors on happy and sad blocks: bars represent the mean, error bars 1 SEM
Separating total BDI score into cognitive and somatic components, the groups differed on both the somatic subscale [F(3,106)=5.3, P=0.001], and the cognitive subscale [F(3,106)=5.4, P=0.002]. On the somatic subscale, post-hoc analysis revealed that the ex-MDMA-using group reported significantly higher scores than the drug naive control group (P<0.001) and the poly-drug control group (P<0.05). The current MDMA-using group reported higher scores than the poly-drug control group, which showed a trend towards significance (P=0.070). There was no difference between the current MDMA-using group and the poly-drug control group on the somatic subscale of the BDI (P=0.68).
On the cognitive subscale, the ex-MDMA-using group reported higher cognitive symptom scores than the drug naïve control group (P<0.05). Additionally, the ex-MDMA-using group reported higher scores than the poly-drug control group (P=0.070), and the current MDMA-using group reported higher scores than the drug naive control group (P=0.099) (see Table 4). There was no difference between the current MDMA-using group and the poly-drug control group on the cognitive subscale (P=0.56). In summary, the results for both the cognitive and somatic subscales of the BDI showed a similar pattern to that of the total BDI score
As the groups differed on age and alcohol consumption, both were entered as covariates in the ANOVA. This did not affect the results.
Affective Go/No-go
Overall, the groups did not differ on affective bias scores [F(3,106)<1, P>0.1]. Indeed, current MDMA users tended to show a positive rather than a negative affective bias, while the other groups showed very little bias toward positive or negative words (see Table 4).
Analysis of reaction time in the repeat measures ANOVA revealed no main effect of valence [F(1,106)<1, P>0.1] or shift [F(1,106)=2.4, P=0.12], no valence×shiftinteraction [F(1,106)=1.7, P=0.2], shift×group interaction [F(3,106)<1, P>0.1] and no valence×shift×group interaction [F(3,106)<1, P>0.1].
Analysis of the error data revealed a main effect of shift for both commission errors [F(1,106=9.7), P=0.002] and omission errors [F(1,106)=7.8, P=0.006], as expected, due to a reduction in errors on non-shift blocks compared to shift blocks across all groups. There was no main effect of valence for either commission errors or omission errors [F(1,106)<1, P>0.1 for both]. Neither the valence×group nor shift×group interactions approached significance for either the commission errors or the omission errors (P>0.2 for all).
Significant valence×switch [F(1,106)=5.1, P=0.026] and valence×switch×group [F(3,106)=2.7, P=0.048] interactions were found for commission errors, but not omission errors [F(1,106)<1, P>0.1]. This was due to the current MDMA-using group and the ex-MDMA using group reducing the number of commission errors on non-shift blocks compared to shift blocks when targets were sad, but not when targets were happy. Both control groups, by contrast, showed a similar difference in errors between shift and non-shift blocks on happy and sad target blocks (see Fig. 2—groups pooled in figure for clarity).
Correlational measures
Within the current MDMA-using group, BDI score correlated with life exposure to MDMA (r=0.43, P=0.02) and frequency of MDMA use (r=0.43, P=0.02). As expected, life exposure to MDMA also correlated with frequency of MDMA use (r=0.4, P=0.004). When controlling for frequency of MDMA use, the correlation between life MDMA exposure and BDI was no longer significant (r=0.24, P=0.1). BDI score also correlated with amyl nitrate exposure in the three drug using groups (ρ=0.44, P=0.001).
On the Affective Go/No-go task, within the three illicit drug-using groups, cannabis use over the last month correlated with total commission errors (ρ=0.30, P=0.007) and total omission errors (ρ=0.38, P=0.0005). However, total commission errors and total omission errors were also correlated (ρ=0.42, P=0.0001), and only the correlation with omission errors survived partial correlation.
Discussion
Depression and cognitive function in MDMA users
The results of this study show that current and ex-MDMA users scored higher on a self-report depression scale than drug naïve controls. However, there was no difference in depression score between the current MDMA-using group and a control group that was well matched for other illicit drug use. The MDMA users did not show the attentional cognitive bias towards negative words seen in clinically depressed populations on the Affective Go/No-go task. Our data do not support the hypothesis that elevated depression rating scale scores in MDMA users are due to the somatic side-effects of MDMA (such as sleep disturbance and weight loss), as the results from the cognitive and somatic subscales mirror the overall BDI results in our data.
To our knowledge, seven other studies have investigated depression in MDMA users while attempting to control for other illicit drug use. One study employing the HDRS reported a difference from a poly-drug control group, but the groups were not well matched for illicit drug use (Gamma et al. 2001). Another, employing the depression sub-scale of the Symptom Check List 90 (SCL-90), showed increased scores for current, but not ex-MDMA users (Morgan et al. 2002). Five studies have reported no difference between a current MDMA-using group and poly-drug controls, three using the depression sub-scale from the SCL-90 and one using the BDI (Parrott et al. 2000, 2001; Verheyden et al. 2002; Thomasius et al. 2003). One study using the BDI reported higher scores in current MDMA users compared to controls, but this difference did not survive statistical correction for baseline differences between the groups (Verkes et al. 2001). All of these studies also failed adequately to match for non-MDMA illicit substance use between the groups. One of these studies also reported cognitive and somatic symptoms of the BDI in MDMA users separately (Verheyden et al. 2002). This study examined the “mid-week low” phenomenon, and found that 4 days after ecstasy exposure, MDMA users showed increased scores for cognitive symptoms, but no change for somatic symptoms.
In this study, the current MDMA using-group was well matched with the poly-drug control group on demographic variables, alcohol and nicotine consumption. In addition, the MDMA-using group was well matched with the poly-drug control group on measures of illicit substances other than MDMA, and both groups had only occasional use of illicit substances other than cannabis. Such matching is important as some of the behavioural measures in this study were correlated with measures of illicit drug use other than MDMA. In particular, total omission errors on the Affective Go/No-go task was correlated with cannabis use, and BDI score was correlated with amyl nitrate use. These correlations with other measures of illicit drug use are worthy of further investigation.
The failure to find a difference between the current MDMA-using group and the poly-drug control group indicates that the increased depression rating scale scores reported by MDMA users may not necessarily be due to MDMA use. One weakness of all studies relying on self-report of drug use is that abused substances are unlikely to be pure. It is therefore possible that subjects in the poly-drug control group in our study who took substances such as amphetamine could have unwittingly been exposed to small amounts of MDMA. However, quantities of MDMA accidentally ingested in this manner are likely to be small and the effects are probably minor.
Our study, like others, did find a correlation between measures of MDMA consumption, in particular frequency of use, and BDI score (MacInnes et al. 2001). However, it is impossible unambiguously to infer from this correlation, or indeed from any result of a cross-sectional study, that MDMA use causes depression, as it is possible that symptoms of depression predated the onset of MDMA use. As many ecstasy users are young men, who are the group least likely to seek help from primary medical care (Carr-Hill et al. 1996), it is possible that subjects who report symptoms of depression are using MDMA more frequently precisely to alleviate those symptoms. Consistent with this hypothesis, at least one study has reported that MDMA users currently suffering from depression experienced symptoms before the onset of MDMA use (Gamma et al. 2001). Nevertheless, it is also possible that MDMA, which is known to cause damage to the serotonin system, could exacerbate existing depressive symptomatology, or even lead to depression in vulnerable individuals. Only a longitudinal study of MDMA use and depression will be able to answer the question of whether MDMA use necessarily causes depression, or if depression increases the likelihood of MDMA use.
Attentional bias and affective processing: comparison with depression and tryptophan depletion
A further important finding in this study is that MDMA users do not resemble other depressed groups in their attentional cognitive bias, as measured by reaction time on the Affective Go/No-go task. Previous studies have shown that depressed patients show slowed responding to positive words on this task (Murphy et al. 1999). MDMA users did not show this negative affective bias.
However, MDMA users did show an interesting pattern of errors. While both control groups showed no difference between happy and sad blocks in terms of reducing the commission error rates from shift to non-shift blocks, the current and ex-MDMA users showed a different pattern. As expected, they exhibited a reduction in commission errors between shift and non-shift blocks when the targets were sad words, but showed no difference between shift and non-shift blocks when targets were happy words (see Fig. 2).
The interpretation of this result is not entirely clear. However, it appears that like adults and adolescents with depression, attention towards negatively toned stimuli is heightened in MDMA users (Murphy et al. 1999; Kyte et al., unpublished data). Rubinzstein et al. (2001) suggested that tryptophan depletion in healthy volunteers, which causes a temporary reduction of central 5-HT, produces a labile affective state that influences memory retrieval in this Affective Go/No-go task, even in the absence of an overt subjective change in mood. It is possible that chronic ecstasy use, which is thought to cause long-lasting changes in the serotonin system, produces a permanent state akin to tryptophan depletion in MDMA users.
Acute tryptophan depletion causes reduced activity in a number of areas including the orbital prefrontal cortex and anterior cingulate cortex (Bremner et al. 1997; Smith et al. 1999). It is possible that chronic ecstasy use may also affect these areas, which are known to be important not only in the regulation of mood in both healthy volunteers (Mayberg et al. 1999) and depressed patients (Drevets et al. 1997), but also in the performance of the Affective Go/No-go task (Elliott et al. 2000, 2002). However, further research is necessary to elucidate the precise nature of this result.
Summary
This study compared a current MDMA-using group, an ex-MDMA-using group, a poly-drug control group and a drug-naive control group on the Beck Depression Inventory and the Affective Go/No-go task. The current MDMA-using group differed from the drug-naive control group, but not from the poly-drug control group, on the BDI, suggesting that the increased depression score in MDMA-using groups reported in previous studies cannot be attributed to ecstasy use alone. The groups did not differ in terms of affective bias on the Affective Go/No-go task. However, the MDMA-using groups did not show an expected difference in commission errors between shift and non-shift trials when targets were happy words. These data do not suggest that ecstasy use alone can account for increased levels of self-reported depression in MDMA-using groups. However, the data from the Affective Go/No-go task are concordant with the hypothesis that MDMA causes long-term reductions in serotonin system function, sufficient to influence cognitive processing of emotionally toned material.
Acknowledgements
This study was funded by a Wellcome Trust Program Grant (no. 019407) to Trevor Robbins, Barbara Sahakian, Barry Everitt and Angela Roberts, and was completed within the MRC Centre for Behavioural and Cognitive Neuroscience. J.P.R. was funded by a Medical Research Council Studentship. Many thanks to Caroline Humphries and all the staff at the Wellcome Trust Clinical Research Facility, Addenbrooke’s Hospital, for their help and support. We would also like to thank Professor Trevor Robbins and Dr. Andrew Blackwell for valuable discussion of the manuscript, and the Cambridge Evening News for their support in recruiting participants.
References
- Beck AT, Ward CH, Medelson M, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- Carr-Hill RA, Rice N, Roland M. Socioeconomic determinants of rates of consultation in general practice based on fourth national morbidity survey of general practices. BMJ. 1996;312:1008–1012. doi: 10.1136/bmj.312.7037.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Travill RA. Mood and cognitive effects of ±3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”): weekend “high” followed by mid-week low. Addiction. 1997;92:821–831. [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Gamma A, Buck A, Berthold T, Vollenweider FX. No difference in brain activation during cognitive performance between ecstasy (3,4-methylenedioxymethamphetamine) users and control subjects: a [H2(15)O]-positron emission tomography study. J Clin Psychopharmacol. 2001;21:66–71. doi: 10.1097/00004714-200102000-00012. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Ferri M, Zambelli U, Timpano M, Neri E, Marzocchi GF, Delsignore R, Brambilla F. Long-lasting effects of (±)3,4-methylene-dioxymethamphetamine (ecstasy) on serotonin system function in humans. Biol Psychiatry. 2000;47:127–136. doi: 10.1016/s0006-3223(99)00180-8. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (±)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci. 1999;19:5096–5107. doi: 10.1523/JNEUROSCI.19-12-05096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS. Striatal serotonin is depleted in brain of a human MDMA (Ecstasy) user. Neurology. 2000;55:294–296. doi: 10.1212/wnl.55.2.294. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Handley SL, Harding GF. Former chronic methylenedioxymethamphetamine (MDMA or ecstasy) users report mild depressive symptoms. J Psychopharmacol. 2001;15:181–186. doi: 10.1177/026988110101500310. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vazquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli—a meta-analytic review. Clin Psychol Rev. 1992;12:227–255. [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Peroutka SJ. Neurochemistry and neurotoxicity of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) J Neurochem. 1990;54:14–22. doi: 10.1111/j.1471-4159.1990.tb13277.x. [DOI] [PubMed] [Google Scholar]
- McLean A, Rubinsztein JS, Robbins TW, Sahakian BJ. The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology. 2003 doi: 10.1007/s00213-003-1586-8. in press. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, McFie L, Fleetwood H, Robinson JA. Ecstasy (MDMA): are the psychological problems associated with its use reversed by prolonged abstinence? Psychopharmacology. 2002;159:294–303. doi: 10.1007/s002130100907. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology. 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Lasky J. Ecstasy (MDMA) effects upon mood and cognition: before, during and after a Saturday night dance. Psychopharmacology. 1998;139:261–268. doi: 10.1007/s002130050714. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Sisk E, Turner JJ. Psychobiological problems in heavy “ecstasy” (MDMA) polydrug users. Drug Alcohol Depend. 2000;60:105–110. doi: 10.1016/s0376-8716(99)00146-5. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Milani RM, Parmar R, Turner JD. Recreational ecstasy/MDMA and other drug users from the UK and Italy: psychiatric symptoms and psychobiological problems. Psychopharmacology. 2001;159:77–82. doi: 10.1007/s002130100897. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Buchanan T, Scholey AB, Heffernan T, Ling J, Rodgers J. Ecstasy/MDMA attributed problems reported by novice, moderate and heavy recreational users. Hum Psychopharmacol. 2002;17:309–312. doi: 10.1002/hup.415. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet. 2001;358:1864–1869. doi: 10.1016/S0140-6736(01)06888-X. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Finnegan KF, Nichols DE, DeLanney LE, Irwin I, Langston JW. 3,4-Methylenedioxyethylamphetamine (MDE), a novel analogue of MDMA, produces long-lasting depletion of serotonin in the rat brain. Eur J Pharmacol. 1987;137:265–268. doi: 10.1016/0014-2999(87)90233-0. [DOI] [PubMed] [Google Scholar]
- Rubinzstein JS, Rogers RD, Riedel WJ, Mehta MA, Robbins TW, Sahakian BJ. Acute dietary tryptophan depletion impairs maintenance of “affective set” and delayed visual recognition in healthy volunteers. Psychopharmacology. 2001;154:319–326. doi: 10.1007/s002130000655. [DOI] [PubMed] [Google Scholar]
- Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (“ecstasy”) users. Br J Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- Smith KA, Morris JS, Friston KJ, Cowen PJ, Dolan RJ. Brain mechanisms associated with depressive relapse and associated cognitive impairment following acute tryptophan depletion. Br J Psychiatry. 1999;174:525–529. doi: 10.1192/bjp.174.6.525. [DOI] [PubMed] [Google Scholar]
- Tavares JV, Drevets WC, Sahakian BJ. Cognition in mania and depression. Psychol Med. 2003;33:959–967. doi: 10.1017/s0033291703008432. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology. 2003;167:85–96. doi: 10.1007/s00213-002-1383-9. [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Hadfield J, Calin T, Curran HV. Sub-acute effects of MDMA (±3,4-methylenedioxymethamphetamine, “ecstasy”) on mood: evidence of gender differences. Psychopharmacology. 2002;161:23–31. doi: 10.1007/s00213-001-0995-9. [DOI] [PubMed] [Google Scholar]
- Verkes RJ, Gijsman HJ, Pieters MS, Schoemaker RC, de Visser S, Kuijpers M, Pennings EJ, de Bruin D, Van de Wijngaart G, Van Gerven JM, Cohen AF. Cognitive performance and serotonergic function in users of ecstasy. Psychopharmacology. 2001;153:196–202. doi: 10.1007/s002130000563. [DOI] [PubMed] [Google Scholar]