Abstract
A novel three-layer microfluidic polydimethylsiloxane (PDMS) device was constructed with two fluid chambers that holds a brain slice in place with microposts while maintaining laminar perfusate flow above and below the slice. Our fabrication technique permits rapid production of PDMS layers that can be applied to brain slices of different shapes and sizes. In this study, the device was designed to fit the shape and thickness (530-700 μm) of a medullary brain slice taken from P0-P4 neonatal rats. Medullary slices in this chamber spontaneously produced rhythmic, respiratory-related motor output for up to 3 h, thereby demonstrating that brain slice viability was maintained for prolonged periods. This design is unique in that it achieves independent control of fluids through multiple channels in two separate fluid chambers. The laminar flow exhibited by the microfluidic chamber allows controlled solutions to target specific areas of the brain slice based on the input flow rates. To demonstrate this capability, a stream of Na+-free solution was focused on one half of a medullary slice to abolish spontaneous neural activity in only that half of the brain slice, while the other half remained active. We also demonstrated that flow of different solutions can be focused over the midline of the brain slice. The multilayer brain slice chamber design can integrate several traditional types of electrophysiology tools that are commonly used to measure neurophysiological properties of brain slices. Thus, this new microfluidic chamber is advantageous for experiments that involve controlled drug or solution delivery at high spatiotemporal resolution.
Introduction
Viable, electrically active brain slices are widely used for neurophysiological studies because they provide access to intact neural networks and permit analysis of underlying cellular and synaptic mechanisms. Maintaining brain slice viability is critical to sustaining the electrophysiological properties of the intrinsic neural networks. Brain slices are typically kept alive in a Haas perfusion chamber with oxygenated artificial cerebrospinal fluid (ACSF) passing over the slice.1 Typical brain slice chambers2-7 immobilize brain slices either by pinning the slices down onto a substrate (e.g., PDMS, nylon net), or by laying a metal mesh on top of the slice. Slices that are superfused only on the top surface will have oxygen, pH, and ion gradients produced within the slice. To circumvent this problem, brain slices have been immobilized on a nylon mesh that allows flow to occur above and below the brain slice. However, these chamber designs do not have finely controlled solution flow over the brain slice surfaces. Instead, they produce unstirred layers near the slice surface and small eddy currents around the brain slice (depending on the chamber volume). In typical brain slice chambers, local drug or solution application is accomplished by local pressure microinjection using a micropipette, which only affects small regions in the brain slice, making its application to larger slice regions problematic.
Several variables must be considered when designing a tissue preparation and perfusion system. For example, thicker slices allow for greater synaptic connectivity between neurons and better dendritic integrity, but the limitation is that tissue oxygen demand must not exceed the diffusion-limited oxygen delivery capacity of the perfusion bath.8 Also, chemicals or drugs are often added to the ACSF to alter neuronal activity and gain insight into various biological phenomena. In most of these experiments, however, the entire brain slice is exposed to the chemicals or drugs, with no provision for applying substances to specific regions of brain slices. Solution application at high spatiotemporal resolution is important because different areas of the brain, such as the cortex, are organized into discrete layers of cell bodies, dendrites, and axonal processes. Likewise, other parts of the brain are organized into separate nuclei that perform different functions. Thus, having the ability to apply different solutions to specific regions of brain slices under in vitro conditions is highly desirable because it provides versatility in addressing specific scientific questions. We have developed a microfluidic brain slice chamber (Fig. 1) that can be used to apply solutions to specific brain slice regions while maintaining adequate viability.
Fig. 1.
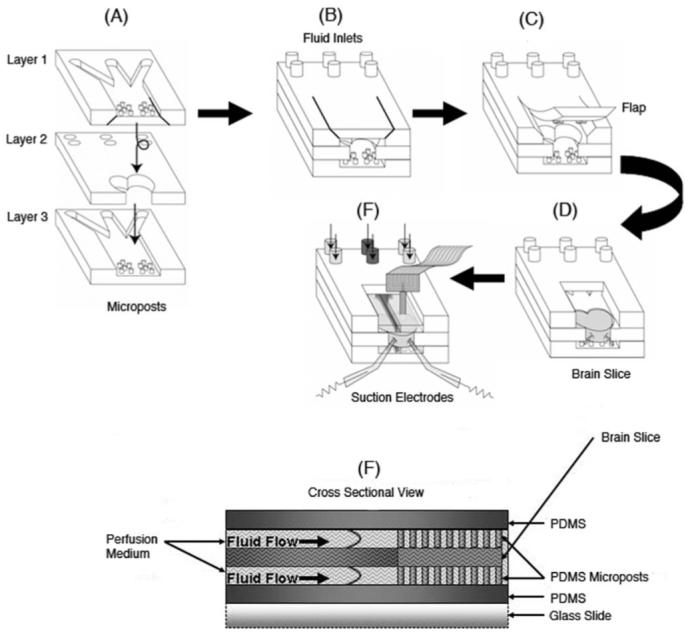
Schematic of the PDMS three-layer microfluidic slice chamber construction. (A) Masks with three distinct designs (1, 2 and 3) were used to pattern the three different PDMS layers. (B) The three layers were assembled and the top layer was cut to generate a flap that could be opened (C) to allow insertion of a brain slice in the middle layer (D). (E) Suction electrodes attached to nerve roots allowed recording of spontaneous respiratory motor output. The flap was removed in (D) and (E) for clarity (F). Cross section displaying the side view of the general fluid velocity profiles for this device. The brain slice fits into the middle layer. Microposts patterned on the top and bottom layers hold the brain slice in place.
A modified version9 of a classical slice chamber10 has been recently developed and been shown to achieve superior perfusion characteristics by using a base layer of microposts instead of the nylon mesh over which the brain slice is typically placed. We have patterned arrays of microposts above and below the brain slice in a novel microfluidic chamber design that also provides independent control of multiple fluids through two separate fluid chambers.
Microfluidics has emerged as a powerful technology in the biological community 11-18 because it offers advantages over classical approaches. A reduction in feature size gives control over fluid phenomena such as laminar flow, shear stresses, and velocity profiles. These characteristics have been manipulated in our novel multilayer perfusion chamber to promote a favorable brain slice environment, both for tissue health and experimental purposes. This microfluidic chamber permits all of the following:
Laminar flow allowing multiple fluids to be independently directed above and below the brain slice.
Localized exposure of both surfaces of the brain slice to fluids. This improves chemical delivery via diffusion to the brain slice, and thus enhances slice viability.
Adaptation to any brain slice shape with short fabrication turnover times using rapid prototyping.
Batch processing to produce inexpensive identical devices.
Compatibility with typical electrophysiology tools found in many laboratories.
Future integration of sensors and microelectrode arrays.
Materials and methods
Design of microfluidic device
Conventional perfusion chambers use common materials, such as Plexiglas, glassware, nylon mesh, and sometimes metallic parts. While these materials are easily accessible, PDMS was chosen as the construction material because it offers several key advantages. The ability to rapid prototype devices with features on the microscale, gives microfluidics a huge advantage over conventional approaches that provide little, if any, fine control over the microenvironment of the brain slice. The material properties of this PDMS perfusion device meet the standards of existing perfusion chambers (sterility, etc.) and additionally offer the flexibility to quickly change experimental parameters (e.g. adding more inputs, modifying center layer to fit different slice shapes or thicknesses, etc.). Rapid protoyping can be used to arrive at the optimum design, after which perfusion devices can be batch-produced with short turnover times. Hence we have developed a microfluidic chamber that uses inexpensive, easily modified materials that provide maximum experimental flexibility.
A three-layered device was fabricated (Fig. 1A-E) in PDMS, and permanently bonded to a standard glass slide to provide a rigid substrate for mounting within an experimental apparatus. The brain slice is inserted in the middle layer, which can be designed to fit different slice shapes and thicknesses. Arrays of microposts9 were patterned on the top and bottom layers. The microposts hold the brain slice in place without compromising its mechanical integrity, keep the perfusate flow restricted to within the chamber by surface tension effects, and ensure maximum surface contact between the brain slice and the perfusate. The microposts were designed so as to optimize the diameter and spacing. The microposts cannot be too large in diameter or too closely spaced since this will inhibit diffusion of nutrients to the brain slice, resulting in a higher rate of neural cell death. The microposts should not be too small in diameter or spaced too far apart, to prevent indentation of the brain slice by unequal distribution of its weight on the microposts. Indentation of the brain slice may alter the mechanical integrity of the neural network residing in the brain slice, leading to distortions in the electrical recordings.
Syringe pumps are used to achieve independent control of flow rates for three inputs in the top and the bottom fluid chambers each, which enables controlled localized exposure to perfusate on both top and bottom of the brain slice. This circumvents the generation of oxygen, pH and ion gradients seen in single chamber devices, and improves viability through controlled diffusion on both the top and bottom of the brain slice. The two fluid chambers were designed with three ∼1mm diameter inlets. The chamber width is 5.5 mm to encompass the medullary brain slice size. The design can be easily modified and fabricated to fit any brain slice dimension within days. This process involves taking a microscope image of a desired slice, scaling it and importing it into the mask design software.
Microfluidic chamber fabrication
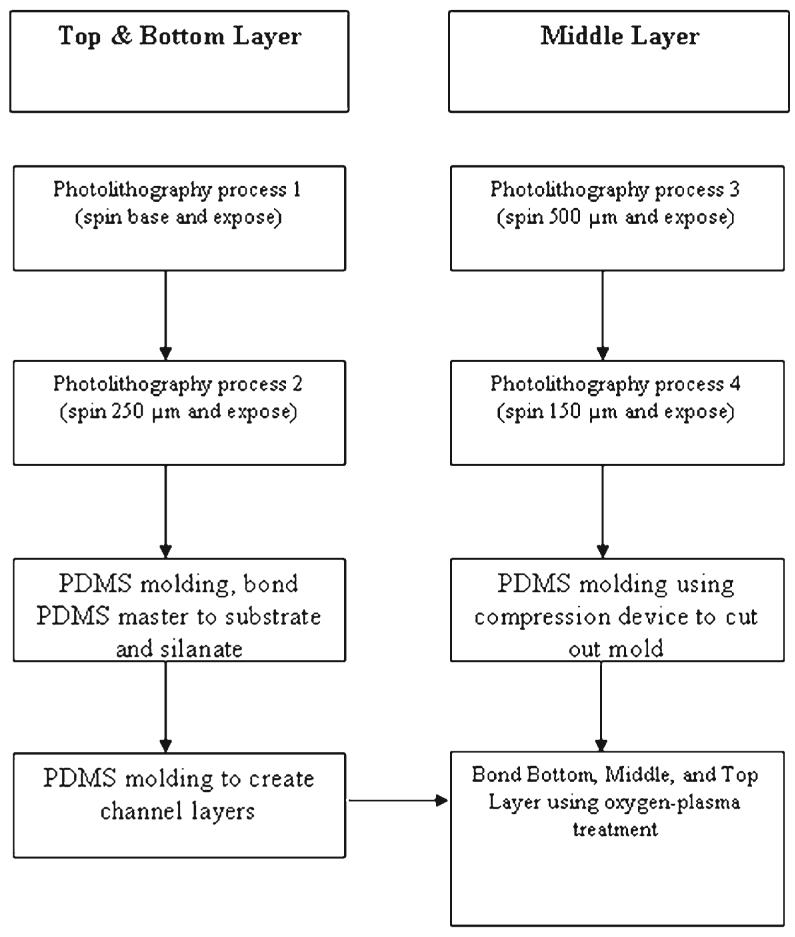
Standard photolithography and microfabrication techniques were implemented for microfluidic device construction. The three different layer patterns were drawn using image software (Adobe Illustrator 10.0) and then printed (3600 dpi) on a transparency film using a high resolution printer. This resolution results in feature sizes down to 10 μm, which is more than adequate for our mask design. Silicon wafers with a diameter of 7.5 cm were used as the substrate material.
The fabrication process (Fig. 2) utilized in the construction of the top and bottom PDMS microfluidic channel layers began with curing a thin base layer (30-40 μm) of negative photoresist (SU-8 50, MicroChem Inc.) to the surface of the silicon wafer. Next, an SU-8 master is created that is a positive of the final PDMS channel design. The base was used to enhance micropost adhesion, thereby peventing them from breaking off the master after removing the cured PDMS. The SU-8 master is used to cast a PDMS master mold (negative). The PDMS master is then used to cast the desired PDMS microfluidic channel layer. The PDMS master is silanized so that the PDMS prepolymer poured on the master can be removed after curing.16 A PDMS master was used due to the short fabrication times (2 h) compared to making multiple similar SU-8 masters (up to 10 h) for batch processing. PDMS is advantageous since it is flexible and releases the microposts more easily than an SU-8 master would.16
Fig. 2.
Process flow chart showing the method of creating the masters for constructing the different layers. The photolithography process consisted of spinning the substrate, prebaking, UV exposure at 365 nm, and postbaking.
The middle layer is constructed differently from the top and bottom layers because of the need to have a slice with the shape of the brain slice cut out of the PDMS. The middle layer of the three-layer device was constructed using a 650 μm negative SU-8 master mold that was fabricated to match the desired brain slice shape and thickness. To achieve the exact dimensions of the brain slice, a brain slice picture was taken through a microscope and imported into Adobe Illustrator 10.0 to make the brain slice layer mask. The fabrication process is delineated in Fig. 2. The hot plate used to cure the PDMS on the brain slice master is integrated into a compression stand (constructed in house). The fabrication process used the following order of stacking materials on the hot plate: master, PDMS prepolymer, transparency, polycarbonate, metal compression foot. The transparency serves as a flexible interface to peel off after curing, while the polycarbonate distributes the pressure and reduces stress on the silicon wafer. The final result was a 500-700 μm middle layer shaped to house a medullary tissue slice, as shown in Fig. 1D.
The three layers were aligned and permanently bonded together (Fig. 1A-B) using an oxygen-plasma treatment. The top layer was cut to generate a flap that could be opened (Fig. 1C) to allow insertion of a brain slice (Fig. 1D). The final device was permanently bonded to a standard glass slide to provide a rigid substrate for mounting within the experimental apparatus which included suction electrodes that can be attached to nerve roots to record spontaneous respiratory motor output (Fig. 1E). The top and bottom channel inputs are completely separated from one another by the middle layer, thus enabling independent control of the fluids and easy flow adjustments to correct any misalignments. The center area that did not contain any microposts on the bottom and top layers was aligned along the midline of the brain slice layer (Fig. 1A). Fluid flow rates could be altered on the syringe pumps to maintain a focused laminar flow down the centerline area of the brain slice for the bottom and top channels (Fig. 1E). Variations in cutting the top layer flap (Fig. 1C) did not have any influence on the experiment because the PDMS was rigid and clean enough to form a seal after inserting the brain slice and pushing the flap down.
Modeling and simulations
A similar post design was analyzed9 with the exception of the space separating the two sets of posts down the midline. Thus finite element analysis was utilized to gain a better understanding of any change in the fluid interactions in the fluid chamber layers of our multilayer perfusion chamber. Before simulations could be performed, the device design was drawn (Fig. 3) using Gambit (version 2.1.6, Fluent Inc.) and meshed using triangular mesh elements.
Fig. 3.
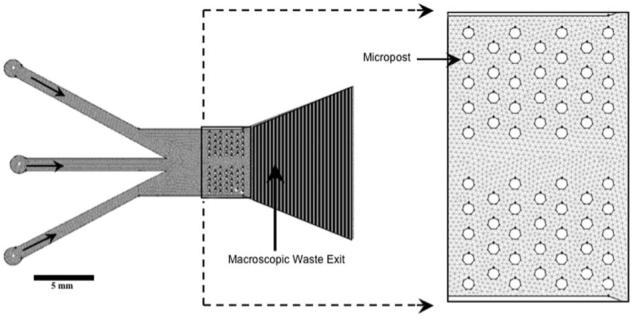
The top and bottom layers of the multilayer perfusion chamber have the following dimensions (in mm): Inlet diameter = 1.75; width of inlet pipes = 1.025; post diameter = 0.22; post height= 0.250. The following parameters were set up for the mesh in Gambit: Interval size of the face mesh = 1 mm; boundary layer width = 10 μm; interval size of the edge mesh of the outer wall = 100 μm; interval size of the edge mesh of the post walls = 50 μm.
The meshed device was imported into Fluent (Release 6.1.22, Fluent Inc.), a commercially available software for computational fluid dynamics. Simulations were performed to analyze and compare variations in velocity profile due to changes in flow velocity (Fig. 4A-D). A multiphase volume of fluid (VOF) model was used. Two phases were defined, both being the same material (liquid water) with no surface tension interaction between them and no wall adhesion forces. The pressure at the outlet was set to zero to indicate free flow uninhibited through the end of the chamber. The velocities of the inlets were varied in two different simulation trials. The two outer inlets were always set to the same velocity (10 mm s-1), while the middle inlet velocity was varied from 0 to 10 mm s-1. These flow rates are comparable to those used in typical perfusion chambers.19
Fig. 4.
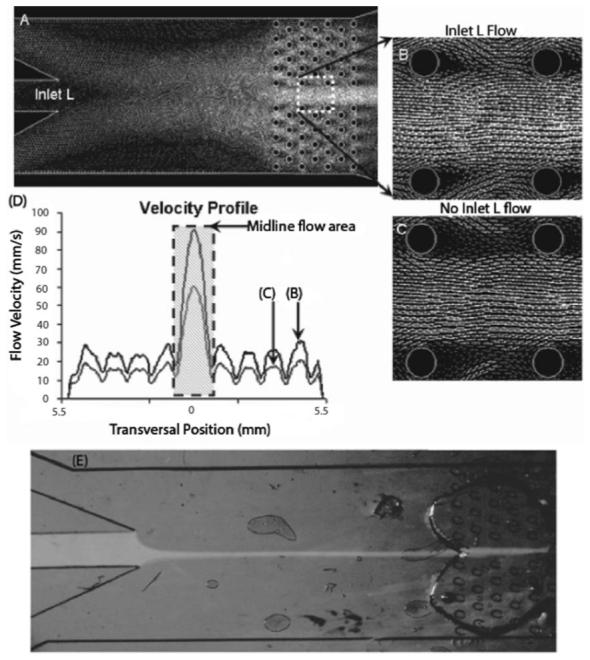
Modeling of the perfusion chamber design was done using Gambit and Fluent (A-C). A plot of the fluid flow velocities (D) during inlet L fluid flow at 10 mm s-1 (B) and 0 mm s-1 (A, C), revealed that removing microposts from the midline achieves more spatial control down the midline of the brain slice (shaded region of D) than through the micropost area. The velocity magnitude is proportional to the gray-scale intensity of the flow vectors. Demonstration of a focused flow of Na+-free solution dye, pumped through the middle inlet by a syringe pump, goes through the midline of the PDMS chamber and brain slice area without any interference from microposts, while clear fluid is pumped through the two side inlets (E).
Several variants of the chamber geometry, micropost size and spacing were modelled and simulated to arrive at an optimum design (see Results and discussion). Simulations were used to quantify areas of stagnant flow and calculate the flow velocities through a transverse cross section of the brain slice area (Fig. 4D). Two cases are depicted in Fig. 4B-C, first in which the fluid flow is 10 mm s-1 for all three inlets (Fig. 4B), and the second, in which the outer two inlets maintain a flow of 10 mm s-1, with no flow through the center inlet (Fig. 4A and C). The velocity of fluid through the uninterupted midline area had a maxima of 90.7 mm s-1 for the first case and a maxima of 60.4 mm s-1 for the second (Fig. 4D).
The fluid velocities through the micropost arrangement consistently varied within 13 mm s-1 for both cases (11-24.5 mm s-1 for the first case; 7.44-20.8 mm s-1 for the second). The design of the microposts allows high flow rates for the midline, while maintaining a stable, non-stagnant flow in the microposts area, providing much more flexibility in altering the desired fluid exchange rates than is possible in existing perfusion chamber systems. The technology can be extended to other experimental studies by altering the micropost design to a specific pattern to provide very flexible fluid exchange rates over specific areas of the brain slice being studied and use focused flow to concentrate a specific solution over the uninterupted area of the brain slice. Fig. 4E demonstrates how a steady, focused flow of Na+-free solution dye, pumped through the middle inlet by a syringe pump, goes through the midline of the PDMS chamber and brain slice area without any interference from microposts, while clear fluid is pumped through the two side inlets. The fluid flow rates through the inlets can be varied independently by the syringe pumps, to maintain a focused laminar flow down the centerline area of the brain slice for either the bottom chamber, or the top chamber, or both.
Medullary slice preparations
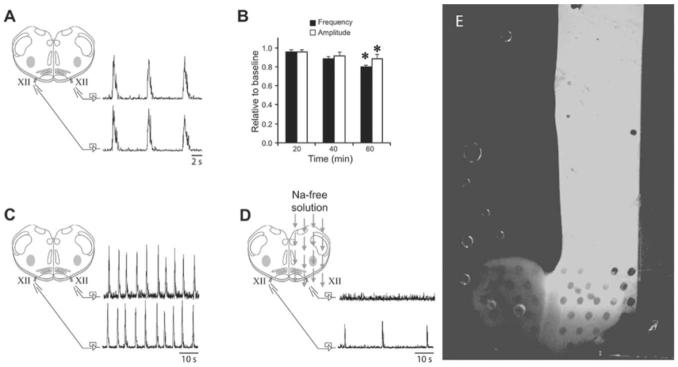
All procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison School of Veterinary Medicine. Sprague-Dawley rats (P1-P4) were anesthetized with isoflurane (5%, balance oxygen) and decerebrated. The brainstem and cervical spinal cord were isolated in ice-cold standard solution and pinned onto a wax-covered plastic chuck. Standard solution contained (in mM): 120 NaCl, 26 NaHCO3, 20 glucose, 1.25 Na2HPO4, 1.0 CaCl2, 2.0 MgSO4, 9.0 KCl (equilibrated with 95% O2/5% CO2 to get pH ∼ 7.35). A vibratome (Leica VT1000S, Leica Microsystems Inc.) was used to serially section the rostral brainstem in the transverse plane to within 100-150 μm of the pre-Bötzinger Complex (preBötC) rostral boundary. The preBötC is hypothesized to be a critical site for respiratory rhythm generation.20 One transverse slice (530-700 μm thick) containing the preBötC (located bilaterally) was cut (Fig. 5A) and transferred to a microfluidic chamber. In microfluidic chambers, slices were bathed with standard solution at room temperature (flow rate = 2-3 ml min-1). In one experiment, Na+-free solution was applied to the right half of the slice in both the top and bottom chambers (Fig. 5D). The Na+-free solution consisted of (in mM): 120 choline chloride, 26 choline bicarbonate, 20 glucose, 1.25 K2HPO4, 1.0 CaCl2, 2.0 MgSO4, 7.75 KCl (equilibrated with 95% O2/5% CO2 to get pH ∼ 7.35). To record respiratory motor bursts, glass suction electrodes were attached to hypoglossal (XII) nerve rootlets (Fig. 5A and C). Signals were amplified (10 000×) and band-pass filtered (1-500 Hz) using a differential AC amplifier (model 1700, A-M Systems, Everett, WA) before being rectified and integrated (time constant = 50 ms) using a moving averager (MA-821/RSP, CWE, Inc., Ardmore, PA) (Fig. 5A-D). Burst analysis was performed using Clampfit 9.0 software (Axon Instruments, Foster City, CA). All measurements were averaged into 20 min bins and reported as mean ± SEM.
Fig. 5.
(A) Rythmic respiratory motor bursts from left and right XII roots during fluid flow in a microfluidic chamber. (B) Time-dependent changes in burst frequency and amplitude are small under these conditions. (C) Standard solution flowing simultaneously over separate halves of the brain slice allowed simultaneous bursts to be recorded on both XII nerve roots. Fig. 5A and C display similar configurations with different time scales to distinguish separate experiments. (D) Superfusion of the right half of the slice with Na+-free solution showed that rhythmic XII activity was blocked on the right half while XII activity could be recorded on the left half, thereby showing that the slice microenvironment can be selectively altered. (E) Distilled solution containing concentrated fluorescent dye was used in conjunction with a standard solution to verify flow over the 650 μm brain slice in a microfluidic chamber. The two solutions maintained equal flows ranging from 0.5 to 1 ml min-1, thereby exposing the two halves of the brain slice to different environments. * = P < 0.05.
Results and discussion
Observing the brain slice through the microscope, the posts did seem to make small impressions (∼220 μm diameter, ∼20 μm depth) on the slice surfaces, but the slice was still able to spontaneously produce rhythmic respiratory-related motor output comparable to that obtained using classical perfusion chambers (Fig. 5A-D). After modeling and simulating several variants of the chamber geometry and microposts pattern, a design was achieved that met the appropriate standards for a brain slice perfusion chamber. The microposts are arranged quincunx (diagonally-alternating pattern) (Fig. 3) to optimize flow distribution of oxygen/nutrients to the tissue.9 The microposts are 250 μm high with a diameter of 200 μm, vertical spacing of 521 μm, and horizontal spacing of 456 μm, spanning the top and bottom area of the brain slice. This configuration adequately supports the brain slice, while maintaining adequate flow volumes above and below the brain slice. The arrangement of the microposts in the chamber consists of a span of 150 μm in the center uninterrupted by microposts (Fig. 3). This layout of the microposts achieves a higher variation in fluid exchange rates down the midline of the brain slice compared to the area consisting of microposts. The uninterupted area in the center of the chamber enables the use of laminar flow to focus a specific solution down the brain slice center at different flow speeds compared to that through the micropost area, while still permitting fine control over the spatial resolution. Different micropost patterns create different laminar flows, demonstrating a means by which specific brain slice areas can be targeted by solutions (Fig. 4E and 5E).
Control of solution in microfluidic chamber
Fluid tests were performed on the brain slice chamber to verify the stability of the brain slice at different laminar flow rates. Fluid flow rates reached as high as 1 ml min-1 for each of the inlets in the fluid chambers. Even at a combined inlet flow rate of 3 ml min-1, the mechanical integrity of the brain slice was not compromised, as evidenced by the brain slice’s ability to spontaneously produce rhythmic respiratory-related motor output. The brain slice stayed in place with high flow rates and did not float away. Next, distilled solution containing concentrated fluorescent dye was used in conjunction with a standard solution to verify that stable laminar flow was maintained over the brain (Fig. 5E). Each of the outer inlets were set to an equal flow rate, 0.5 ml min-1, and it was observed that the two halves of the brain slice of the neonatal rat were exposed to different solutions simultaneously. Laminar flow can be further exploited to perform pulses or gradients of different solutions and monitor the changes in electrical activity of the targeted neural network.21 More inlets may be added to the device to further increase combinations of solutions exposed over the brain slice region, thereby increasing productivity in having multiple experimental environments within one brain slice chamber that can be monitored.
Brain slice viability and control of function
Slices (n = 5) placed in the multilayer PDMS microfluidic chamber spontaneously produced rhythmic motor bursts which reached a stable frequency and amplitude within 20 min (Fig. 5A). Two slices were allowed to produce rhythmic motor bursts for >3 h. Burst frequency (baseline = 0.18 ± 0.02 Hz) and amplitude significantly decreased by 12 ± 1% and 20 ± 5%, respectively, after 60 min (Fig. 5B), but optimizing conditions to maintain stable respiratory motor output were beyond the scope of this manuscript. The main point was to show that the brain slices were viable for sufficient time to perform typical experiments, and the rhythmic burst activity observed prove that this was the case.
In a separate experiment (n = 1), baseline activity was recorded from both left and right XII roots while the slice was bathed in standard solution (Fig. 5C). The fluid bathing one half of the slice was then switched to Na+-free solution to test whether rhythmic activity could be blocked on one half of the slice. After 50 min, the XII root on the half bathed with Na+-free solution was silent while the other XII root continued to produce bursts (Fig. 5D), demonstrating that rhythmic activity was successfully blocked on one half of the slice.
These experiments demonstrate how this perfusion device can be successfully used to conduct novel electrophysiology experiments by passing multiple solutions simultaneously over brain slices. For example, a focused flow of Na+-free or ice-cold solution down the midline of medullary slices (as in Fig. 4E) may uncouple the preBotC respiratory rhythm generators such that each half of the slice bursts asynchronously. Different drugs or solutions could then be applied to one half of the slice (as in Fig. 5E) to alter or transform respiratory motor activity. The midline blockade could then be reversed to determine the extent to which respiratory motor output produced by the coupled preBotCs represents a hybrid motor output. 22Alternatively, in cortical slices with highly organized layers, different solutions could be applied to layers containing either only dendrites or somas, or targeting specific types of neurons. This clearly illustrates the fine control over the microfluidic environment that can provide new methods to study brain slice preparations.
Conclusions
A new multilayer perfusion chamber is demonstrated that perfuses brain slices in a fine and controlled manner using multiple solutions, while recording and monitoring the tissue’s electrical properties with common electrophysiological laboratory equipment. The microfluidic environment promotes controlled laminar flow that offers fast localized exchange rates of solutions. The flexibility in both the fabrication process and flow control allows users to have superior control over the microenvironment and the diffusion parameters they seek to expose to a specific brain slice area. Flow rates of up to 1 ml min-1 for each inlet do not alter the position of the brain slice due to the microposts holding the brain slice in place, while maintaining the viability of the brain slice as displayed in the electrical recordings.
Microfluidics has emerged as a useful tool for biological studies. The novel multilayer PDMS perfusion chamber design has much to offer for brain slice experiments. Microfluidics can be easily integrated with all types of instrumentation 23-27 (e.g., microelectrode array, thermal sensors) to further improve the precision of brain slice experiments, and add to the simplification of the experimental setup and quantification of results.
References
- 1.Dingledine R, Dodd J, Kelly JS. The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J. Neurosci. Methods. 1980;2(4):323–362. doi: 10.1016/0165-0270(80)90002-3. [DOI] [PubMed] [Google Scholar]
- 2.Corrigall WA, Lucato RM. A simple modification to permit fast-flow perfusion of brain slices. Brain Res. Bull. 1980;5(4):481–482. doi: 10.1016/s0361-9230(80)80020-7. [DOI] [PubMed] [Google Scholar]
- 3.Fagni L, Hugon M, Folco A, Imbert GA. Versatile chamber for microphysiologic studies with gas mixtures under high pressure. Undersea Biomed. Res. 1987;14(2):161–168. [PubMed] [Google Scholar]
- 4.Kelso SR, Nelson DO, Silva NL, Boulant JA. A slice chamber for intracellular and extracellular recording during continuous perfusion. Brain Res. Bull. 1983;10(6):853–857. doi: 10.1016/0361-9230(83)90219-8. [DOI] [PubMed] [Google Scholar]
- 5.Koerner JF, Cotman CW. A microperfusion chamber for brain slice pharmacology. J. Neurosci. Methods. 1983;7(3):243–251. doi: 10.1016/0165-0270(83)90006-7. [DOI] [PubMed] [Google Scholar]
- 6.Matthies H, Schulz S, Thiemann W, Siemer H, Schmidt H, Krug M, Hollt V. Design of a multiple slice interface chamber and application for resolving the temporal pattern of CREB phosphorylation in hippocampal long-term potentiation. J. Neurosci. Methods. 1997;78(12):173–179. doi: 10.1016/s0165-0270(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 7.Palovcik RA, Phillips MI. A constant perfusion slice chamber for stable recording during the addition of drugs. J. Neurosci. Methods. 1986;17(23):129–139. doi: 10.1016/0165-0270(86)90066-x. [DOI] [PubMed] [Google Scholar]
- 8.Jiang C, Agulian S, Haddad GG. O2 tension in the adult and neonatal brain slices under several experimental conditions. Brain Res. 1991;568(102):159–164. doi: 10.1016/0006-8993(91)91392-e. [DOI] [PubMed] [Google Scholar]
- 9.Passeraub PA, Almeida AC, Thakor NV. Design, micro-fabrication and analysis of a microfluidic chamber for the perfusion of brain tissue slices. Biomed. Microdev. 2003;5(2):147–155. [Google Scholar]
- 10.Haas HL, Schaerer B, Vosmansky MA. Simple perfusion chamber for the study of nervous tissue slices in vitro. J. Neurosci. Methods. 1979;1(4):323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- 11.Atencia J, Beebe DJ. Controlled microfluidic interfaces. Nature. 2005;437(7059):648–655. doi: 10.1038/nature04163. [DOI] [PubMed] [Google Scholar]
- 12.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 13.Beebe DJ, Moore JS, Yu Q, Liu RH, Kraft ML, Jo BH, Devadoss C. Microfluidic tectonics: A comprehensive construction platform for microfluidic systems. Proc. Natl. Acad. Sci. U. S. A. 2000;97(25):13488–13493. doi: 10.1073/pnas.250273097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruger J, Singh K, O’Neill A, Jackson C, Morrison A, O’Brien P. Development of a microfluidic device for fluorescence activated cell sorting. J. Micromech. Microeng. 2002;12(4):486–494. [Google Scholar]
- 15.Pantoja R, Nagarah JM, Starace DM, Melosh NA, Blunck R, Bezanilla F, Heath JR. Silicon chip-based patch-clamp electrodes integrated with PDMS microfluidics. Biosens. Bioelectron. 2004 15;20(3):509–517. doi: 10.1016/j.bios.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor AM, Rhee SW, Jeon NL. Microfluidic chambers for cell migration and neuroscience research. Methods Mol. Biol. 2006;321:167–177. doi: 10.1385/1-59259-997-4:167. [DOI] [PubMed] [Google Scholar]
- 18.Walker GM, Zeringue HC, Beebe DJ. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4(2):91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 19.Croning MD, Haddad GG. Comparison of brain slice chamber designs for investigations of oxygen deprivation in vitro. J. Neurosci. Methods. 1998;81(12):103–111. doi: 10.1016/s0165-0270(98)00023-5. [DOI] [PubMed] [Google Scholar]
- 20.Onimaru H, Homma I, Feldman JL, Janczewski WA. The para-facial respiratory group (pFRG)/pre Botzinger Complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. J. Appl. Physiol. 2006 doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- 21.Pearce TM, Williams JJ, Kruzel SP, Gidden MJ, Williams JC. Dynamic control of extracellular environment in in vitro neural recording systems. IEEE Trans. Neural Syst. Rehabil. Eng. 2005;13(2):207–212. doi: 10.1109/TNSRE.2005.848685. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: Role of gi/o protein-mediated mechanisms. J. Appl. Physiol. 1996;80(6):2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- 23.Duport S, Millerin C, Muller D, Correges PA. Metallic multisite recording system designed for continuous long-term monitoring of electrophysiological activity in slice cultures. Biosens. Bioelectron. 1999;14(4):369–376. doi: 10.1016/s0956-5663(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 24.Heuschkel MO, Fejtl M, Raggenbass M, Bertrand D, Renaud PA. Three-dimensional multi-electrode array for multisite stimulation and recording in acute brain slices. J. Neurosci. Methods. 2002;114(2):135–148. doi: 10.1016/s0165-0270(01)00514-3. [DOI] [PubMed] [Google Scholar]
- 25.Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP. Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol. Bioeng. 2005;89(1):1–8. doi: 10.1002/bit.20289. [DOI] [PubMed] [Google Scholar]
- 26.Stoppini L, Duport S, Correges PA. New extracellular multirecording system for electrophysiological studies: Application to hippocampal organotypic cultures. J. Neurosci. Methods. 1997;72(1):23–33. doi: 10.1016/s0165-0270(96)00151-3. [DOI] [PubMed] [Google Scholar]
- 27.Stopps M, Allen N, Barrett R, Choudhury HI, Jarolimek W, Johnson M, Kuenzi FM, Maubach KA, Nagano N, Seabrook GR. Design and application of a novel brain slice system that permits independent electrophysiological recordings from multiple slices. J. Neurosci. Methods. 2004;132(2):137–148. doi: 10.1016/j.jneumeth.2003.08.015. [DOI] [PubMed] [Google Scholar]