Abstract
This study used a combination of zonal elution and frontal affinity chromatography on immobilized human serum albumin (HSA) high-performance affinity chromatography (HPAC) column to examine the association constants of various compounds that have been studied by equilibrium dialysis or ultra filtration. A standard plot was generated from retention factors of reference compounds using zonal elution chromatography against association constants of reference compounds using frontal affinity chromatography. The linear relationship was established (r2 = 0.9993) between retention factors and association constants of reference compounds. This standard plot was later used for rapid determination of association constants of various drugs which show low to medium binding affinity to HSA. Association constants of those drugs from this study were compared to that of more generally used methods (i.e., equilibrium dialysis or ultra filtration) from literature and resulted in a relatively high correlation (r2 = 0.945) value. This combination of zonal elution and frontal affinity chromatography method for determining association constants showed several advantages against traditional methods. Depending on drugs of interest, an association constant of drug to HSA can be measured as fast as 1.5 min. Other notable advantages include an ease of automation and its ability to distinguish association constants of chiral compounds at the same time. The same approach could be used for studying interaction of other drugs and proteins and should further improve overall drug screening process.
Keywords: Frontal chromatography, Zonal elution, Human serum albumin, Association constants, Drug–protein interaction, Affinity chromatography
1. Introduction
Quantitative characterization of interactions between small solutes such as drugs to proteins using association constants is routinely performed as part of the drug discovery process [1,2]. Human serum albumin (HSA) is the most abundant plasma protein and many small drugs reversibly bind to HSA [3–5]. These include both endogenous compounds such as bilirubin, long chain fatty acids and steroids as well as exogenous compounds such as warfarin, diazepam and ibuprofen [3–5]. Binding of these small compounds to HSA occur at relatively well-defined regions on HSA and two well characterized binding regions are the warfarin-azapropazone site, which is located in the IIA subdomain of HSA and the indole-benzodiazepine site, which is located in the IIIA subdomain of HSA [3–8].
Equilibrium dialysis and ultra filtration are the most widely used techniques for determining HSA binding constants [9,10]. Other techniques include spectrofluorometry, crystallography, and affinity capillary electrophoresis [11–14]. One major problem associated with these techniques, especially in equilibrium dialysis, is the presence of wide ranges of reference binding constants (i.e., inter-laboratory differences in binding constants) for a given compound to HSA. For example, the reported binding constants of the non-steroidal anti-inflammatory drug (NSAID) ibuprofen range from 2 × 105 M−1 to 3.5 × 106 M−1 [15–17], while the reported binding constants for phenylbutazone, another NSAID, range from 1.4 × 105 M−1 to 1.5 × 106 on HSA [16,18,19]. These values were all obtained using equilibrium dialysis with phosphate buffer (0.067 M, pH 7.4) at 37°C or similar conditions. The large variation in binding constant for a given compound from different laboratories may be due to non-specific binding to HSA and/or the dialysis bag or container, different concentration of HSA, and insufficient eqilibration time.
Another technique for the determination of binding constant between drugs and proteins is high-performance affinity chromatography (HPAC) [20–24]. Its general advantages over other traditional methods are high precision, automation, speed, and good correlation versus solution-based methods [6]. HPAC is based on examining the retention and competition of solutes as they pass through an immobilized HSA column. Both frontal and zonal chromatographies can be used to detemine binding constants in HPAC.
Fronatal analysis utilizes continous application of a known concentration of pure solute to a column containing a fixed amount of an immobilized ligand [6]. As the amount of bound solute in the column increases, it begins to saturate the ligand and form a breakthrough curve. The mean point of this curve is directly related to the concentration of applied solute and the amount of immobilized ligand [6,20,21].
In zonal elution, a known concentration of a competing agent is continuously applied in the mobile phase to a column containing an immobilized ligand while small amounts of the analyte are injected. The binding constant is then calculated from the shift of retention factor on injected analyte [6,25].
Although frontal analysis and zonal elution are effective individual approaches for determining binding constants in HPAC, HPAC can be further improved by combining two approaches to one. In this study, we generated a standard plot using binding constants obtained from the frontal analysis and retention factors from the zonal elution. A linear relationship was observed in such plot from the analysis of reference compounds. This standard plot was later used for determining binding constants of several compounds, and high correlation was observed compared to reference data. The analysis time and the amount of analyte used are significantly reduced and precision is also increased.
2. Experimental
2.1. Reagents
The phenytoin, furosemide, tolbutamide, R/S-warfarin, l-tryptophan, salicylic acid, carbamazepine, imipramine, ibuprofen, diazepam, aspirin, 3-acetylcoumarin, sulindac, verafamil, propranolol, chlorpromazine, chlorpropamide, triflupromazine, benzoic acid, 4-chromanol, valproic acid, bupivacaine, cefazolin, etodolac, digitoxin were from Sigma (St. Louis, MO, USA); all of these agents were highest grade possible from the supplier. R-Warfarin and S-warfarin were purchased from Gentest (Woburn, MA, USA). The HSA (Cohn fraction V, essentially fatty acid and globulin free) was obtained from Fluka (Milwaukee, WI, USA). The Nucleosil Si-300 (7 µm particle diameter, 300 Å pore size)was from Macherey Nagel (Düren, Germany). Reagents for the BCA protein assay were from Pierce (Rockford, IL, USA). All other chemicals were of the highest grades available. All buffers and aqueous solutions were prepared using water from a MilliQ water system (Millipore, Billerica, MA, USA) and filtered using Osmonics 0.22 µm nylon filters from Fisher (Pittsburgh, PA, USA).
2.2. Apparatus
The chromatographic system used in this study consisted of Shimadzu (Columbia, MD, USA) LC-10AD isocratic pumps, a Shimadzu SPD-10AV UV absorbance detector, a Shimadzu SIL-10 AD auto-injector, a Shimadzu CTO-10AS column oven, an HSA column (30 mm × 4.6 mm I.D.). Chromatographic data were collected and processed using Class-VP software Version 5.032 from Shimadzu. Empty columns used to prepare the HSA columns were purchased from Alltech and columns were downward slurry packed using an Alltech Slurry Packer (Deerfield, IL, USA). BCA protein assay was performed using a Model 680 microplate reader from BioRad (Hercules, CA, USA).
2.3. Methods
2.3.1. Preparation of HSA support
HSA support was prepared using Nucleosil Si-300 silica according to a literature [20,21]. Briefly, Nucleosil Si-300 silica was converted into a diol-bonded form and this diol-bonded silica (2.0 g) was then converted to an aldehyde form through oxidation with sodium periodate (2.0 g) in 90% glacial acetic acid (20 mL). The HSA was immobilized using a Schiff base method by mixing 0.5 g of aldehyde silica with 100 mg HSA in the presence of 50 mg sodium cyanoborohydride in 10 mL of potassium phosphate buffer (KPB) (0.067 M, pH 7.4). The immobilization reaction was allowed to proceed for 4 days in a cold room. The HSA silica was then washed with PBS buffer and treated with three portions of 10 mg sodium borohydride to convert the excess aldehyde groups on the support into alcohols. The support was then washed several times with PBS buffer and stored in the cold room until use.
Non-specific interaction of drug to silica support was measured using a control support which was prepared by following the same Schiff base method except no HSA was added during the immobilization step. This control material was washed and stored in the same manner as the immobilized HSA support. A small portion of both the HSA silica and control support was washed several times with deionized water and dried under vacuum at room temperature. These dried samples were analyzed in triplicate using a BCA protein assay (Pierce) in which HSA was the standard and the control silica acted as the blank.
2.3.2. Chromatographic procedures
The immobilized HSA silica and control support were downward slurry packed at 3000 psi (207 bar) into separate stainless steel columns (30 mm × 4.6 mm I.D.) using KPB as the packing solution. For high affinity HSA binding compounds a shorter column (10 mm × 2.1 or 4.6 mm I.D.) was used. Each column was placed into a column oven for temperature control at 37 °C.
All drugs are dissolved in ethanol or KPB at concentration of 10 mM as stock solutions and further diluted with KPB for working solutions. Drugs dissolved in ethanol were stored in a cold room (4°C) not more than 1 week while drugs dissolved in KPB were prepared daily. Chromatographic mobile phase was KPB and degassed for 20 min before use.
Frontal analysis was performed using KPB that contained 0–10 µM various compounds (tolbutamide, furosemide and phenylbutazone). This solution was applied at a flow rate of 0.5 mL/min. This flow rate was found to be well within the range needed to establish a local equilibrium in the HSA column, in agreement with earlier results reported for other solutes [22,26]. All experiments were performed in triplicate under each set of tested conditions. The retained compounds were eluted and the column regenerated between studies by passing KPB through the column. The amount of compounds required to saturate a column were determined from the mean position of the resulting breakthrough curve [6]. The results obtained for the control column were subtracted from those obtained for an HSA column of identical size to correct for the column void time and to correct for secondary interactions between drugs and the support. A correction for the system void time was made by performing similar experiments using sodium nitrate as a non-retained solute.
Zonal elution was typically performed at 1.0 mL/min (4.6 mm I.D. column) or 0.2 mL/min (2.1 mm I.D. column). The concentrations of injected compounds range from 1.0 to 30 µM. These levels were found to be sufficiently low to avoid any significant changes in the retention factor due to overloading effects, thus indicating that linear elution conditions were present. The mean retention time was obtained by calculating the first statistical moment for each peak. The column void time was found by injecting sodium nitrate as a non-retained solute.
3. Results and discussion
3.1. Frontal analysis
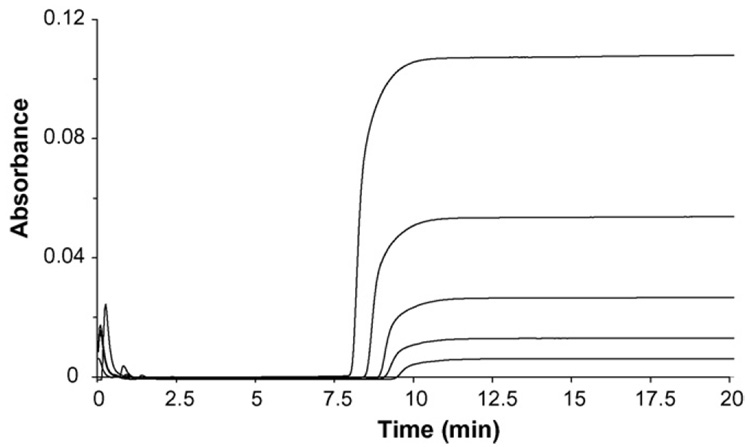
Binding of reference drugs to human serum albumin was first examined by frontal affinity chromatography. In this method, a mobile phase containing known concentration of reference drugs was applied to a column containing immobilized human serum albumin. As the immobilized HSA becomes saturated by binding of the applied drugs, the amount of drugs eluting from the column gradually increases forming a characteristic breakthrough curve. Typical breakthrough curves obtained from the application of increasing concentration of tolbutamide on immobilized HSA column are shown in Fig. 1. As the concentration of tolbutamide on immobilized HSA column increased, it results in a faster saturation of HSA and produces a breakthrough curve that appears at faster elution times. This shift of breakthrough time was related to the moles of binding sites in the column and the concentration of tolbutamide by analyzing the results according to Eq. (1) [6,22],
| (1) |
where mL is related to the true number of binding sites on the column while mLapp is the apparent moles of solute required to reach the mean point of the breakthrough curve.
Fig. 1.
Frontal analysis of tolbutamide on immobilized HSA column at pH 7.4 and 37 °C, where the concentrations of applied tolbutamide (from right to left) are 2 µM, 4 µM, 6 µM, 8 µM, 10 µM. Other experimental details are in text.
In this relationship,Ka is the association equilibrium constant for the binding of A to L, and [A] is the concentration of solute applied to the column. This equation predicts that a plot of 1/mLapp versus 1/[A] will give a straight line with a slope equal to 1/(KamL) and an intercept of 1/mL. This makes it possible to obtain the association constant (Ka) from the ratio of the intercept to the slope. In addition, the true number of binding sites (mL) in the column can be determined from the inverse of intercept.
The plots obtained for 1/mLapp versus 1/[tolbutamide] gave linear relationships with correlation coefficients ranging from0.997 to 0.999 (n = 5). According to Eq. (1), this suggested that only a single type of binding site was present for tolbutamide on the immobilized HSA. The association constant for tolbutamide determined from above equation was 8.2 (±0.2) × 104M−1, which is close to the value obtained from solution-based equilibrium dialysis. The association constants obtained for furosemide and phenylbutazone to HSA in this study using frontal analysis are listed in Table 1. These compounds also bound to HSA at a single binding location(r2 = 0.993–0.999, n = 5) under the conditions used in this study.
Table 1.
Association constants (frontal analysis) and retention factors (zonal elution) of standard compounds obtained from HSA column
| Standard compounds | Association constant (Ka, M−1) | Retention factor (k′) |
|---|---|---|
| Phenytoina | 8.5 (±0.5) × 103 | 6.58 (±0.02) |
| l-Tryptophanb | 1.1 (±0.3) × 104 | 13.4 (±0.2) |
| Tolbutamide | 8.2 (±0.2) × 104 | 58.5(±0.6) |
| Furosemide | 1.4 (±0.3) × 105 | 95 (±3) |
| R-Warfarinc | 2.1 (±0.2) × 105 | 122 (±3) |
| S-Warfarinc | 2.6 (±0.4) × 105 | 161 (±4) |
| Phenylbutazone | 1.2 (±0.5) × 106 | 664 (±11) |
Table 1 also includes association constants for several other drugs obtained with frontal analysis performed by other groups under the similar chromatographic conditions. This comparison was possible since the above equation implies that the measurement of association constants from frontal analysis is independent of the number of immobilized protein in column because the value it provides for Ka can be determined from the ratio of the intercept to the slope. This can be advantageous for comparing values from HSA columns with different HSA immobilization coverage or when precise measurement of association constants are required over the time if the number of immobilized HSA or the binding capacity of column gradually decrease over the time.
3.2. Zonal elution
Zonal elution chromatography of reference drugs was also used to observe the binding of drugs to HSA. Zonal elution is the most common method used in affinity chromatography to study drug–protein interactions and differs from frontal analysis in aspects that a small plug of sample (linear elution condition) rather than a continuous application is introduced into HPAC column. Chromatographic data obtained from zonal elution is generally characterized by retention factor (k′) of injected solute and the value of k′ is related to how strongly a compound interacts with immobilized HSA in column by Eq. (2) [6],
| (2) |
where and mL tot are global association constant and the moles of protein immobilized in column while tr, tm and Vm are retention time, void time and void volume of column, respectively.
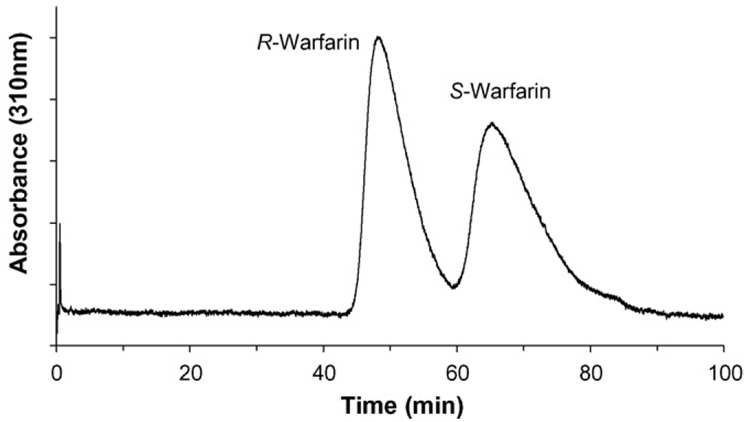
Representative chromatogram obtained during the zonal elution chromatography studies are shown in Fig. 2. One advantage of immobilized HSA column over traditional binding approaches (i.e., equilibrium dialysis or ultracentrifugation) is its ability to separate enantiomers of racemic drugs. Chiral separation of racemic warfarin using immobilized HSA column is shown in Fig. 2.
Fig. 2.
Zonal elution profile of racemic warfarin on immobilized HSA column at pH 7.4 and 37 °C. The concentration of injected warfarin is 20 µM. Other experimental details are in text.
Table 1 shows retention factors obtained from several reference compounds. In general, less than a 5% variation in the retention of all injected compounds except phenylbutazone was noted as the flow rate was varied from 0.2 mL/min to 1.0 mL/min, confirming that a local equilibrium had been established on the HSA and control columns under these conditions. A tendency of higher variation in retention times, however, was observed as the drug–HSA interaction increased. Notice that the capacity factor of phenylbutazone is over 660 and this represent the retention time of over 300 min (6 h). No further study was carried for drugs showing retention times over 300 min in HSA column due to practical reasons.
3.3. Combination of frontal and zonal chromatography
Although frontal analysis and zonal elution are effective ways to measure the association constant of drug–protein interaction, they also suffer from several drawbacks. The amount of reagent required for both methods are disadvantageous for some occasions such as analysis of certain metabolites which are not commercially available. Both methods suffer from relatively a long analysis time with each costing more than several hours or days to process one compound. This certainly is not tolerable for high throughput analysis required on drug screening department. One way to avoid this problem is to combine pros of two methods.
Eq. (1) and Eq. (2) imply that the association constant measured from frontal chromatography can be directly related to the retention factor obtained from zonal elution chromatography using the same HSA column. Indeed, a linear relationship (r2 = 0.9993, n = 7) was observed between the standard association constants from frontal analysis and retention factors from zonal elution using reference drugs in Table 1.
Retention factors of phenytoin, l-tryptophan, R-and S-warfarin were obtained from current study and plotted against their association constants from literature values [22,26,27]. No significant deviation was observed even if columns and conditions used for obtaining retention factors and association constants were different. This was further tested later with a different column with smaller amount of immobilized HSA. A linear decline in retention factors of reference compounds was observed with the column with smaller amount of HSA. This further confirms the versatility of immobilized HSA column for studying such drug–protein interaction.
4. Correlation study
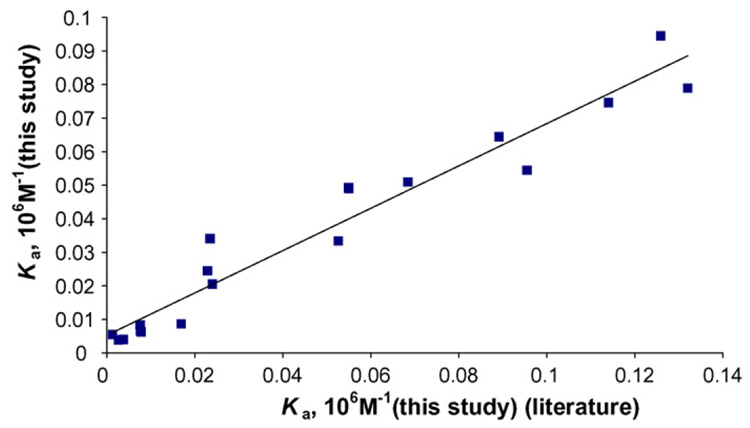
Further characterization of the current method (frontal and zonal chromatography combination) was performed by comparison of association constants from literature with more traditional approaches (i.e., equilibrium dialysis or ultra filtration). Association constants of current method were obtained by inserting retention factors of each compound on HSA column directly to the linear equation obtained from the standard plot obtained from Table 1. The variations of retention factors for those compounds on HSA column were 3% or less with triplicate measurements. All chromatographic data were obtained at 37°C with KPB as mobile phase. In some compounds such as carbamazepine and imipramine, for example, the non-specific interaction to silica support (i.e., the retention times on control column) is significant compared to the specific interaction to HSA. This can be corrected by simple subtraction of retention factors [21]. Association constants of same compounds from literatures were extracted with similar experimental conditions (i.e., similar buffer pH, ionic strength and temperature) and relatively recent publications.
The values in Table 2 were used to construct the correction plot for two groups in Fig. 3. The correlation coefficient of this plot is 0.945 (n = 19). This indicates that two groups are in good agreement with each other. This confirms that the combination of frontal and zonal chromatography might be used as an alternative method for equilibrium dialysis or ultra filtration for rapid screening of drug–protein interaction. The analysis times for compounds with Ka in ranges of 103M−1 were less than 10 min and for compounds with Ka in ranges of 104M−1 were longer (20–60 min). However, for compounds with Ka higher than 105 M−1 (e.g., ibuprofen and diazepam), the retention times exceeded 500 min under the current experimental conditions. Use of organic modifier such as methanol or acetonitrile might help to further decrease retention times of these strongly bound compounds on immobilized HSA column but this may also pose a problem if the target compounds were bound to HSA by hydrophobic interaction. Small cartridge type HSA columns (10 mm × 4.6 or 2.1 mm I.D.) were prepared in attempt to reduce overall retention times of ibuprofen and diazepam. However, the retention times of these compounds still exceeded more than 300 min. Possible solutions for rapid analysis of these strong bound compounds to HSA are either to make a micro dimension column or manipulate the amount of immobilized HSA. These are currently under investigation.
Table 2.
Comparison on the association constants of various compounds obtained from this study and association constants extracted from literature
| Compounds | Ka (HPAC) | Ka (ED) |
|---|---|---|
| (M−1, current study) | (M−1, literature value) | |
| Carbamazepine | 5.5 × 103 | 1.4 × 103 |
| Salicylic acid | 7.9 × 104 | 1.3 × 105 |
| Imipramine | 2.0 × 104 | 2.4 × 104 |
| Coumarin | 6.5 × 103 | 7.7 × 103 |
| 3-Acetylcoumarin | 6.2 × 103 | 7.8 × 103 |
| Sulindac | 7.5 × 104 | 1.1 × 105 |
| Aspirin | 3.4 × 104 | 2.3 × 104 |
| Chlorpromazine | 6.4 × 104 | 8.9 × 104 |
| Triflupromazine | 4.9 × 104 | 5.5 × 104 |
| 4-Chormanol | 5.0 × 104 | 6.8 × 104 |
| Chlorpropamide | 5.4 × 104 | 9.5 × 104 |
| Benzoic acid | 8.3 × 103 | 1.7 × 103 |
| Verafamil | 3.7 × 103 | 2.7 × 103 |
| Propranolol | 3.9 × 103 | 3.8 × 103 |
| Valproic acid | 3.3 × 104 | 5.3 × 104 |
| Bupivacaine | 8.4 × 103 | 7.5 × 103 |
| Cefazolin | 2.4 × 104 | 2.3 × 104 |
| Etodolac | 9.3 × 104 | 1.3 × 105 |
| Digitoxin | 4.9 × 104 | 5.5 × 104 |
Fig. 3.
The correlation plot between association constants for various drugs in Table 2. The correlation coefficient of plot is 0.945 (n = 19).
5. Conclusions
This methodology used a combination of frontal and zonal chromatography on immobilized HSA column to quantitatively characterize drug–protein interaction. The linear equation obtained from the standard plot of retention factors versus association constants on HSA column showed a high correlation (r2 = 0.9993) and this equation was further used for rapid determination of association constants for the number of drugs on HSA. Determination of association constants was as fast as 1.5 min and showed a high correlation to equilibrium dialysis or ultra filtration. The combination of frontal and zonal chromatography for determining association constants showed several advantages, one being rapid determination of association constant of drug to HSA. Other notable advantages include an ease of automation and simultaneous ability to distinguish association constants of chiral compounds at the same time. The same approach could be used for studying interaction of other drugs and proteins and should further improve overall drug screening process.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Morelock MM, Ingraham RH, Betageri R, Jakes S. J. Med. Chem. 1995;38:1309. doi: 10.1021/jm00008a009. [DOI] [PubMed] [Google Scholar]
- 2.Peng SX, Henson C, Wilson LJ, Chromatogr J. B Biomed. Sci. Appl. 1999;732:31. doi: 10.1016/s0378-4347(99)00253-4. [DOI] [PubMed] [Google Scholar]
- 3.Sudlow G, Birkett DJ, Wade DN. Mol. Pharmacol. 1976;12:1052. [PubMed] [Google Scholar]
- 4.Kragh-Hansen U. Pharmacol. Rev. 1981;33:17. [PubMed] [Google Scholar]
- 5.Peters TJ. All about Albumin: Biochemistry, Genetics, and Medical Applications. San Diego: Academic Press; 1996. [Google Scholar]
- 6.Hage DS, Chromatogr J. B Anal. Technol. Biomed. Life Sci. 2002;768:3. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 7.Kragh-Hansen U, Chuang VT, Otagiri M. Biol. Pharm. Bull. 2002;25:695. doi: 10.1248/bpb.25.695. [DOI] [PubMed] [Google Scholar]
- 8.Sowell J, Mason JC, Strekowski L, Patonay G. Electrophoresis. 2001;22:2512. doi: 10.1002/1522-2683(200107)22:12<2512::AID-ELPS2512>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Mazoit JX, Samii K. Br. J. Clin. Pharmacol. 1999;47:35. doi: 10.1046/j.1365-2125.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vita M, Abdel-Rehim M, Nilsson C, Hassan Z, Skansen P, Wan H, Meurling L, Hassan M, Chromatogr J. B Anal. Technol. Biomed. Life Sci. 2005;821:75. doi: 10.1016/j.jchromb.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Austin J, Hage DS. Electrophoresis. 2002;23:956. doi: 10.1002/1522-2683(200203)23:6<956::AID-ELPS956>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Tillement JP, Zini R, d’ Athis P, Vassent G. Eur. J. Clin. Pharmacol. 1974;7:307. doi: 10.1007/BF00560349. [DOI] [PubMed] [Google Scholar]
- 13.Sjodahl J, Hjalmarsson S. FEBS Lett. 1978;92:22. [Google Scholar]
- 14.Carter DC, He XM, Munson SH, Twigg PD, Gernert KM, Broom MB, Miller TY. Science. 1989;244:1195. doi: 10.1126/science.2727704. [DOI] [PubMed] [Google Scholar]
- 15.Kosa T, Maruyama T, Otagiri M. Pharm. Res. 1997;14:1607. doi: 10.1023/a:1012138604016. [DOI] [PubMed] [Google Scholar]
- 16.Honore B, Brodersen R. Mol. Pharmacol. 1984;25:137. [PubMed] [Google Scholar]
- 17.Montero MT, Estelrich J, Valls O. Int. J. Pharm. 1991;62:21. [Google Scholar]
- 18.Chignell CF. Mol. Pharmacol. 1969;5:244. [PubMed] [Google Scholar]
- 19.Yamasaki K, Maruyama T, Kragh-Hansen U, Otagiri M. Biochim. Biophys. Acta. 1996;1295:147. doi: 10.1016/0167-4838(96)00013-1. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Kye YS, Hage DS. J. Chromatogr.A. 2004;1049:51. [PubMed] [Google Scholar]
- 21.Kim HS, Hage DS, Chromatogr J. B Anal. Technol. Biomed. Life Sci. 2005;816:57. doi: 10.1016/j.jchromb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Loun B, Hage DS. Anal. Chem. 1994;66:3814. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 23.Noctor TA, Wainer IW, Hage DS. J. Chromatogr. 1992;577:305. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 24.Walters RR. Anal. Chem. 1985;57:1099A. doi: 10.1021/ac00288a001. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta A, Hage DS. Anal. Chem. 1999;71:3821. doi: 10.1021/ac9903499. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Hage DS. J. Chromatogr. 1993;645:241. doi: 10.1016/0021-9673(93)83383-4. [DOI] [PubMed] [Google Scholar]
- 27.Ohnmacht CM, Chen S, Tong Z, Hage DS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006;836:83. doi: 10.1016/j.jchromb.2006.03.043. [DOI] [PubMed] [Google Scholar]