Abstract
We report the first G-quadruplex structure formed in the promoter region of the human bcl-2. Bcl-2 is a potent oncoprotein that functions as an inhibitor of cell apoptosis and has been found to be aberrantly overexpressed in a wide range of human tumors. A highly GC-rich region upstream of the P1 promoter plays an important role in the regulation of the transcriptional activity of the bcl-2 oncogene. The purine-rich strand of this region contains multiple runs of guanines and can form three distinct intramolecular G-quadruplexes in K+-containing solution. Of these, the G-quadruplex formed within the middle four consecutive guanine runs has been shown to be the most stable G-quadruplex structure, while it is also a mixture of loop isomers. This predominate G-quadruplex structure formed in this region was studied by NMR. Our results demonstrate a novel folding of a unique intramolecular G-quadruplex structure with mixed parallel/antiparallel G-strands. This G-quadruplex structure contains three G-tetrads connected with a single-nucleotide double-chain-reversal side loop and two lateral loops. The three-nucleotide CGC loop in the bcl-2 promoter sequence forms a lateral loop, as opposed to a double-chain-reversal side loop observed in a similar sequence in the c-MYC promoter, which appears to largely determine the overall folding of the bcl-2 G-quadruplex. Furthermore, both the bcl-2 and c-MYC promoter sequences contain the G3NG3 sequence motif, which forms a stable double-chain-reversal, parallel-stranded structural motif. This predominant bcl-2 G-quadruplex represents an attractive novel target for the design of new anticancer drugs that specifically modulate bcl-2 gene expression.
Bcl-2 (B-cell CLL/lymphoma 2) is a potent oncoprotein that plays an essential role in cell survival and functions as an inhibitor of cell apoptosis.1 The bcl-2 proto-oncogene was first discovered in human follicular lymphoma and has been mapped to chromosome 18q21 based on a t(14;18) translocation to the immunoglobulin heavy chain (IgH) locus at 14q32.2 Bcl-2 has been found to be aberrantly overexpressed in a wide range of human tumors, including B-cell and T-cell lymphomas, breast, prostate, cervical, colorectal, sand non-small cell lung carcinomas.3 Elevation of bcl-2 level has also been associated with poor prognosis.3 Thus the bcl-2 transcriptional control has emerged as an attractive target for anticancer therapeutics.
The P1 promoter located 1386–1423 base pairs upstream of the translation start site is the major transcriptional promoter for bcl-2.4 This is a TATA-less, GC-rich promoter that contains multiple transcriptional start sites. The 5′-end of the P1 promoter, including a highly GC-rich region, has been implicated in playing a major role in the regulation of bcl-2 transcription.4 This GC-rich element is a 39-base-pair sequence that is located 58 to 19 base pairs upstream of the P1 promoter. Deletion or mutation of this element has been shown to increase promoter activity by 2.1-fold and 2.6-fold, respectively.4 This 39-mer guanine-rich strand (bcl2Pu39) contains six runs of guanines, with one run of five guanines, two runs of four guanines each, and three runs of three guanines each (Figure 1A). This G-rich strand bcl2Pu39 can form a mixture of three distinct intramolecular G-quadruplexes in K+-containing solution.5 The G-quadruplex formed on the middle four consecutive runs of guanines (bcl2MidG4Pu23, Figure 1A) has been shown to be the most stable and is suggested to be the major G-quadruplex structure formed in the bcl-2 promoter region.5 Furthermore, the bcl2MidG4Pu23 contains a run of five guanines that gives rise to the possibility of three different loop isomers (Figure 1A), which can each be isolated by specific dual G-to-T mutations. Of the three loop isomers, the G15T/G16T dual mutant was the most stable as shown by NMR (Figure S1). The formation of this major G-quadruplex structure was also confirmed by biochemical studies. DMS footprinting data show pronounced cleavage at the G15 and G16 in potassium solution, indicating that these two guanines are not involved in the formation of the major G-quadruplex under physiological conditions.5 Polymerase stop assays show that the major G-quadruplex is formed with 15,16-G-to-T mutant sequence, which is markedly more stable than the minor G-quadruplex formed with 15,19-G-to-T mutant sequence, while 18,19-G-to-T mutant sequence does not form a stable G-quadruplex (Figure 1A).5 Thus a 23-mer DNA oligomer containing the middle four G-runs with G15T/G16T dual mutations (bcl2MidG4Pu23-G15T/G16T) was prepared and used for our NMR structural analysis of this major G-quadruplex formed in the bcl-2 promoter6 (Figure 1A). Our results demonstrate a novel folding of a unique intramolecular G-quadruplex structure with mixed parallel/antiparallel G-strands. This G-quadruplex structure contains three G-tetrads connected with a single-nucleotide double-chain-reversal side loop and two lateral loops. The first three-nucleotide CGC loop in the bcl-2 promoter sequence forms a lateral loop, as opposed to a double-chain-reversal side loop observed in a similar sequence in the c-MYC promoter, and appears to largely determine the overall folding of the bcl-2 G-quadruplex. Possible rules governing various folding patterns of intramolecular G-quadruplexes are also implicated by this study.
Figure 1.

(A) The promoter sequence of the bcl-2 gene and its modifications. The top sequence is the wild-type bcl-2 39-mer sequence. The six G-runs are underlined and numbered using Roman numerals. Bcl2MidG4Pu23 represents the 23mer sequence containing the middle four consecutive runs of guanines, which forms the most stable G-quadruplex structure. Bcl2MidG4Pu23-G15T/G16T represents the mutant 23mer with 15,16-G-to-T mutations. (B) Imino and aromatic regions of 1D 1H NMR spectra of bcl2MidG4Pu23 (upper) and bcl2MidG4Pu23-G15T/G16T (lower), at 7 °C, 20 mM K-phosphate, 40 mM KCl, pH 7.0.
The 1D NMR spectrum of the wild-type 23mer bcl-2 promoter sequence bcl2MidG4Pu23 in potassium solution (Figure 1B top) shows a broad envelope with some fine lines, indicating the presence of a dynamic equilibrium of multiple conformers. The imino protons at 10–12 ppm indicate the formation of G-quadruplex structures.7 The 1D NMR spectrum of the 15,16-G-toT mutant sequence bcl2MidG4Pu23-G15T/G16T for the major G-quadruplex structure formed in the bcl-2 promoter shows much improved line width and better resolution in potassium solution (Figure 1B, bottom). The well-resolved imino proton resonances located between 10.5–12 ppm clearly indicate the formation of a stable G-quadruplex structure. The melting temperature of this G-quadruplex (~75 °C) is independent of the concentration, indicating the formation of a monomeric structure (data not shown). The sharpest linewidths of ~5 Hz for the imino resonances of this intramolecular G-quadruplex are observed around 20 °C. The sharp spectral line widths are also indicative of the presence of a monomeric structure. The presence of a unimolecular structure was confirmed by the EMSA experiment as well.5 The CD spectra of the wide-type and the mutant 23mers are very similar (Figure 2).
Figure 2.
CD spectra of bcl2MidG4Pu23 (WT) and bcl2MidG4Pu23-G15T/G16T (M1) in the absence and presence of 100 mM KCl.
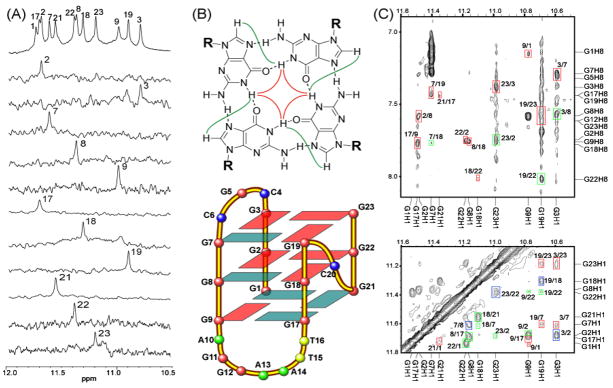
Twelve imino peaks are present in the 10.5–12 ppm region (Figure 1B), indicating that all twelve guanines of the four consecutive guanine runs of the bcl2MidG4Pu23-G15T/G16T are involved in the intramolecular monomeric G-quadruplex formation, and that this G-quadruplex structure contains three G-tetrads. The imino and base aromatic H8 protons of 12 guanine residues were unambiguously assigned by the site-specific low-concentration (6%) incorporation of 1, 2, 7-15N, 2-13C-labeled guanine nucleoside at each guanine position of the sequence.6,8 The guanine imino H1 proton resonance has one-bond coupling to N1, and the guanine base aromatic H8 proton resonance has two-bond coupling to N7. Both H1 and H8 protons of the site-specific labeled guanine are readily detected by 1D 15N-filtered experiments. The assignment of each imino proton of the twelve guanines involved in the three G-tetrads is shown in Figure 3A. The assignment of each guanine aromatic H8 proton is shown in Figure S2.
Figure 3.
(A) Imino proton assignments of bcl2MidG4Pu23-G15T/G16T using 1D 15N-filtered experiments on site-specific labeled oligonucleotides. The imino proton of G1 is assigned using elimination method, in combination with the exchangeable-proton 2D-NOESY (see Figure 3C). (B) (Top) A G-tetrad with H1-H1 and H1–H8 connectivity pattern detectable in NOESY experiments. (Bottom) Schematic drawing of the folding topology of the bcl2MidG4Pu23-G15T/G16T G-quadruplex. Red boxes represent guanines with anti configuration, and blue boxes represent guanines with syn configuration. (C) H1–H8 region (top) and H1-H1 region (bottom) of 2D-NOESY spectrum of bcl2MidG4Pu23-G15T/G16T in H2O at 25 °C. Red boxes represent intra-tetrad connectivity, green boxes represent inter-tetrad connectivity, and blue boxes represent sequential connectivity.
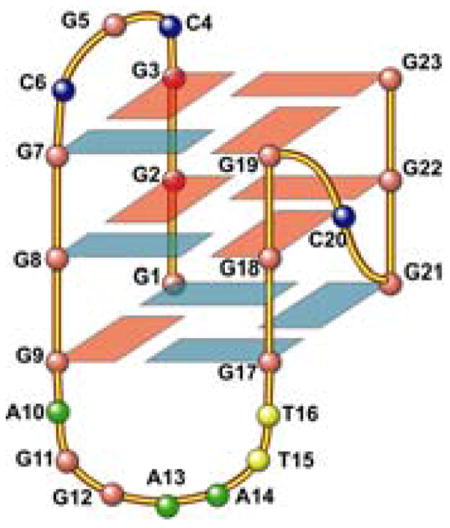
The assignment of the imino and base H8 protons of guanines leads to the direct determination of the folding topology of this bcl-2 G-quadruplex structure. The glycosidic torsion angles of the twelve G-quadruplex guanines are defined as indicated by the intraresidue H8-H1′ NOE intensities6 (Figure S3) and are shown in Figure 3B bottom. In a G-tetrad plane with the Hoogsteen H-bond network, the imino proton NH1 of each guanine is in close spatial vicinity to the NH1s of the two adjacent guanines, and to the base H8 of one of the adjacent guanines (Figure 3B top). The through-space NOE connectivities of guanine H1-H1 and H1–H8 determine the arrangement and topology of a G-tetrad plane. Three G-tetrad planes, G3–G7–G19–G23, G2–G8–G18–G22, and G1–G9–G17–G21, were determined based on the NOE connectivities (Figure 3C). For example, the G3H1/G7H1, G7H1/G19H1, G19H1/G23H1, G23H1/G3H1 (GH1/GH1); and G3H1/G7H8, G7H1/G19H8, G19H1/G23H8, and G23H1/G3H8 (GH1/GH8) NOE interactions (Figure 3C) define a tetrad plane of G3–G7–G19–G23 (Figure 3B bottom). The G-quadruplex alignment is further defined based on the inter-tetrad NOE connections from residues that position far apart in the DNA sequence. For example, the strong NOE interactions of G2H1/G9H1, G8H1/G17H1, G18H1/G21H1, and G22H1/G1H1 connect the middle and bottom G-tetrad planes, as well as reflecting the reversed nucleotide glycosidic torsion angles related with the middle and bottom G-tetrads (Figure 3C, Figure S3). The G3H1/G8H1, G7H1/G18H1, G19H1/G22H1, and G23H1/G2H1 NOEs connect the top and middle G-tetrad planes and reflect the right-handed twist of the DNA backbone. From these data, the folding topology of the bcl2MidG4Pu23-G15T/G16T G-tetrads is determined, as shown in Figure 3B bottom. This G-quadruplex contains mixed parallel/antiparallel G-strands, with the first, third and fourth G-strands being parallel with each other, and the second G-strand being antiparallel with the rest of the strands. The first three G-strands (from 5′-end) are linked with two lateral loops (CGC and AGGAATT), while the third and fourth G-strands are linked with a single nucleotide, double-chain-reversal side loop (C). This bcl-2 G-quadruplex has a similar G-tetrad arrangement, but a different loop arrangement, as compared to that of the Tetrahymena Telomeric (Tet-Tel) sequence (Table 1).9
Table 1.
Comparison of G-quadruplex-forming sequences.
| Name | Sequence (5′to 3′) | ||||||
|---|---|---|---|---|---|---|---|
| Tet-Tel | GGG | GTTG | GGG | TTG | GGG | TT | GGG |
| c-MYC(a) | GGG | T | GGG | TA | GGG | T | GGG |
| c-MYC(b) | GGG | A | GGG | TTTTTA | GGG | T | GGG |
| VEGF | GGG | C | GGG | CCGG | GGG | C | GGG |
| HIF-1α | GGG | A | GGG | GAGAGG | GGG | C | GGG |
| Bcl-2 | GGG | CGC | GGG | AGGAATT | GGG | C | GGG |
It is significant to note that the bcl-2 G-quadruplex represents another well-defined DNA G-quadruplex formation, in addition to c-MYC, in the promoter regions of human proto-oncogenes. In comparison with the recently determined parallel-stranded G-quadruplex structures formed in the c-MYC promoter6,10 (Table 1, Figure S4), the third loops of both G-quadruplexes adopt single-nucleotide (nt) double-chain-reversal side loops, with residue C in bcl-2 and T in c-MYC, respectively; whereas the first and second loops adopt different conformations in the two G-quadruplex structures. In fact, one of the G-quadruplexes formed in the c-MYC promoter [c-MYC(b)] exhibits some degree of sequence similarity to the bcl-2 G-quadruplex (Table 1), both of which contain an extended second loop (7-nt in bcl-2 and 6-nt in c-MYC(b)). However, the first loop of bcl-2 is a three-nt loop (CGC) and adopts a lateral loop conformation, while the first loop of c-MYC(b) is a single-nt loop (A) and adopts a double-chain-reversal side loop conformation10 (Figure S4). Consequently, the extended second loop of bcl-2 forms a lateral loop conformation, while that of c-MYC forms a double-chain-reversal loop. Therefore it appears that the first three-nucleotide lateral loop determines the overall folding of the bcl-2 G-quadruplex, namely the mixed parallel/antiparallel-stranded G-quadruplex, as opposed to the parallel-stranded c-MYC G-quadruplex. Furthermore, it is interesting to note that both the bcl-2 and c-MYC promoter sequences contain the same GGGNGGG sequence motif, which forms a stable double-chain-reversal parallel-stranded structural motif. Indeed, the promoter regions of VEGF and HIF-1α also contain the same sequence motif (Table 1), and are suggested to form parallel-stranded G-quadruplexes.10 In addition, the third double-nt loop (TT) in the Tet-Tel sequence (Table 1) forms the only double-chain-reversal side loop in the Tet-Tel G-quadruplex.9 Thus it appears that the single- or double-nucleotide sized loops are more favored for the formation of the double-chain-reversal side loop. The detailed NMR solution structure determination of this predominant G-quadruplex in the bcl-2 promoter will be published in a separate full paper.
In summary, our study demonstrates the formation of a unique intramolecular G-quadruplex structure in the promoter region of the human bcl-2 gene. Our NMR results define a novel folding pattern of this predominate G-quadruplex in the bcl-2 promoter, which adopts a mixed parallel/antiparallel-stranded G-quadruplex structure. This major G-quadruplex in the bcl-2 promoter represents an attractive target for the design of new anticancer drugs that specifically target this secondary structure and modulate bcl-2 gene expression. Furthermore, the bcl-2 G-quadruplex has a different folding pattern as compared to the c-MYC G-quadruplex, albeit with some degree of sequence similarity. It thus appears that different G-quadruplex structures will form based on different promoter sequences, making such regions attractive targets for pathway-specific drug design.
Supplementary Material
Experimental Methods, 1D NMR spectra of three dual G-to-T mutants of bcl2MidG4Pu23, HMQC experiments for base H8 proton assignments and H8/H6-H1′ region of 2D-NOESY with assignments of bcl2MidG4Pu23-G15T/G16T. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported by the National Institutes of Health (1K01CA83886 and 1S10 RR16659). We are grateful to Dr. Laurence H. Hurley and his lab for valuable assistance and discussion.
References
- 1.(a) Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]; (b) Vaux DL, Cory S, Adams JM. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 2.Yunis JJ. Science. 1983;221:227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]
- 3.(a) Akagi T, Kondo E, Yoshino T. Leukemia & Lymphoma. 1994;13:81–87. doi: 10.3109/10428199409051655. [DOI] [PubMed] [Google Scholar]; (b) Joensuu H, Pylkkanen L, Toikkanen S. American Journal of Pathology. 1994;145:1191–1198. [PMC free article] [PubMed] [Google Scholar]; (c) Tjalma W, De Cuyper E, Weyler J, Van Marck E, De Pooter C, Albertyn G, van Dam P. American Journal of Obstetrics & Gynecology. 1998;178:113–117. doi: 10.1016/s0002-9378(98)70636-2. [DOI] [PubMed] [Google Scholar]; (d) Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY. New England Journal of Medicine. 1993;329:690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]; (e) McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML. Cancer Research. 1992;52:6940–6944. [PubMed] [Google Scholar]; (f) Baretton GB, Diebold J, Christoforis G, Vogt M, Muller C, Dopfer K, Schneiderbanger K, Schmidt M, Lohrs U. Cancer. 1996;77:255–264. doi: 10.1002/(SICI)1097-0142(19960115)77:2<255::AID-CNCR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; (g) Reed JC, Kitada S, Takayama S, Miyashita T. Annals of Oncology. 1994;5 Suppl 1:61–65. doi: 10.1093/annonc/5.suppl_1.s61. [DOI] [PubMed] [Google Scholar]
- 4.(a) Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, Korsmeyer SJ. EMBO Journal. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Young RL, Korsmeyer SJ. Molecular & Cellular Biology. 1993;13:3686–3697. doi: 10.1128/mcb.13.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Heckman C, Mochon E, Arcinas M, Boxer LM. J Biol Chem. 1997;272:19609–19614. doi: 10.1074/jbc.272.31.19609. [DOI] [PubMed] [Google Scholar]
- 5.Dexheimer TS, Sun D, Hurley LH. Journal of the American Chemical Society. 2005 doi: 10.1021/ja0563861. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Dai JX, Punchihewa C, Mistry P, Ooi AT, Yang DZ. Journal of Biological Chemistry. 2004;279:46096–46103. doi: 10.1074/jbc.M404053200. [DOI] [PubMed] [Google Scholar]; (b) Ambrus A, Chen D, Dai J, Jones RA, Yang DZ. Biochemistry. 2005;44:2048–2058. doi: 10.1021/bi048242p. [DOI] [PubMed] [Google Scholar]
- 7.(a) Smith FW, Feigon J. Nature. 1992;356:164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]; (b) Matsugami A, Ouhashi K, Kanagawa M, Liu H, Kanagawa S, Uesugi S, Katahira M. Journal of Molecular Biology. 2001;313:255–269. doi: 10.1006/jmbi.2001.5047. [DOI] [PubMed] [Google Scholar]; (c) Scaria PV, Shire SJ, Shafer RH. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10336–10340. doi: 10.1073/pnas.89.21.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Marathias VM, Bolton PH. Nucleic Acids Research. 2000;28:1969–1977. doi: 10.1093/nar/28.9.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gavathiotis E, Heald RA, Stevens MFG, Searle MS. Journal of Molecular Biology. 2003;334:25–36. doi: 10.1016/j.jmb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 8.(a) Zhao H, Pagano AR, Wang W, Shallop A, Gaffney BL, Jones RA. Journal of Organic Chemistry. 1997;62:7832–7835. [Google Scholar]; (b) Phan AT, Patel DJ. Journal of the American Chemical Society. 2002;124:1160–1161. doi: 10.1021/ja011977m. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Patel DJ. Structure. 1994;2:1141–1156. doi: 10.1016/s0969-2126(94)00117-0. [DOI] [PubMed] [Google Scholar]
- 10.(a) Seenisamy J, Rezler EM, Powell TJ, Tye D, Gokhale V, Joshi CS, Siddiqui-Jain A, Hurley LH. Journal of the American Chemical Society. 2004;126:8702–8709. doi: 10.1021/ja040022b. [DOI] [PubMed] [Google Scholar]; (b) Phan AT, Modi YS, Patel DJ. Journal of the American Chemical Society. 2004;126:8710–8716. doi: 10.1021/ja048805k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sun D, Guo K, Rusche JJ, Hurley LH. Nucl Acids Res. 2005;33:6070–6080. doi: 10.1093/nar/gki917. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) De Armond R, Wood S, Sun D, Hurley LH, Ebbinghaus SW. Biochemistry. 2005 doi: 10.1021/bi051618u. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental Methods, 1D NMR spectra of three dual G-to-T mutants of bcl2MidG4Pu23, HMQC experiments for base H8 proton assignments and H8/H6-H1′ region of 2D-NOESY with assignments of bcl2MidG4Pu23-G15T/G16T. This material is available free of charge via the Internet at http://pubs.acs.org.