Abstract
Our previous work indicates that the intrathecal administration of neuropeptide Y (NPY) acts at its cognate receptors to reduce behavioral signs of nociception in several models of inflammatory pain, including the formalin test. The present study extends these findings to a rat model of peripheral neuropathic pain, and then evaluates the hypothesis that NPY inhibits inflammation- and nerve injury-induced activation of spinal nociceptive transmission. Here we show that NPY dose-dependently reduced behavioral signs of mechanical and cold hypersensitivity in the spared nerve injury (SNI) model. Intrathecal administration of either a Y1 (BIBO3304) or a Y2 (BIIE0246) receptor antagonist dose-dependently reversed the anti-allodynic actions of NPY. To monitor the effects of NPY on the stimulus-induced activation of spinal nociresponsive neurons, we quantified protein expression of the immediate-early gene c-fos in lamina I–VI of the L4–L5 dorsal horn, with special attention to the mediolateral pattern of Fos immunohistochemical staining after SNI. Either tactile stimulation of the hindpaw ipsilateral to nerve injury, or intraplantar injection of noxious formalin, increased the number of Fos-like immunoreactive profiles. Tactile stimulation evoked a mediolateral pattern of Fos expression corresponding to the innervation territory of the uninjured (sural) nerve. We found that intrathecal NPY reduced both formalin- and SNI-induced Fos expression. NPY inhibition of SNI-induced Fos expression was localized to the sural (uninjured) innervation territory, and could be blocked by intrathecal BIBO3304 and BIIE0246. We conclude that NPY acts at spinal Y1 and Y2 receptors to reduce spinal neuron activity and behavioral signs of inflammatory or neuropathic pain.
Keywords: spared nerve injury, formalin, allodynia, hyperalgesia, rat, c-fos
INTRODUCTION
Neuropeptide Y (NPY) is an abundant and widespread neuroactive peptide that exerts numerous physiological actions, including pain modulation. NPY and its Y1 and Y2 receptors are located at key pain signalling centers throughout the nervous systems, particularly the dorsal horn of the spinal cord (Gibson et al. 1984; Ji et al. 1994). Several studies indicate that NPY, when administered at the spinal level, inhibits transient or inflammatory pain (Taylor 2005; Smith et al. 2007). For example, intrathecal administration of NPY Y2 receptor agonists reduces not only behavioral responsiveness to noxious heat (Hua et al. 1991; Taiwo and Taylor 2002), but also the hyperalgesia associated with inflammation (Taiwo and Taylor 2002; Mahinda and Taylor 2004). Much less clear are the effects of NPY receptor agonists in models of neuropathic pain. Evidence from pharmacological studies suggest that intrathecally applied Y1 and Y2 agonists attenuate the flexor reflex in axotomized animals (Xu et al. 1999), but comparable studies in behavioral models of partial peripheral nerve injury are not available. Therefore, the present studies were designed to determine whether intrathecal administration of NPY dose-dependently reduces behavioral signs of neuropathic pain, and whether such effects are mediated by Y1 or Y2 receptors. Furthermore, we asked whether intrathecal NPY inhibits formalin- and nerve injury-induced activation of spinal nociresponsive neurons. To address these questions, we mapped the population of neurons in the dorsal horn that respond to somatosensory stimulation with induction of the proto-oncogene, c-fos (Coggeshall 2005). We began our Fos studies with a commonly-used stimulus involving the intraplantar injection of dilute formalin. Formalin first produces a short-lived, rapid-onset period (Phase 1) of peripheral nerve activity, sympathetic activity, and pain-like behaviors (Tjolsen et al. 1992; Taylor et al. 1995b; Puig and Sorkin 1996). Phase 1 behaviors are a direct result of tissue injury and / or chemical activation of primary afferent terminals. Phase 1 is followed by a brief quiescent period essentially devoid of nociception (“interphase”), and then by a more persistent period (Phase 2) of licking, lifting and flinching behavior (Tjolsen et al. 1992). Peripheral sensitization coincides with the release of inflammatory mediators, and likely contributes to the ongoing activation of peripheral inputs that contribute to the maintenance of Phase 2 (Taylor et al. 1995b; Taylor et al. 2000). We then extended our analysis to the spared nerve injury model. This model of peripheral neuropathic pain produces robust signs of allodynia, and here we show for the first time that this is accompanied with stimulus-induced Fos expression in the dorsal horn.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles Rivers Laboratories, Inc) were housed in individual cages in a temperature controlled room on a 12-hour light/dark cycle (6am/6pm), and were given food and water ad libitum. All animal use protocols were approved by the IACUC of Tulane University.
Intrathecal Drug Delivery Methods
Rats (280–300 g) were fitted with intrathecal catheters to deliver drugs or saline to the lumbar spinal cord. Anesthesia was induced and maintained throughout surgery with 5% and 2–3% isoflurane, respectively.
Formalin studies
This procedure is a modification of that originally described by Storkson et al. (Storkson et al. 1996; Pogatzki et al. 2000; Taiwo and Taylor). A 32G polyurethane (PU) catheter was used to minimize morphological changes that may be associated with the use of polyethylene (PE) tubing (Sakura et al. 1996). To construct the catheter, the distal end of a 15 cm 32G PU catheter was inserted into a 15 cm piece of PE-10 tubing and the connection was sealed using super glue and 5-min epoxy, which was allowed to dry for 12 hours. A longitudinal 2–3 cm midline incision was made at the level of the iliac crest. A 23G guide needle (Lazlo Bocksai, UCSF Physiology machine shop) was advanced between the L5 and L6 vertebrae. Following confirmation of correct placement with a tail or paw flick, the 32 g PU end of the catheter, which was reinforced with a Teflon-coated stainless steel stylet, was inserted through the needle and cranially advanced 4 cm. The 23G needle was removed and the catheter withdrawn, leaving 26 cm exteriorized. A 1-cm2 sterilized cloth mesh (Instech Laboratories Inc., Plymouth Meeting, PA) was placed about the catheter and a drop of Dermabond (Ethicon, Inc., Somerville, NJ) secured the catheter and mesh to the underlying tissue. A 2 cm diameter loop of catheter was secured to muscle with 2 loosely tied 4-0 sutures. The stylet was removed. The catheter was tunnelled under the skin, exteriorized at a 1 cm incision at the nape, and secured to the neck muscle with 4-0 suture. The exteriorized end of the catheter was cauterized to prevent leakage. Placement was tested two days after catheterization with the intrathecal administration of 10 µl of 4% lidocaine. Animals not responding to lidocaine with temporary hind limb paralysis were euthanized and excluded from further study (~25%).
NPY antagonist studies in the SNI model
Due to the suboptimal success rate of the lumbar approach towards i.t. catheterization (~75%), subsequent studies used the classic cisternal approach (Yaksh and Rudy 1976; LoPachin et al. 1981). Under isoflurane anaesthesia, rats (290–310 g) were placed in a stereotaxic instrument (Stoelting, Wood Dale, IL) with the snout angled downwards 45° relative to the horizontal. A 1.5 cm midline incision extended from the ears to the atlas. Fascia and superficial neck muscles were bluntly dissected and retracted to expose the atlanto-occipital membrane. This membrane was punctured with the tip of a 27 g needle. A PE-10 catheter was inserted and advanced caudally 7.5 cm through the intrathecal space. When necessary, tension was applied to the tail to straighten the spine. The retractor was removed, the catheter was sutured to the neck muscle (5-0 Ethicon), and then the skin incision was closed. Finally, the external end of the catheter was cauterized to prevent any leakage or environmental contamination. Animals with signs of paralysis or other motor impairment lasting more than 24 hr were euthanized and excluded from further study (<20%).
Spared Nerve Injury (SNI) surgery
Immediately following intrathecal PU or PE catheterization, nerve injury was performed as described previously (Decosterd and Woolf 2000). An incision was made in the skin at the level of the trifurcation of the left sciatic nerve. The overlying muscles were retracted, exposing the common peroneal, tibial, and sural nerves. With care taken to avoid mechanical stimulation of the sural branch, the common peroneal and tibial nerves were loosely ligated with 6.0 silk (Ethicon, Somerville, NJ), followed by transection of 1 mm of each nerve at either end of each knot. The muscle was sutured with absorbable 5-0 sutures (Ethicon) and the wound was closed with 9mm metal clips.
Formalin studies: Drug Administration and Behavioral Testing
Drug Administration
One wk after surgery, a 4-cm piece of PE-10 tubing was connected to the intrathecal catheter assembly. The PE10 was loaded with 10 µl of drug or saline, a 1 µl air bubble, and a 10 µl saline flush. After a 30–60 min acclimation period, the rat was gently restrained, and then saline, NPY (30 µg) or morphine (20 µg) were intrathecally injected in total volume of 10 µl. Thirty min later, formalin (5% solution of 37% formaldehyde, 50µl) was subcutaneously injected into the plantar aspect of the right hindpaw, followed by behavioral measurement for 60 min.
Behavioral Testing
As previously described (Taylor et al. 1995a; Peterson et al. 1997), the number of flinches and time spent licking the injured paw during the second, third, fourth, and fifth min after injection were counted during Phase 1 (time points 1–4). Time points 1–3 and 3–5 were summed for graphical presentation. During the Interphase and Phase 2 (time points 8–60), the number of flinches and time spent licking were counted every five min, in two min bins, e.g. 8–10, 13–15, 18–20, … 58–60 (Mahinda and Taylor 2004).
Nerve Injury Studies: Drug Administration
One wk after surgery, drugs were administered as in the formalin studies. Seven d after SNI, allodynia and hyperalgesia were assessed before and after intrathecal administration of saline, NPY (3.0, 6.5, 10, or 30 µg), the Y1 antagonist BIBO3304 (1.0 µg), the Y2 antagonist BIIE0246 (1.0 or 10 µg), or a combination of NPY (30 µg) + BIBO (1.0 or 3.0 µg) or NPY (30 µg) + BIIE (0.01, 0.03, 0.3, 1.0, 3.0, or 10 µg). BIBO3304 was administered concomitantly with NPY. To take advantage of the extended duration of action of BIIE0246 (El Bahh et al. 2002), it was administered 90 min prior to NPY injection.
Some animals in this study (Fig 7–Fig 8) were not implanted with indwelling catheters. Instead, saline or drugs were administered via direct intrathecal injection (Mestre et al. 1994; Fairbanks 2003). Under light isoflurane anaesthesia (1.5%), a small midline skin incision (< 1 cm) exposed the muscle above the L5–L6 lumbar vertebrae. A 25 gauge needle (1.5 in), attached to a 50 µl Hamilton syringe, was inserted between the L5 and L6 vertebrae at an ~80 degree angle. Following contact with bone, the needle angle was lowered to ~30 degrees and advanced slightly until a reflexive tail flick was observed. Saline or drug was rapidly injected in a volume of 10 µl.
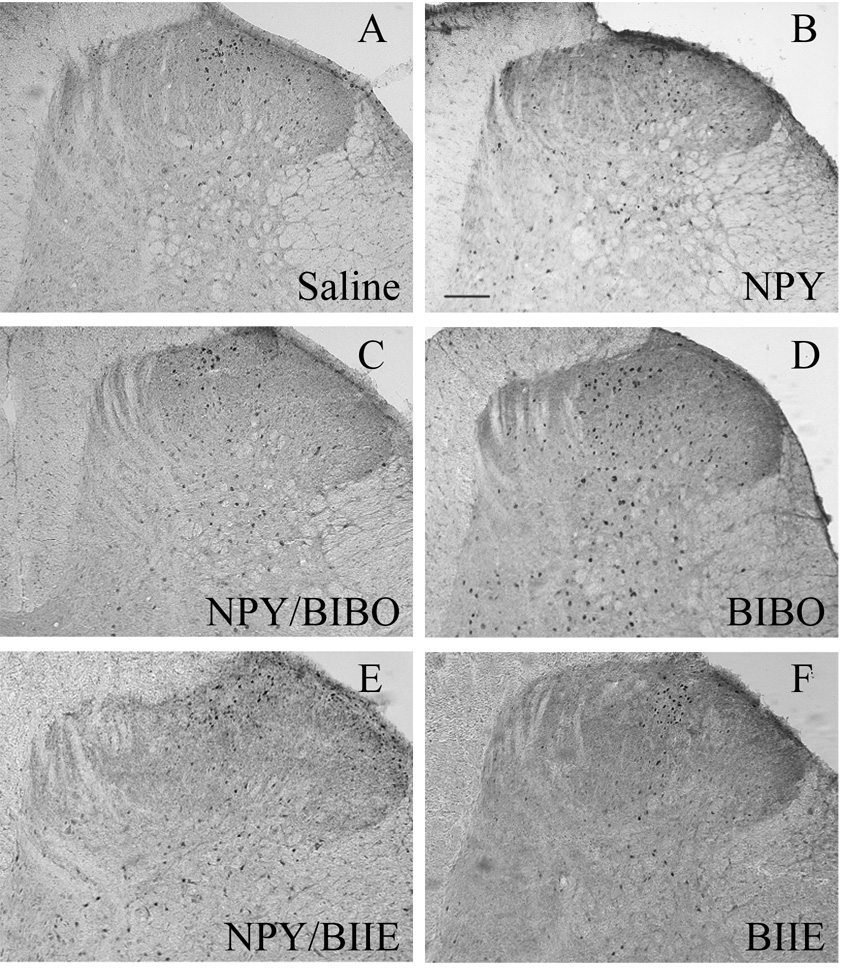
Figure 7. Tactile stimulus-induced Fos expression in rats with SNI.
These panels depict representative photomicrographs of the L4 dorsal horn from rats treated with intrathecal: (A) saline; (B) NPY; (C) NPY + the Y1 receptor antagonist BIBO3304 injected together; and (D) BIBO alone; (E) NPY + the Y2 receptor antagonist BIIE0246 given 90 minutes prior and (F) BIIE0246 alone. NPY reduced Fos in the dorsal horn. Magnification = 100x, scale bar = 200 µm. Only the side ipsilateral to SNI is shown.
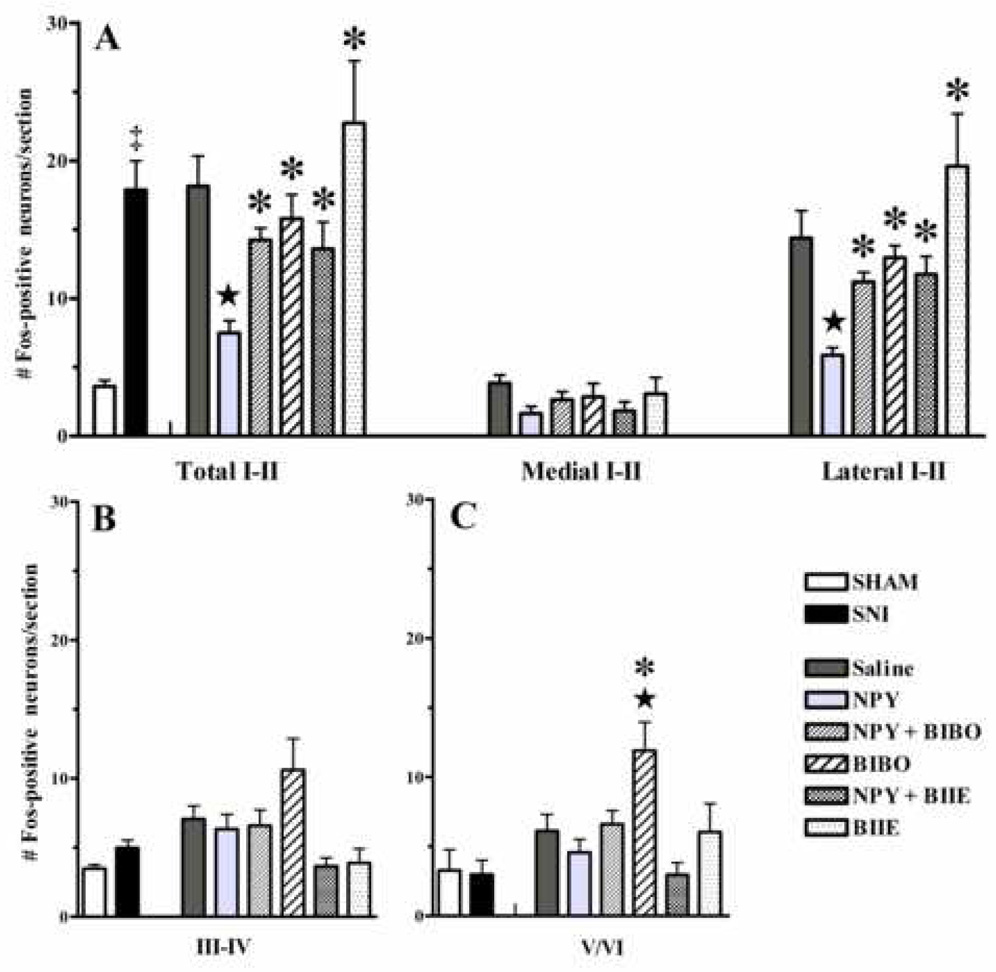
Figure 8. NPY acts at Y1 receptors to reduce non-noxious tactile stimulus-induced spinal Fos expression in animals with SNI.
Panel A illustrates that sham-injured rats (open bars) express only a small number of Fos-positive neurons in laminae I–VI following mechanical stimulation. SNI (solid bars) increases the number of Fos-positive neurons in I–II. NPY decreased Fos-LI in laminae I–II of the dorsal horn. This was reversed by concomitant administration of both the Y1 receptor antagonist BIBO3304 (BIBO, 3 µg) and prior (90 min) administration of the Y2 antagonist BIIE0246 (3 µg). NPY decreased Fos-LI in the lateral region of laminae I–II, which was reversed by both BIBO3304 and BIIE0246. Similar trends were observed in the medial region but did not reach statistical significance. n=5–13. Values represent mean ± SEM; ★P<0.05 vs saline control, *P<0.05 vs NPY, ‡P<0.05 vs sham.
Nerve Injury Studies: Behavioral Testing
Intrathecal injections were followed by either: 1. Behavioral testing for allodynia and hyperalgesia or 2. Somatosensory stimulation to induce Fos expression. Drug administration was randomized and the observer was blind to experimental condition.
Mechanical allodynia
Animals were acclimated to their testing boxes (transparent Plexiglass boxes placed on a raised metal mesh grid) for 30–60 min. A series of 8 von Frey (VF) monofilaments (Stoelting, Inc, Wood Dale, IL) were applied to the lateral aspect of the plantar surface of the hind paw. This area is innervated by the sural branch of the sciatic nerve. A modified version of the 50% withdrawal threshold was determined using the up-down method of Dixon, modified by Chaplan et al (Chaplan et al. 1994). First, an intermediate monofilament (number 4.31, exerts ~2.0g of force) was gently applied with enough force to cause it to bend. This was repeated up to three times in distinct areas along the lateral paw. In case of a positive response (rapid withdrawal of the paw within 2 sec), a smaller filament was tested. If no response was recorded in any of the three different areas, a larger filament was tested. Occasionally, animals did not develop mechanical allodynia on the day of pharmacological testing after nerve injury (VF threshold > 5.0 g on the nerve-injured side). In such cases, von Frey testing was either terminated immediately or its data was not included in the final analysis (<5%).
Cold allodynia
Using a syringe connected to PE-90 tubing, flared at the tip to a diameter of 3½ mm, we applied a drop of acetone to the plantar paw. Surface tension maintained the volume of the drop to 10–12 µl. The length of time the animal lifted or shook its paw was recorded. The duration of paw response was recorded for 30 sec. Three observations were averaged. Occasionally, animals did not develop cold allodynia on the day of pharmacological testing after nerve injury (<3 sec). In such cases, acetone testing was either terminated or its data was not included in the final analysis (<5%).
Mechanical hyperalgesia
Next, we gently applied the sharp edge of a diaper pin to the footpad, avoiding damage to the skin. The duration of paw withdrawal was recorded for 30 sec. Three observations were averaged. If animals did not develop mechanical hyperalgesia on the day of pharmacological testing (<2.5 sec), testing was terminated or its data was excluded from the study (7%).
Fos Stimulation
We were unable to evoke Fos expression with 3 applications/min for 10 min of the number 4.34, 5.5g von Frey hair or application of 1 drop of acetone every 30 sec for 10 min (data not shown). Therefore, we utilized the method of Ma et al (Ma and Woolf 1996). Briefly, while gently restraining the rat, the experimenter manually stroked the ventrolateral aspect of the hindpaw with their thumb for 2 sec. This was repeated every 4 sec for 10 min. Animals were perfused 2 hr after formalin injection or initiation of non-noxious stimulation.
Immunohistochemistry
Animals were deeply anesthetized with an intramuscular ketamine/xylazine (Vedco, St. Joseph, MO, Henry Schein, Melville, NY, respectively) injection (1 ml/kg of 88.9 mg/ml ketamine/11.1 mg/ml xylazine) and perfused with 150–250 ml PBS followed by 250–500 ml of 10% buffered formalin. Spinal cords were removed, post-fixed for 4–16 hr, and cryoprotected in 30% sucrose overnight. Forty micron sections were cut on a freezing microtome (Leica, Bannockburn, IL). After washing 4 × 15 min in 0.1 M PB and blocking for 1 hr in normal goat serum from the Vectastain ABC-AP kit (AK-5001, Vector Labs, Burlingame, CA), sections were incubated with a rabbit anti-Fos primary antibody (1:20,000, Calbiochem, San Diego, CA) at RT for 16–24 hr or at 4° for 48–72 hr. Next, the tissue was washed again with 0.1 M PB (4 × 15 min) and incubated with a goat anti-rabbit biotinylated secondary antibody, also from the Vectastain ABC-AP kit, for 2 hr at 4°. After 3 × 5 min washes in 0.1 M PB, the tissue was incubated with an avidin/biotinylated enzyme complex from the Vectastain Elite ABC kit (PK-6100, Vector Labs) for 1 hr at 4°. The tissue was again washed 4 × 15 min in 0.1 M PB. For visualization of the Fos protein product, tissue slices were incubated in a DAB substrate kit (SK-4100, Vector Labs) for approximately 5 min, and then rinsed in 0.01 M PB for 3 × 10 min. Sections were mounted on Superfrost/Plus glass slides (Fisherbrand, Houston, TX) and then cover-slipped using Permount (Fisherbrand, Houston, TX). Using a Nikon TE2000-E microscope with Metamorph software (Version 6.1r4, Universal Imaging Corp.), digital photomicrographs of 4–5 randomly selected sections were taken from lumbar segment L4–L5. Pictures were printed and Fos-like immunoreactive (Fos-LI) profiles were manually counted by an observer blind to treatment, as described previously (Abbadie et al. 1997).
To quantify Fos-LI for mediolateral analysis, we used a technique developed by Sugimoto et al. (Sugimoto et al. 1993), based on the anatomic findings of Swett and Woolf (Swett and Woolf 1985). We measured the mediolateral extent of the dorsal horn and divided it into eight equal horizontal segments. The most medial 3 segments roughly correspond with the tibial territory, while the next 3 segments correspond to the common peroneal/sural innervation territories. After dividing the images in this manner, the neurons within the segments were manually counted by an observer blind to treatment. The most lateral two segments are not innervated by the sciatic nerve and therefore not analyzed further.
Materials
Human NPY, obtained from Anaspec (Cat #22465, San Jose, CA), was diluted in distilled water, divided into aliquots, and frozen at −80°C until use. The Y2 receptor antagonist BIIE0246 (Cat # 1700, Tocris, Ellisville, MO) was diluted in 10% DMSO and made fresh as needed. The Y1 receptor antagonist BIBO 3304 was generously provided by H. Doods (Boehringer Ingelheim, Biberach, Germany) and diluted in saline as needed. Lidocaine was obtained from Bimeda (Dublin, Ireland). Isoflurane was from Abbott Labs (Chicago, Ill).
Data Analysis
To analyze the formalin behavior studies (Fig. 1), we used GraphPad Prism 4 software (GraphPad Software, Inc. San Diego, CA), to conduct two-way ANOVAs across the entire 60 min period, with Drug as the between-subjects factor and Time as the repeated measure. When F values were significant (p<.05), Bonferroni’s post hoc tests were used to determine differences between drugs at each time point. Separate ANOVAs were conducted for Phase 1 and Phase 2, comparing NPY and morphine to saline. Bonferroni post hoc tests were used to determine differences between drugs in each Phase.
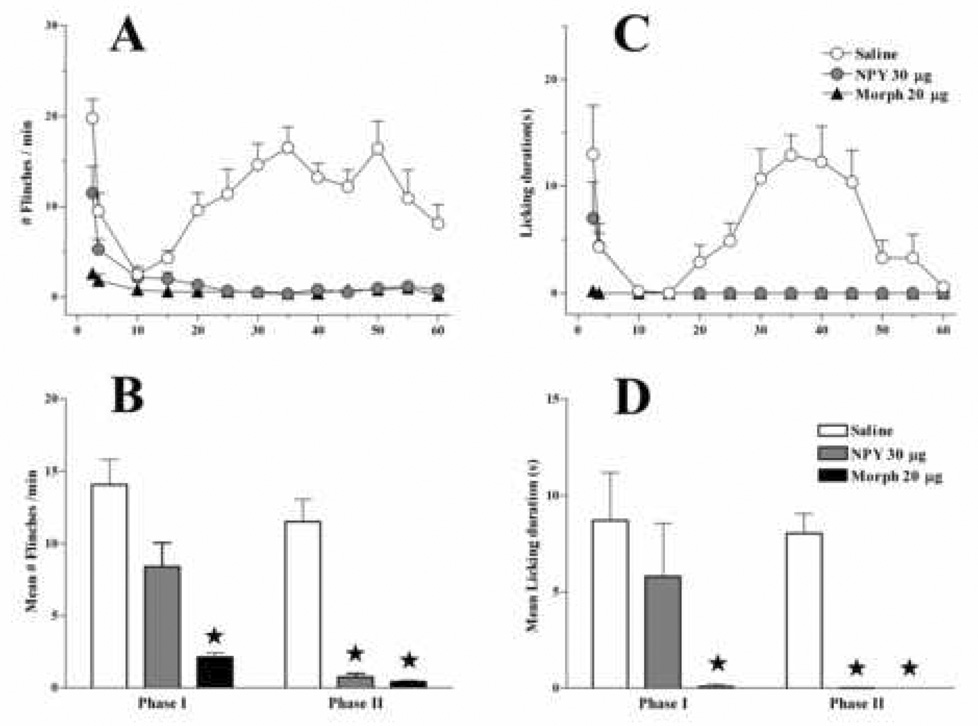
Figure 1. Intrathecal NPY reduces formalin-induced behaviors.
Intraplantar administration of formalin (5% in 50 µl injected at time 0) elicits biphasic increases in both the number of flinches/min (A) and seconds spent licking the injured paw (C) following intrathecal injection of saline (n=10). Intrathecal NPY 30 min prior to formalin injection significantly reduced Phase I flinching and licking and completely blocked Phase II (n=6). Intrathecal morphine (n=5) almost completely abolished behavior during both phases. Values for Phase I represent averages from time 0–5; Phase II values are averages from time 20–55 (B, D). Values represent mean ± SEM; * = P<0.05 vs saline.
For the dose-response SNI studies (Fig. 4), two-way ANOVAs were conducted, with Dose as the between-groups factors with five levels (saline, NPY 3 µg, NPY 6.5µg, NPY 10µg, and NPY 30 µg). Significant main effects involving Dose were further investigated with one-way ANOVA comparing the saline group with each dose of NPY from time 0–90 min. For the NPY antagonist studies (Fig. 5), we conducted one-way ANOVA with Drug as the between groups factor. When F values were significant (P<0.05), post hoc Bonferroni or Student’s t-test was used to determine differences between two groups.
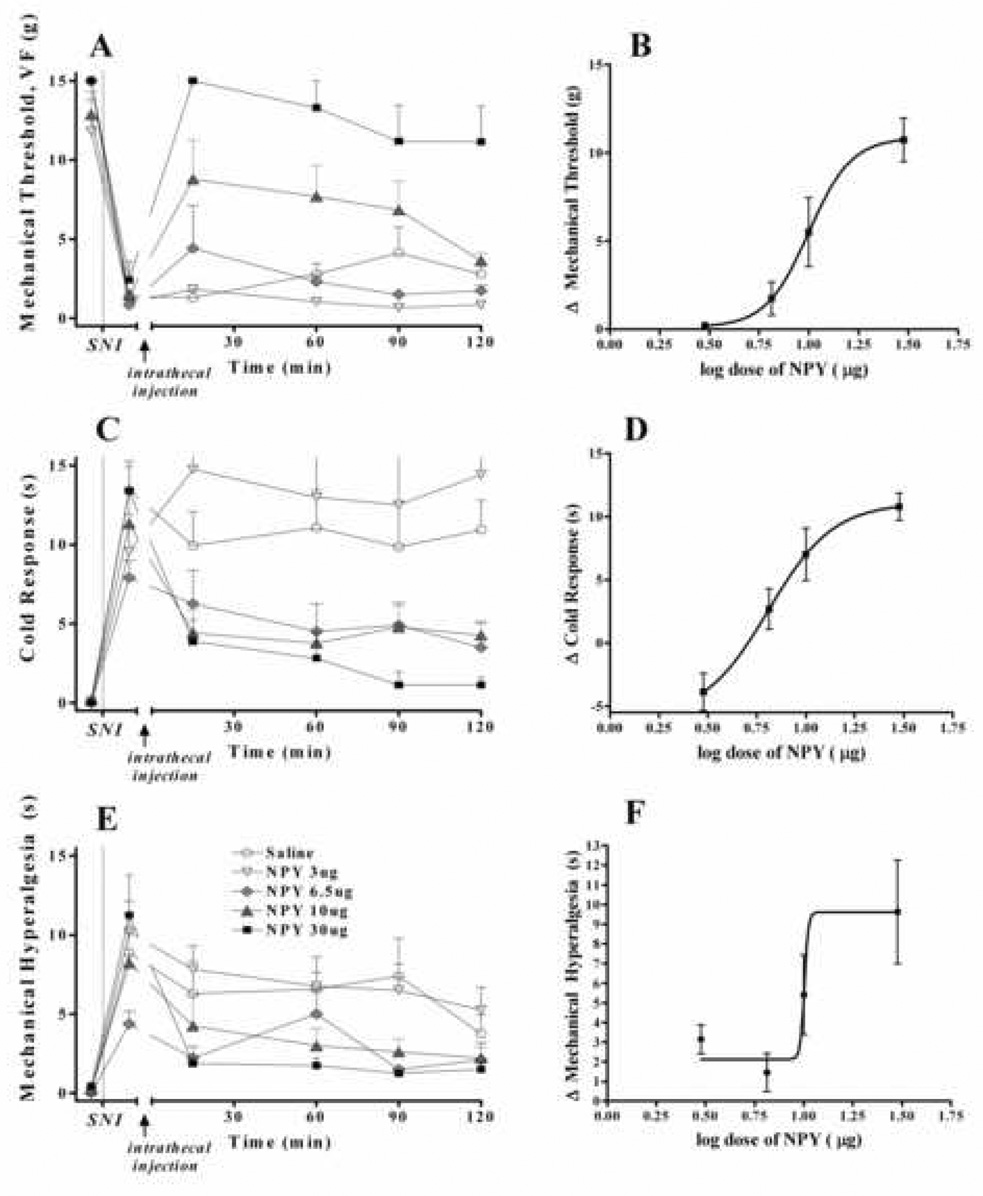
Figure 4. NPY dose dependently attenuates behavioral signs of neuropathic pain.
Two wk after spared nerve injury, intrathecal administration of NPY reduced paw withdrawal responses to the plantar application of von Frey filaments (Panels A–B). Post-hoc comparison of baseline value with the averaged value of time points 15–90 revealed a statistically significant difference (P<0.0001) between saline (1.3±0.7) and 30 µg NPY (10.7±1.3). Panels C–D illustrate that NPY produced a strong trend towards reduction of paw withdrawal duration in response to the intraplantar application of acetone (cold response). The difference between baseline value and the averaged value of time points 15–90 for the saline group (3.0 ± 2.5) and the 30 µg NPY group (10.8±1.1) just missed statistical significance (P=0.063). Panels E–F illustrate that NPY reduced paw withdrawal responses to the plantar application of a diaper pin (mechanical hyperalgesia). Post-hoc comparison of baseline value with the averaged value of time points 15–90 revealed a statistically significant difference (P<0.05) between saline (2.1±1.2) and 30 µg NPY (9.6±2.6). Figures B, D and F show log dose-response curves for NPY. n=4–8. Values represent mean ± SEM.
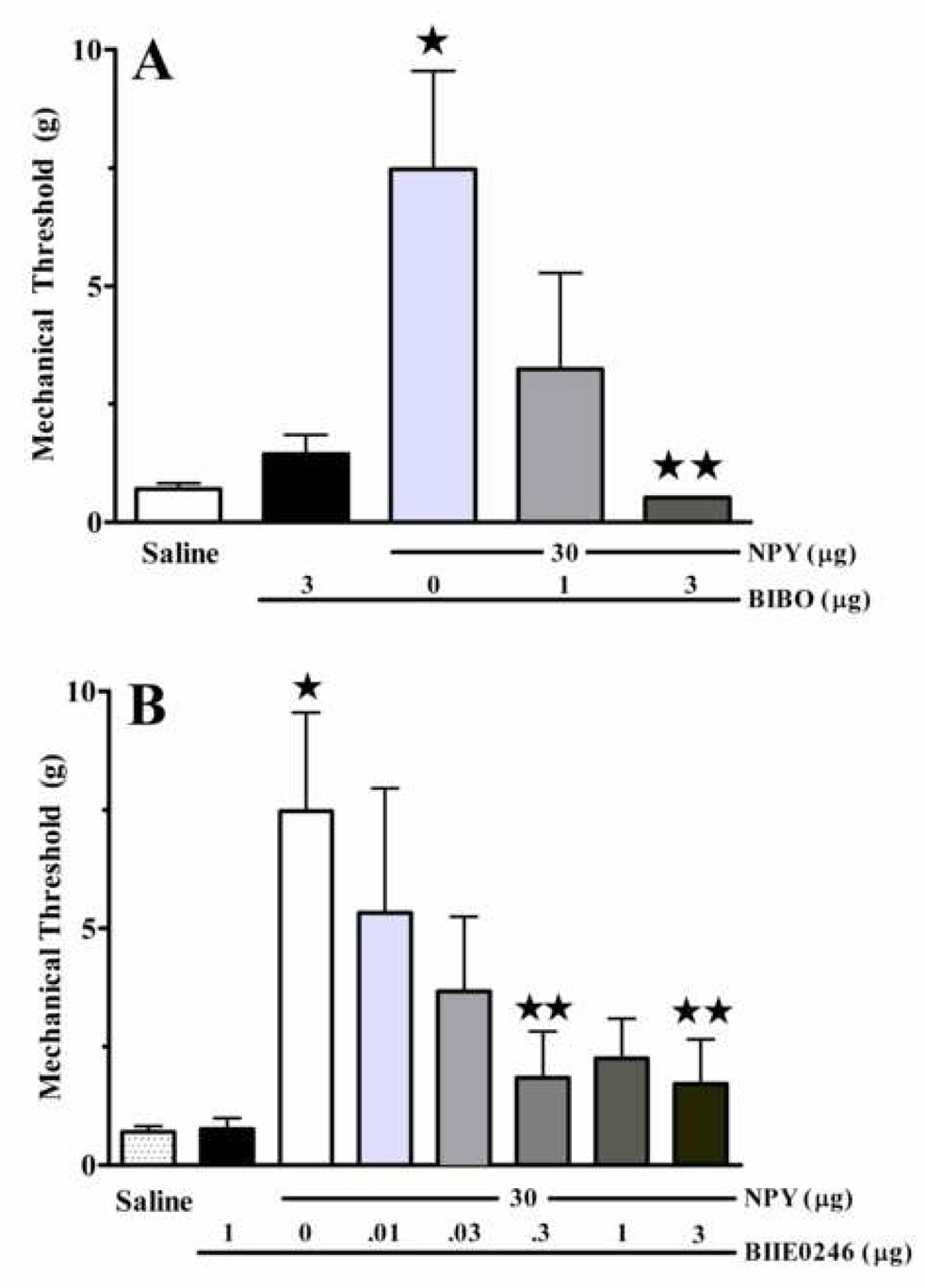
Figure 5. Both Y1 and Y2 receptors contribute to the anti-allodynic effects of NPY.
Mechanical allodynia after spared nerve injury was reduced 45 min after intrathecal administration of NPY. Concurrent administration of the Y1 receptor antagonist BIBO3304 (A), or pre-administration of the Y2 receptor antagonist BIIE0246 (B), dose-dependently reversed the anti-allodynic effect of NPY. Each antagonist had little effect when administered alone. n= 4–8. Values represent mean ± SEM. * p<0.05 vs saline; ** p<0.05 vs NPY.
For the Fos studies, we conducted two-way ANOVA to analyze the effect of Drug and laminar region of the dorsal horn (Laminae I–II, Laminae III–IV, and Laminae V–VI) on the number of Fos-positive neurons. Further one-way ANOVAs were conducted on the effect of Drug within each laminar group. When F values were significant (P<0.05), post-hoc Bonferroni and Student’s t-tests were used to determine the differences between two drugs groups within each laminar regions: saline vs NPY or morphine (Fig. 3); saline vs NPY or NPY/BIBO or BIBO and NPY/BIIE or BIIE (Fig. 8). All data are presented as mean ± SEM.
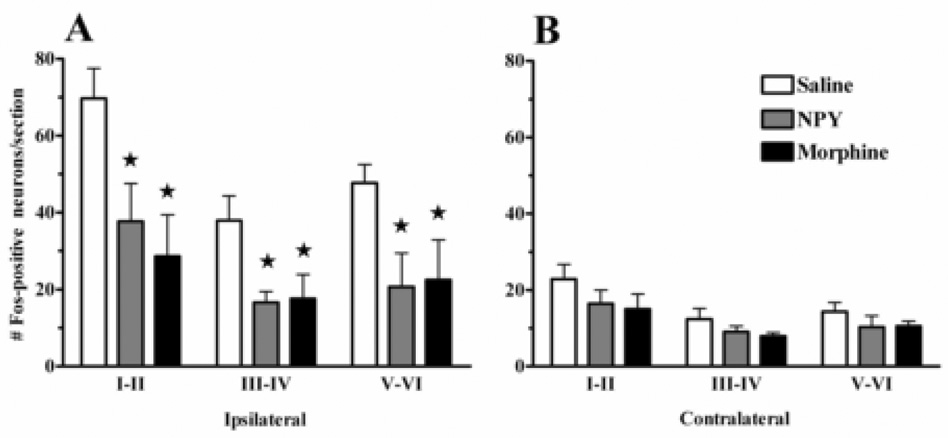
Figure 3. NPY reduces formalin-induced Fos expression in the dorsal horn.
Both NPY (30 µg) and morphine (20 µg) decreased Fos expression in all laminae of the L4–L5 dorsal horn on the side ipsilateral (A) but not contralateral (B) to formalin injection. Values represent mean ± SEM. * p<.05 vs saline. n = 5–9.
RESULTS
Intrathecal NPY reduces formalin-induced behavior and spinal Fos expression
Noxious stimulus-induced Fos expression is the best method to map populations of nociresponsive neurons in the dorsal horn of the awake animal (Coggeshall 2005). To test the hypothesis that intrathecal administration of NPY inhibits spinal nociceptive transmission, we evaluated formalin-induced behavior and Fos expression. Based on previous dose-response and toxicity studies, we chose an analgesic dose of NPY (30 µg) that lacks non-specific motor effects (Taiwo and Taylor 2002; Mahinda and Taylor 2004). For comparative purposes, we evaluated the effects of morphine at a dose (20 µg) that is equi-analgesic to NPY in terms of inhibition of Phase 2 formalin behavior (Yamamoto and Yaksh 1992). Morphine served as a positive control because it consistently reduces formalin-induced behavior and Fos expression (Menetrey et al. 1989).
As illustrated in Fig. 1, formalin produced overt behavioral signs of nociception with the expected biphasic profile in saline control animals, and two-way ANOVA revealed that Drug reduced flinching [F(2,216) = 29, P<0.0001] and licking [F(2,216) = 25, P<0.0001]. As observed previously (Mahinda and Taylor 2004), NPY did not significantly reduce Phase I flinching or licking (P>0.05), while morphine completely inhibited flinching and licking during Phase I (P<0.05). In contrast, both NPY and morphine blocked Phase II: one-way ANOVA revealed that Drug reduced Phase II flinching [F(2,18) = 26, P<0.001] and licking [F(2,18) = 34, P<0.001]. Qualitative assessment of gross motor behavior indicated that morphine, but not NPY, produced mild signs of sedation.
As described previously, basal Fos expression in the dorsal horn is quite low (data not shown). Fig 2 illustrates that unilateral hindpaw injection of formalin produced a robust increase in Fos expression throughout the ipsilateral L4–5 dorsal horn, particularly in laminae I–II. Fig 2 and Fig 3 illustrate that both NPY [F(2,17) = 10.89, P<0.01] and morphine [F(2,17) = 10.89, P<0.01] reduced Fos-LI by roughly 50% throughout the dorsal horn (laminae I–VI), and further post-hoc tests indicated significant effects within each laminar region. Lower numbers of Fos-LI was observed on the contralateral side and was changed by neither NPY nor morphine (P>0.05).
Figure 2. Formalin-induced Fos expression in the dorsal horn.
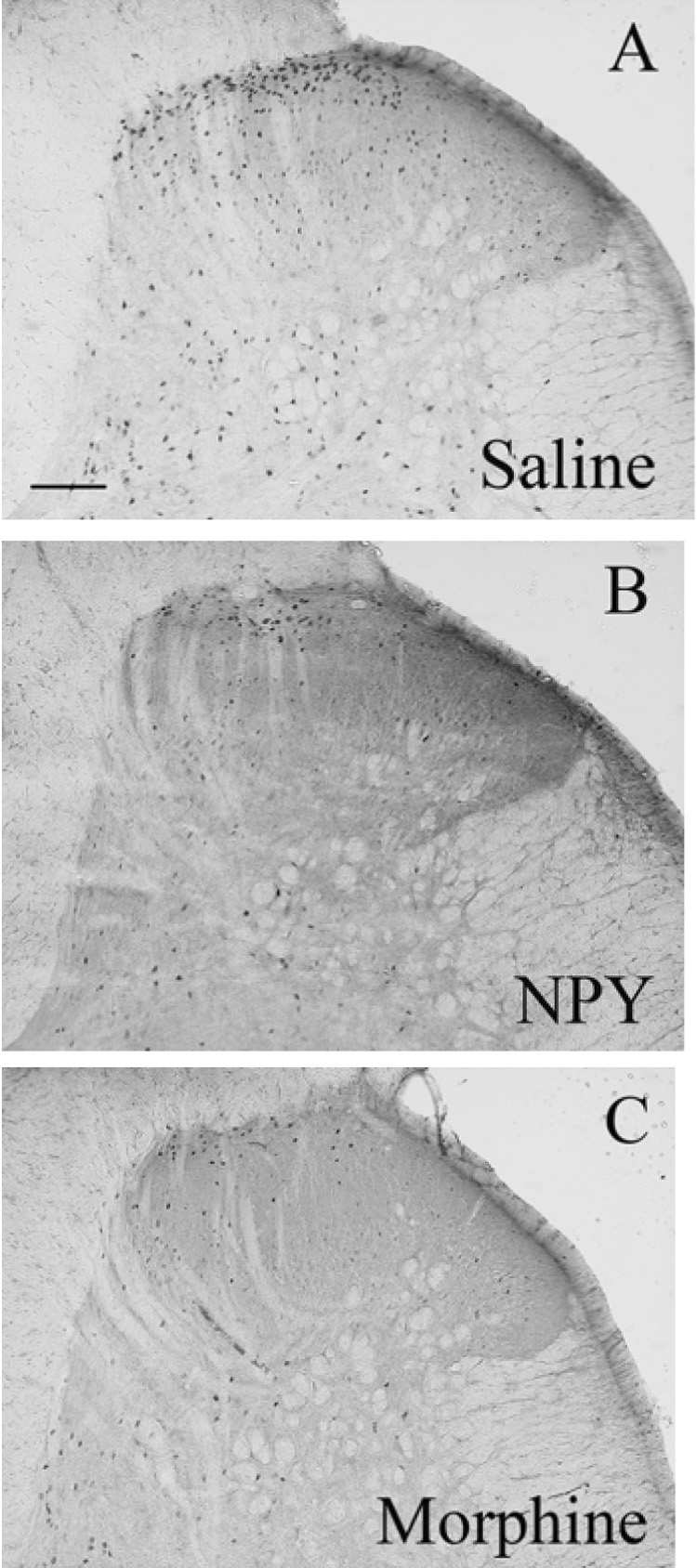
These panels depict representative photomicrographs of the L4 dorsal horn for each group of animals treated with intrathecal saline (A), NPY (B) or morphine (C). Animals were perfused 2 hr after intraplantar formalin. Only the side ipsilateral to formalin injection is shown. Magnification = 100x, scale bar = 200 µm.
Our previous study investigated the contribution of NPY Y1 and Y2 receptors to formalin nociception, and we found that BIBO partially reversed flinching behavior, while BIIE had no effect (Mahinda and Taylor, 2004). Thus, the Fos studies described below focus on the actions of NPY receptor antagonists in neuropathic pain, rather than in formalin-induced pain.
Intrathecal NPY dose-dependently attenuates behavioral signs of neuropathic pain
To test the hypothesis that NPY inhibits behavioral signs of neuropathic pain, we evaluated allodynia and hyperalgesia after intrathecal injection of saline or drug. As illustrated in Fig 4, NPY dose-dependently inhibited mechanical allodynia [F(4,25) = 7.8, P<0.001], mechanical hyperalgesia [F(4,24) = 3.1, P<0.05], and cold allodynia [F( 4,27), = 4.98 P<0.05]. In general, these effects peaked at 15 min and lasted over 120 min (Fig 4A, C, E). For example, the 30 µg dose significantly reduced mechanical allodynia at the 15 min (P<0.01) and 60 min (P<0.05) time points. When responses were averaged between 15–90 min, the 30 µg dose significantly reduced mechanical allodynia (P<0.0001) and mechanical hyperalgesia (P < 0.05), and almost reached significance in reducing cold allodynia (P = 0.063). The 10 µg dose also reduced mechanical allodynia, but again this effect missed statistical significance (P = 0.077). The ED50 for NPY was 9.98 µg (mechanical allodynia) and 6.45 µg (cold allodynia) (Fig 4B, D). We could not determine ED50 for mechanical hyperalgesia due to the non-sigmoidal relationship between dose and response (Fig 4F). NPY did not change paw withdrawal behavior on the contralateral paw at any dose (data not shown).
As an additional control, we next determined whether NPY changes sensitivity in animals without nerve injury. We observed no significant difference in VF (15±0 vs 14.7±0.3), acetone (0.21±0.03 vs 0.06±0.06) or pin prick (0.36±0.07 vs 0.23±0.06) responses between the saline vs 30 µg NPY groups, respectively (P>0.05).
Both Y1 and Y2 receptors contribute to the anti-allodynic effect of intrathecal NPY
To evaluate the NPY receptor subtype that mediates the anti-allodynic effects of NPY, we tested subtype-selective Y1 and Y2 receptor antagonists (BIBO3304 and BIIE0246, respectively.) As illustrated in Fig 5A, BIBO3304 dose-dependently reversed the anti-allodynic effects of NPY [F(4,26) = 4.04, P<0.05], with complete inhibition at the 3 µg dose. Similarly, Fig 5B illustrates that BIIE0246 dose-dependently attenuated the anti-allodynic effects of NPY [F(9,39) = 2.216 P<0.05], with a maximal effect at the 0.3 and 3 µg doses (the 1 µg dose yielded an effect that missed statistical significance, p=0.062, n=6). Neither antagonist had an appreciable effect on behavioral responses elicited from the contralateral paw (data not shown).
Comparison of formalin-vs SNI-induced Fos expression
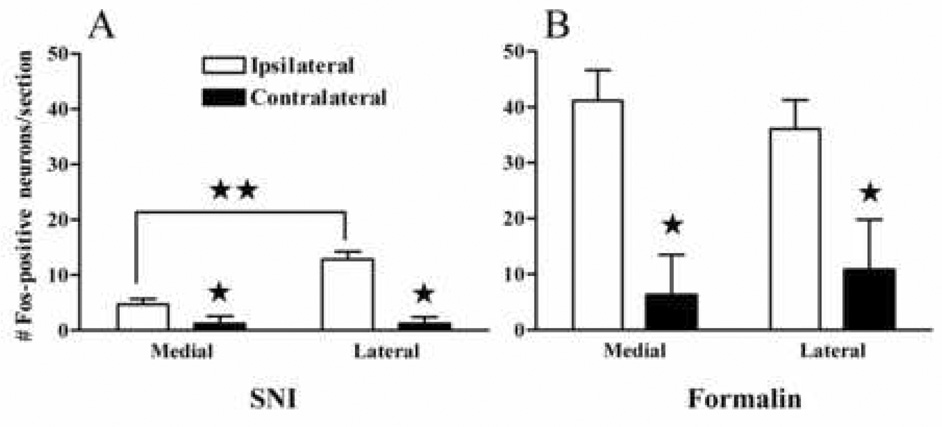
We divided the dorsal horn into 8 mediolateral subdivisions so as to more closely examine the somatotopic pattern of Fos-LI. The mediolateral distribution of Fos in the dorsal horn was quite different in formalin and SNI animals. Formalin evoked Fos-LI throughout the medial and central regions of the superficial dorsal horn (Fig 2 and Fig 6b) indicate a consistent mediolateral distribution of Fos-LI. In contrast, Fos-LI evoked by tactile stimulation + SNI occurred primarily within the central region. Thus, as illustrated in Fig. 6a and Fig 7a, the number of Fos-positive neurons was significantly lower in the medial region as compared to the lateral region (5.0 ± 1.0 vs 13.0 ± 1.6, respectively; P<0.001). The medial region corresponds to the innervation territory of the transected tibial nerve in our SNI model, as predicted by the seminal work of Swett and Woolf (Swett and Woolf 1985; Shields et al. 2003).
Figure 6. Distinct mediolateral patterns of Fos expression are expressed between the formalin and SNI models.
Non-noxious tactile stimulation induced nearly a 3-fold increase in Fos expression in the area of the dorsal horn innervated by the common peroneal and sural nerves as compared to the area innervated by the tibial nerve (A). After formalin injection, there was no difference in Fos expression between either of the ipsilateral regions that were analyzed (B). n=7–12. Values represent mean ± SEM; *p<0.05 vs ipsilateral. **p<0.05 medial vs lateral.
We were concerned that variability of Fos-LI in the SNI model could reduce analytical power. To address this, we roughly compared variability in total ipsilateral Fos-LI following SNI with a more established paradigm, the formalin test. We found that variability (standard deviation divided by the group mean) was 42% and 39% in the formalin and SNI groups, respectively. The similar variability indicates that SNI-induced Fos is at least as reliable a measure of spinal neuron activation as is formalin-induced Fos.
Further characterization of Fos-LI after SNI
Although nerve injury alone does not trigger Fos expression (Rodella et al. 2005), mechanical stimulation of the hindpaw after peripheral nerve injury does (Catheline et al. 1999). Fig 8 illustrates robust Fos expression in laminae I–II of nerve-injured rats at the ipsilateral side. This was greater than that evoked either on the contralateral side of SNI rats (17.9±2.1 vs 5.1±1.4; P<0.01, data not shown) or on the ipsilateral side of sham rats (17.9±2.1 vs 3.6± 0.4; P<0.005). As compared to sham, nerve injury induced a small, statistically insignificant change in contralateral Fos-LI in laminae I–II (5.1±1.5 vs 1.8±0.5; P>0.05).
Previous studies have reported that either intrathecal catheterization or vehicle injection bilaterally increases Fos expression in the dorsal and ventral horns (Luo et al. 1995). However, Fig 8A shows that stimulus-induced Fos expression in the setting of SNI did not differ between non-catheterized rats (17.9±2.1, second bar from the left) and intrathecally-catheterized rats given saline (18.1±2.2, third bar from the left).
NPY reduces stimulus-induced Fos expression in animals with SNI
Fig 7 and Fig 8 illustrate that drug treatment changed the number of Fos-positive neurons in laminae I–VI. Further analysis of laminar regions revealed an effect in laminae I–II [one-way ANOVA: F(5,41) = 4.76; P=0.0019], laminae III–IV [one-way ANOVA: F(5, 41) = 3.2; P<0.05], and laminae V–VI [one-way ANOVA: F(5, 41) = 3.6; P<0.05]. Further analysis in the mediolateral plane revealed an overall drug effect in the lateral region [F(5,41) = 4.79; P=0.0018] but not the medial region (P>0.05). Indeed, all subsequent analyses (see below) suggest that drug effects in lamina I–II were localized to the lateral aspects of the dorsal horn (Fig 8A).
Fig 7B and Fig 8A–C illustrate that, compared to saline, intrathecal NPY significantly reduced the total number of Fos-positive neurons in laminae I–II by 59% (18.1±2.2 vs 7.5±0.9; P<0.01), but not laminae III–IV or V–VI (P>0.05). As illustrated in Fig 7C and Fig 8, the Y1 receptor antagonist BIBO3304 reversed the effect of NPY, and Fos-LI in laminae I–II was similar to those of the saline group (14.2±0.9 vs 18.1±2.2, respectively; P>0.05). Fig 7D and Fig 8 indicates that BIBO alone had no effect in laminae I–II or III–IV, but increased Fos-LI in laminae V–VI (P<0.05, Fig 8C).
As shown in Fig 7E and Fig 8A, the Y2 receptor antagonist BIIE0246 also reversed the effect of NPY; Fos-LI in laminae I–II was similar to those of the saline group (13.6±1.9 vs 18.1±2.2, respectively; P>0.05). Fig 7F and Fig 8 indicates that BIIE alone had no significant effect as compared to saline (P>0.05).
DISCUSSION
NPY reduces noxious stimulus-induced behavior
Early nociception in the formalin test
The present studies demonstrate that intrathecal administration of morphine (20 µg) abolished Phase 1. This agrees with previous findings indicating that intrathecal morphine and other opioid receptor agonists, like remifentanil, decrease formalin-induced behavior (Yamamoto and Yaksh 1992; Taylor and Basbaum 2000). By comparison, the 30 µg dose of NPY only partially attenuated Phase 1 and did not disrupt motor coordination (Taiwo and Taylor 2002). The present results suggest NPY is no better than morphine at inhibiting formalin-induced nociception during Phase 1.
Persistent nociception in the formalin test
The present studies indicate that both NPY (30 µg) and morphine (20 µg) eliminated Phase 2 (Yamamoto and Yaksh 1992; Mahinda and Taylor 2004). Unlike morphine, NPY did not produce sedation. These results confirm our previous conclusion that NPY is efficacious in reducing the persistent nociception associated with formalin injection (Mahinda and Taylor 2004).
NPY reduces noxious stimulus-induced spinal Fos expression
While many studies indicate that systemic morphine reduces noxious stimulus-induced Fos expression (Menetrey et al. 1989), far fewer studies have utilized the intrathecal route of administration. One article indicated that a 10 µg dose of morphine reduced formalin-induced Fos expression by roughly 40% (Labuz et al. 2003). Here, we administered doses of morphine and NPY that produce maximal reduction in formalin-induced behavior, and asked whether NPY would be as effective as morphine in reducing Fos-LI. We found that 20 µg of morphine reduced Fos-LI in profiles of laminae I–VI by 56%, while 30 µg NPY reduced Fos by 51%. Because both phases of the formalin test substantially contribute to Fos-LI (Abbadie et al. 1997), the ability of NPY to reduce Fos to the same extent as morphine, without eliminating Phase 1, suggests that this peptide is an outstanding candidate for the interruption of spinal nociceptive transmission.
NPY reduces behavioral signs of neuropathic pain
Although Naveilhan et al. reported that intrathecal NPY decreased the heat hypersensitivity associated with partial sciatic nerve injury in mice, this study only evaluated a single dose of NPY and a single behavioral endpoint (Naveilhan et al. 2001). Our studies extend these results to the SNI model and to the rat, and show that NPY dose-dependently inhibits behavioral signs of neuropathic pain. NPY increased von Frey threshold and reduced response duration to both noxious mechanical and cold stimuli, indicating that its inhibitory effects span multiple sensory modalities. NPY inhibition does not result from non-specific behavioral effects, because these doses of NPY do not disrupt motor coordination (Taiwo and Taylor 2002).
We conclude that intrathecal NPY reduces behavioral signs of neuropathic pain. This conclusion neither supports nor contradicts the findings of Ossipov et al, who found that intracranial administration of anti-NPY antiserum or BIBO3304 into the nucleus gracilis reversed nerve injury-induced mechanical allodynia (Ossipov et al. 2002). A harmonious explanation is that NPY can either inhibit or facilitate neuropathic pain, depending on whether it is administered in the spinal cord or nucleus gracilis, respectively.
NPY Y1 receptors contribute to the anti-allodynic actions of NPY
The Y1 antagonist BIBO3304 dose-dependently reversed the anti-allodynic and antihyperalgesic actions of NPY. Furthermore, BIBO3304 reversed the inhibitory effect of NPY on Fos-LI, a molecular correlate of spinal neuron activation. We conclude that intrathecal NPY acts at spinal Y1 receptors to inhibit the activation of pain-related neurons in the dorsal horn, ultimately leading to a reduction in behavioral signs of neuropathic pain.
NPY Y2 receptors contribute to the anti-allodynic actions of NPY
The present studies indicate that the Y2 receptor antagonist BIIE0246 dose-dependently reversed the anti-allodynic effects of NPY in the SNI model of neuropathic pain. We also found that BIIE0246 reversed the inhibitory effect of intrathecal NPY on Fos-LI in the superficial dorsal horn. Taken in combination with our Y1 antagonist data, our results suggest that NPY acts at both receptors to inhibit spinal neuron activation. Our results extend previous studies indicating that Y2-preferring agonists inhibit the flexor reflex in axotomized, anesthetized rats, an alternative model of neuropathic pain (Xu et al. 1999). In contrast to the cellular localization of Y1 receptors, spinal Y2 receptors are located on primary afferent terminals (Brumovsky et al. 2005). Because axotomy increases Y2-mediated inhibition of Ca2+ channel currents in dorsal root ganglion neurons (Abdulla and Smith 1999), and NPY attenuates excitatory postsynaptic currents in substantia gelatinosa cells via presynaptic Y2 receptors (Moran et al. 2004), we suggest that NPY acts at presynaptic Y2 receptors to inhibit the release of pronociceptive neurotransmitters. One such neurotransmitter could be glutamate, since Y2 agonists inhibit glutamate release from spinal synaptosomes (Martire et al. 2000).
Spinal Fos expression in the spared nerve injury model of neuropathic pain
This report is the first to validate stimulus-evoked Fos expression in the dorsal horn after SNI. Variability was no greater than that produced in a more established paradigm, the formalin test. As in other models of peripheral neuropathic pain (Catheline et al. 1999), SNI alone induced little Fos-LI. However, the application of a non-noxious mechanical stimulus to the hindpaw substantially increased the number of Fos-positive profiles in the substantia gelatinosa. Similar increases in laminae I–II Fos expression were obtained following the application of a non-noxious tactile stimulus in the sciatic nerve crush and chronic constriction injury models of peripheral neuropathic pain (Bester et al. 2000; Catheline et al. 2001).
We found that immunoreactive cells were particularly abundant in the mediolateral area innervated by central terminals of the spared sural nerve, as predicted by the anatomical studies of Swett and Woolf (Swett and Woolf 1985; Shields et al. 2003). By contrast, only a few Fos-positive profiles were found in the three medial-most subdivisions, areas of the dorsal horn likely innervated by the (cut) tibial nerve. We found that NPY significantly reduced Fos-LI in the lateral region; this was reversed by both BIBO3304 and BIIE0246. Because stimulus-induced Fos expression did not differ between non-catheterized and catheterized rats, intrathecal catheterization did not confound our results as might have occurred in previous studies (Luo et al. 1995). We conclude that NPY acts at Y1 and Y2 receptors in the region of the spinal cord that receives afferent input from the uninjured sural nerve.
Our studies revealed that intrathecal BIBO3304 not only reverses NPY-induced reduction of Fos-LI in laminae I–II, but also, when administered alone, significantly increases Fos-LI in laminae V–VI. Y1 receptors label numerous laminae within the dorsal horn, including “Type 1” small fusiform neurons of laminae I–II, and large “Type 5” multipolar lamina V neurons (Brumovsky et al. 2006). We speculate that the Y1 receptor antagonist BIBO3304 increased Fos-LI in lamina V by: 1. Indirect actions at Type I Y1 interneurons in lamina II that project to lamina V (Eckert et al. 2003); or 2. Direct actions at Type 5 Y1 projection neurons. Thus, Y1 receptor-mediated inhibition of nociceptive transmission may occur at multiple laminar levels: at laminae I–II to inhibit the actions of exogenously-administered NPY, and ultimately at deeper lamina V to inhibit the actions of endogenous neurotransmitters.
Although nerve injury produced an almost complete absence of Fos staining in the medial portion of the dorsal horn, we did observe more neurons in this region in SNI animals than in controls. We discuss two explanations for this unexpected finding. First, Sugimoto et al. found that tibial neurotomy acutely ablated intraplantar formalin-induced Fos staining in this region at one week; interestingly, staining began to normalize after just two weeks (Sugimoto et al. 1993; Sugimoto et al. 1994). We speculate that after SNI, afferent terminals from the sural innervation territory sprout into the tibial region, thus allowing peripheral stimulation of the uninjured sural nerve to activate Fos in the tibial innervation territory. Our study falls within the suggested timeframe for sprouting after peripheral nerve injury (Woolf et al. 1995; Kohama et al. 2000). A second hypothesis for the appearance of Fos-LI in the medial dorsal horn after SNI is an increase in synaptic input, due to trans-synaptic dendritic atrophy. Sugimoto et al. (Sugimoto et al. 1990) found that chronic constriction injury was sufficient to induce transsynaptic degeneration, which, if it occurred on interneurons, would cause a loss of inhibitory tone and ultimately increase projection neuron activation. However, an important caveat to this conclusion is that not all cells that express Fos-LI are projection neurons; some are GABA- or glycinergic, and therefore are inhibitory (Blomqvist and Craig 2000).
Summary
Taken together, our results suggest that intrathecal NPY acts at Y1/Y2 receptors to inhibit spinal neuron activation, leading to antinociceptive and anti-allodynic effects. NPY inhibition of SNI-induced Fos expression was localized to the sural (uninjured) innervation territory. Such conclusions cannot be drawn from Fos-LI experiments using other common models of neuropathic pain, such as chronic constriction injury or partial sciatic nerve ligation (due to the mixed topographic distribution of spinal axon terminals of injured afferents). Our results illustrate the value of using SNI to study neuroplasticity in the dorsal horn following peripheral nerve injury. In conclusion, Y1- or Y2-selective receptor agonists may be clinically effective in the treatment of neuropathic pain.
Acknowledgements
The study was supported by NIH grant NS45954 to BKT. We thank Christine Robinson for her technical help with the behavioral pharmacology studies in SNI rats following intrathecal NPY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the patter of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Smith PA. Nerve injury increases an excitatory action of neuropeptide Y and Y2- agonists on dorsal root ganglion neurons. Neuroscience. 1999;89(1):43–60. doi: 10.1016/s0306-4522(98)00443-6. [DOI] [PubMed] [Google Scholar]
- Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol. 2000;428(1):45–61. doi: 10.1002/1096-9861(20001204)428:1<45::aid-cne5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Craig AD. Is neuropathic pain caused by the activation of nociceptive-specific neurons due to anatomic sprouting in the dorsal horn? J Comp Neurol. 2000;428(1):1–4. [PubMed] [Google Scholar]
- Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hokfelt T. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138(4):1361–1376. doi: 10.1016/j.neuroscience.2005.11.069. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol. 2005;489(3):328–348. doi: 10.1002/cne.20639. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Besson JM. Intravenous morphine does not modify dorsal horn touch-evoked allodynia in the mononeuropathic rat : a Fos study. Pain. 2001;93(3):389–398. doi: 10.1016/S0304-3959(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Honore P, Besson JM. Are there long-term changes in the basal or evoked Fos expression in the dorsal horn of the spinal cord of the mononeuropathic rat? Pain. 1999;80(1–2):347–357. doi: 10.1016/s0304-3959(98)00234-6. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77(5):299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Hope PJ, Lang CW. Microinjection of neuropeptide Y into the superficial dorsal horn reduces stimulus-evoked release of immunoreactive substance P in the anaesthetized cat. Neuroscience. 1991;44(3):733–740. doi: 10.1016/0306-4522(91)90092-3. [DOI] [PubMed] [Google Scholar]
- Eckert WA, 3rd, McNaughton KK, Light AR. Morphology and axonal arborization of rat spinal inner lamina II neurons hyperpolarized by mu-opioid-selective agonists. J Comp Neurol. 2003;458(3):240–256. doi: 10.1002/cne.10587. [DOI] [PubMed] [Google Scholar]
- El Bahh B, Cao JQ, Beck-Sickinger AG, Colmers WF. Blockade of neuropeptide Y(2) receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br J Pharmacol. 2002;136(4):502–509. doi: 10.1038/sj.bjp.0704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55(8):1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Flores CM, Hargreaves KM. Neuropeptide Y inhibits capsaicin-sensitive nociceptors via a Y1-receptor-mediated mechanism. Neuroscience. 2004;125(3):703–709. doi: 10.1016/j.neuroscience.2004.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Flores CM, Hargreaves KM. Attenuation of capsaicin-evoked mechanical allodynia by peripheral neuropeptide Y Y1 receptors. Pain. 2006;124(1–2):167–174. doi: 10.1016/j.pain.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. J Comp Neurol. 1984;227(1):78–91. doi: 10.1002/cne.902270109. [DOI] [PubMed] [Google Scholar]
- Hua XY, Boublik JH, Spicer MA, Rivier JE, Brown MR, Yaksh TL. The antinociceptive effects of spinally administered neuropeptide Y in the rat: systematic studies on structure-activity relationship. J Pharmacol Exp Ther. 1991;258(1):243–248. [PubMed] [Google Scholar]
- Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation B. J Neurosci. 1994;14(11 Pt 1):6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama I, Ishikawa K, Kocsis JD. Synaptic reorganization in the substantia gelatinosa after peripheral nerve neuroma formation: aberrant innervation of lamina II neurons by Abeta afferents. J Neurosci. 2000;20(4):1538–1549. doi: 10.1523/JNEUROSCI.20-04-01538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuz D, Chocyk A, Wedzony K, Toth G, Przewlocka B. Endomorphin-2, deltorphin II and their analogs suppress formalin-induced nociception and c-Fos expression in the rat spinal cord. Life Sci. 2003;73(4):403–412. doi: 10.1016/s0024-3205(03)00309-6. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Rudy TA, Yaksh TL. An improved method for chronic catheterization of the rat spinal subarachnoid space. Physiology & Behavior. 1981;27(3):559–561. doi: 10.1016/0031-9384(81)90350-4. [DOI] [PubMed] [Google Scholar]
- Luo L, Ji RR, Zhang Q, Iadarola MJ, Hokfelt T, Wiesenfeld-Hallin Z. Effect of administration of high dose intrathecal clonidine or morphine prior to sciatic nerve section on c-Fos expression in rat lumbar spinal cord. Neuroscience. 1995;68(4):1219–1227. doi: 10.1016/0306-4522(95)00197-q. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996;67(2–3):307–316. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Mahinda TB, Taylor BK. Intrathecal neuropeptide Y inhibits behavioral and cardiovascular responses to noxious inflammatory stimuli in awake rats. Physiol Behav. 2004;80(5):703–711. doi: 10.1016/j.physbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Martire M, Altobelli D, Maurizi S, Preziosi P, Fuxe K. K(+)-Evoked [(3)H]D-aspartate release in rat spinal cord synaptosomes: modulation by neuropeptide Y and calcium channel antagonists. Journal of neuroscience research. 2000;62(5):722–729. doi: 10.1002/1097-4547(20001201)62:5<722::AID-JNR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Gannon A, Levine JD, Basbaum AI. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989;285(2):177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. Journal of Pharmacological & Toxicological Methods. 1994;32(4):197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Thoren P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409(6819):513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22(22):9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MA, Basbaum AI, Abbadie C, Rohde DS, McKay WR, Taylor BK. The differential contribution of capsaicin-sensitive afferents to behavioral and cardiovascular measures of brief and persistent nociception and to Fos expression in the formalin test. Brain Res. 1997;755(1):9–16. doi: 10.1016/s0006-8993(97)00068-1. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4(1):111–113. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Rodella LF, Borsani E, Rezzani R, Ricci F, Buffoli B, Bianchi R. AM404, an inhibitor of anandamide reuptake decreases Fos-immunoreactivity in the spinal cord of neuropathic rats after non-noxious stimulation. Eur J Pharmacol. 2005;507(1–3):139–146. doi: 10.1016/j.ejphar.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Sakura S, Hashimoto K, Bollen AW, Ciriales R, Drasner K. Intrathecal catheterization in the rat. Improved technique for morphologic analysis of drug-induced injury. Anesthesiology. 1996;85(5):1184–1189. doi: 10.1097/00000542-199611000-00028. [DOI] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4(8):465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;25(2):464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65(2):167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42(2):205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Ichikawa H, Hijiya H, Mitani S, Nakago T. c-Fos expression by dorsal horn neurons chronically deafferented by peripheral nerve section in response to spared, somatotopically inappropriate nociceptive primary input. Brain Res. 1993;621(1):161–166. doi: 10.1016/0006-8993(93)90314-d. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Ichikawa H, Mitani S, Hitsu A, Nakago T. Changes in c-fos induction in dorsal horn neurons by hindpaw formalin stimulation following tibial neurotomy. Brain Res. 1994;642(1–2):348–354. doi: 10.1016/0006-8993(94)90942-3. [DOI] [PubMed] [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231(1):66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Taylor BK. Antihyperalgesic effects of intrathecal neuropeptide Y during inflammation are mediated by Y1 receptors. Pain. 2002;96(3):353–363. doi: 10.1016/S0304-3959(01)00481-X. [DOI] [PubMed] [Google Scholar]
- Taylor BK. NPY analgesia: moving from acute to chronic pain. In: Feuerstein GZ, Zukowska Z, editors. |. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- Taylor BK, Basbaum AI. Early antinociception delays edema but does not reduce the magnitude of persistent pain in the formalin test. J Pain. 2000;1(3):218–228. doi: 10.1054/jpai.2000.7308. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Basbaum AI. Exaggerated cardiovascular and behavioral nociceptive responses to subcutaneous formalin in the spontaneously hypertensive rat. Neurosci Lett. 1995a;201(1):9–12. doi: 10.1016/0304-3940(95)12157-y. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Basbaum AI. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci. 1995b;15(11):7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Roderick RE, Tate J, Green PG, Levine JO, Basbaum AI. Opioid inhibition of formalin-induced changes in plasma extravasation and local blood flow in rats. Pain. 2000;84(2–3):263–270. doi: 10.1016/s0304-3959(99)00212-2. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge O-G, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360(1):121–134. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- Xu IS, Hao JX, Xu XJ, Hokfelt T, Wiesenfeld-Hallin Z. The effect of intrathecal selective agonists of Y1 and Y2 neuropeptide Y receptors on the flexor reflex in normal and axotomized rats. Brain Res. 1999;833(2):251–257. doi: 10.1016/s0006-8993(99)01551-6. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic Catheterization of the Spinal Subarachnoid Space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Comparison of the antinociceptive effects of pre- and post-treatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology. 1992;77:757–763. doi: 10.1097/00000542-199210000-00021. [DOI] [PubMed] [Google Scholar]