Abstract
Immune complexes in the lungs are capable of inducing adverse responses. Herein we have detailed the formation of immune complexes in the lungs of influenza virus-infected mice and examined their effect on alveolar macrophage defenses. On days 3, 7, 10, 15, and 30 after aerosol infection with influenza A/PR8/34 virus, the acellular pulmonary lavage fluid was tested for viral antigen, specific viral antibody, and immune complexes by immunoassays. Whereas peak viral antigen (day 3) diminished to undetectable levels by day 10, specific viral antibody remained at a low concentration until day 10, after which it rapidly increased. Immune complex concentrations increased through day 7, peaked at day 10, and gradually returned to the control level by day 30. These data demonstrate that immune complexes of detectable size are induced by influenza virus infection during the interface between antigen excess and antibody excess conditions. Since alveolar macrophages are the pivotal phagocytic defense cells in the lung, the ability of normal alveolar macrophages to ingest opsonized erythrocytes was quantitated in the presence of immune complexes from lavage fluid. Immune complexes from day 10 virus-infected lungs caused a dose-dependent suppression of antibody-mediated phagocytosis to 30% of control values. In contrast, although these immune complexes also markedly decreased the phagocytosis of antibody-coated yeast cells, they did not significantly impair the antibody-independent ingestion of unopsonized yeast cells by macrophages. the suppressive effects of immune complexes on alveolar macrophages may, in part, explain the phagocytic dysfunction that occurs 7 to 10 days after influenza virus pneumonia.
Full text
PDF

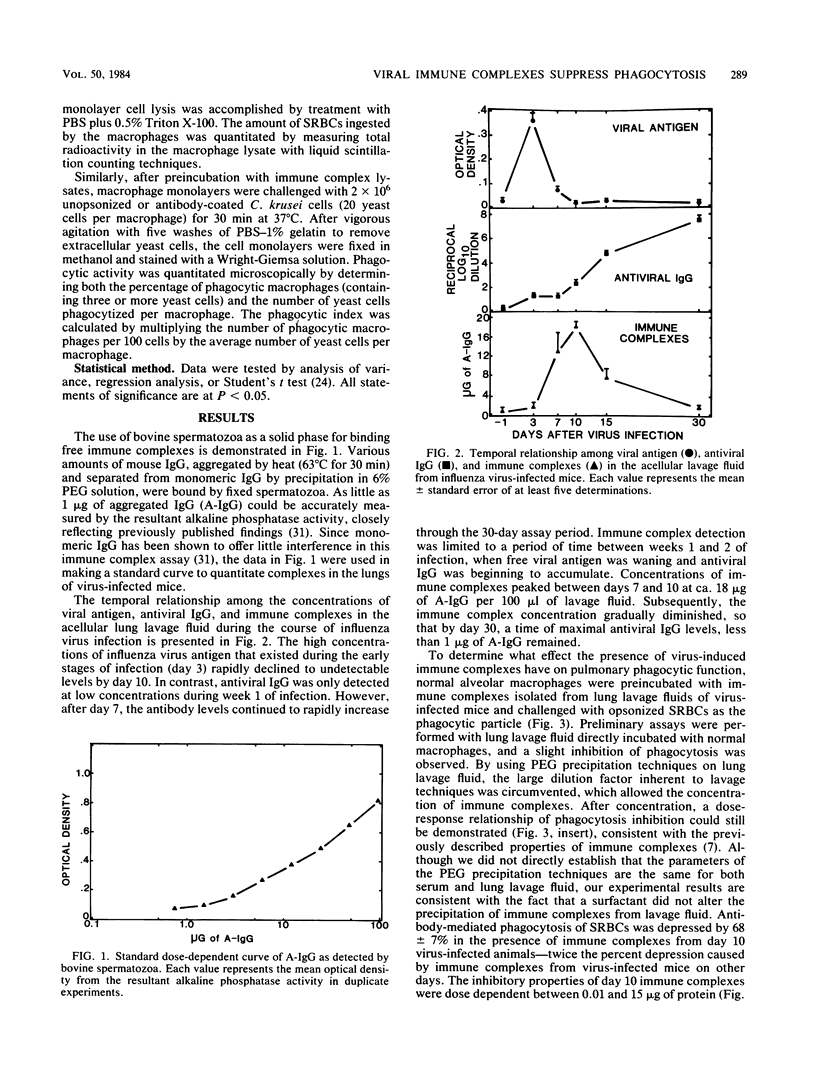
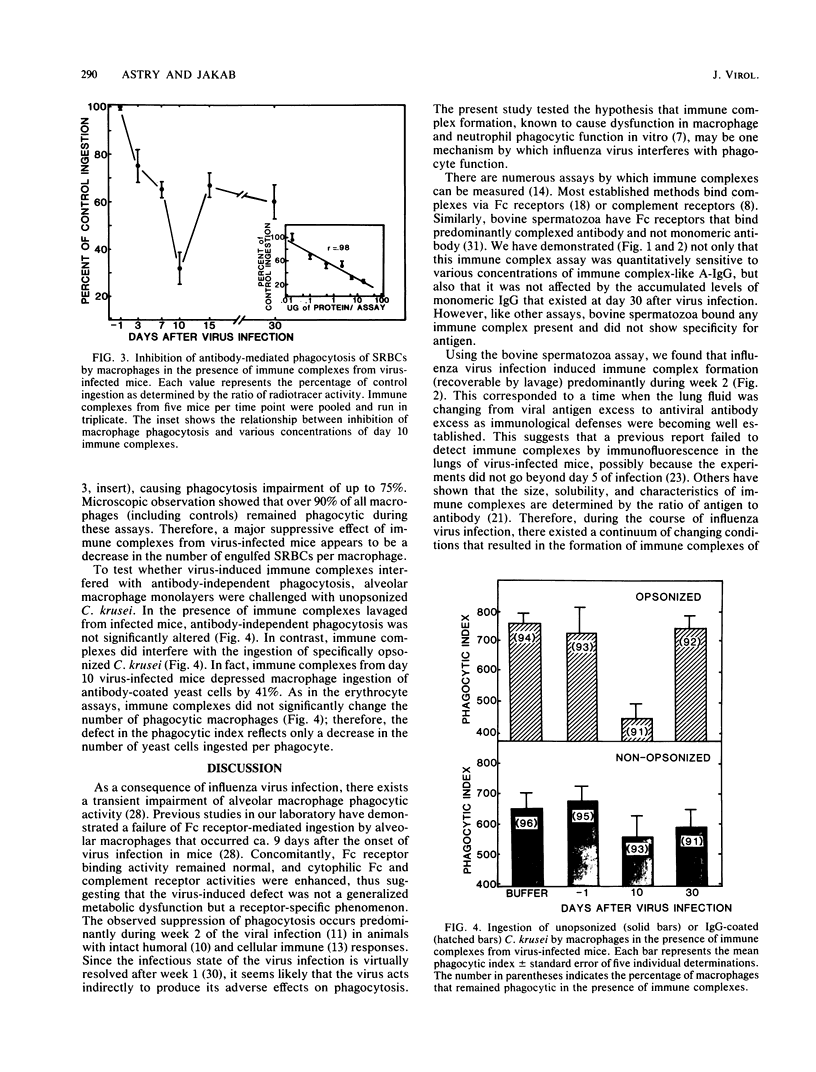


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astry C. L., Warr G. A., Jakab G. J. Impairment of polymorphonuclear leukocyte immigration as a mechanism of alcohol-induced suppression of pulmonary antibacterial defenses. Am Rev Respir Dis. 1983 Jul;128(1):113–117. doi: 10.1164/arrd.1983.128.1.113. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J. A simple procedure to use whole serum as a source of either IgG- or IgM-specific antibody. J Immunol Methods. 1980;32(1):51–58. doi: 10.1016/0022-1759(80)90116-7. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Creighton W. D., Lambert P. H., Miescher P. A. Detection of antibodies and soluble antigen-antibody complexes by precipitation with polyethylene glycol. J Immunol. 1973 Oct;111(4):1219–1227. [PubMed] [Google Scholar]
- Gardner I. D. Suppression of antibacterial immunity by infection with influenza virus. J Infect Dis. 1981 Sep;144(3):225–231. doi: 10.1093/infdis/144.3.225. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr Effects of soluble immune complexes on Fc receptor- and C3b receptor-mediated phagocytosis by macrophages. J Exp Med. 1980 Oct 1;152(4):905–919. doi: 10.1084/jem.152.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada Y., Kamiyama M., Kanemitsu T., Clark W. S. Detection of circulating immune complexes: a new application of immune adherence haemagglutination. Ann Immunol (Paris) 1981 Mar-Apr;132C(2):181–190. doi: 10.1016/0769-2625(81)90026-x. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J. Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am Rev Respir Dis. 1982 Nov;126(5):778–782. doi: 10.1164/arrd.1982.126.5.778. [DOI] [PubMed] [Google Scholar]
- Jakab G. J. Pulmonary defense mechanisms and the interaction between viruses and bacteria in acute respiratory infections. Bull Eur Physiopathol Respir. 1977 Jan-Feb;13(1):119–135. [PubMed] [Google Scholar]
- Jakab G. J. Viral-bacterial interactions in pulmonary infection. Adv Vet Sci Comp Med. 1982;26:155–171. [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A. The participation of antiviral immune mechanisms in alveolar macrophage dysfunction during viral pneumonia. Bull Eur Physiopathol Respir. 1983 Mar-Apr;19(2):173–178. [PubMed] [Google Scholar]
- Meretey K., Böhm U., Falus A., Bozsoky S. Radioimmune double PEG precipitation technique for detecting complexed IgE. J Immunol Methods. 1979;26(3):223–228. doi: 10.1016/0022-1759(79)90247-3. [DOI] [PubMed] [Google Scholar]
- Michl J., Unkeless J. C., Pieczonka M. M., Silverstein S. C. Modulation of Fc receptors of mononuclear phagocytes by immobilized antigen-antibody complexes. Quantitative analysis of the relationship between ligand number and Fc receptor response. J Exp Med. 1983 Jun 1;157(6):1746–1757. doi: 10.1084/jem.157.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom F., Kano K. Comparison of various procedures for the detection of antigen-antibody complexes. Int Arch Allergy Appl Immunol. 1978;56(3):224–231. doi: 10.1159/000232025. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Effect of influenza infection on the phagocytic and bactericidal activities of pulmonary macrophages. Infect Immun. 1979 Nov;26(2):651–657. doi: 10.1128/iai.26.2.651-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestel J., Joseph M., Dessaint J. P., Capron A. Macrophage triggering by aggregated immunoglobulins. I. Delayed effect of IgG aggregates or immune complexes. J Immunol. 1981 May;126(5):1887–1891. [PubMed] [Google Scholar]
- Poskitt P. K., Poskitt T. R. A quantitative enzyme-linked immunoassay for serum immune complexes. J Clin Lab Immunol. 1981 Mar;5(2):125–128. [PubMed] [Google Scholar]
- Semkow R., Wilczyński J. Detection and tissue localization of components of the immune complex in animals infected and immunized with influenza virus. Acta Virol. 1979 Jan;23(1):52–58. [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Jakab G. J., Hearst J. E. Alterations in lung macrophage immune receptor(s) activity associated with viral pneumonia. J Reticuloendothel Soc. 1979 Oct;26(4):357–366. [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Akers T., Lippert W., Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Albrecht P., Ennis F. A. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981 Mar;126(3):1036–1041. [PubMed] [Google Scholar]
- Witkin S. S., Shahani S. K., Gupta S., Good R. A., Day N. K. Demonstration of IgG Fc receptors on spermatozoa and their utilization for the detection of circulating immune complexes in human serum. Clin Exp Immunol. 1980 Sep;41(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Torsch V. Enzyme-linked immunosorbent assay for the detection and identification of coxsackie B antigen in tissue cultures and clinical specimens. J Med Virol. 1980;6(1):45–52. doi: 10.1002/jmv.1890060107. [DOI] [PubMed] [Google Scholar]


