Abstract
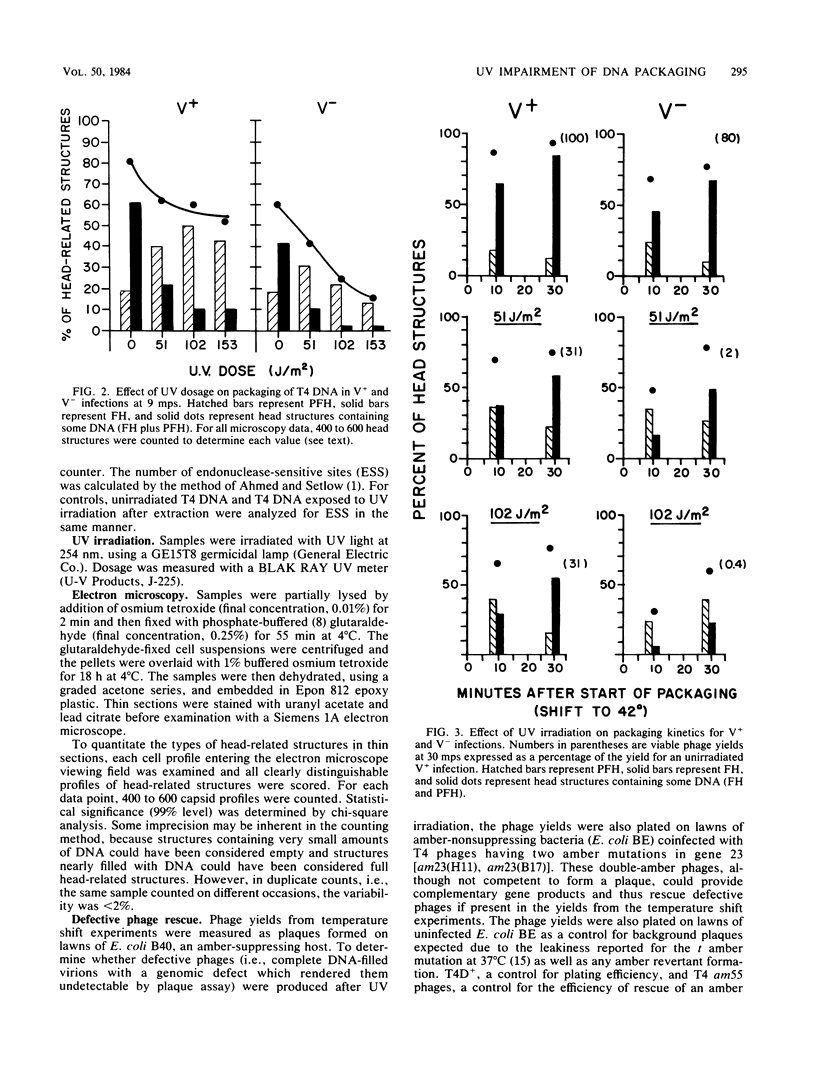
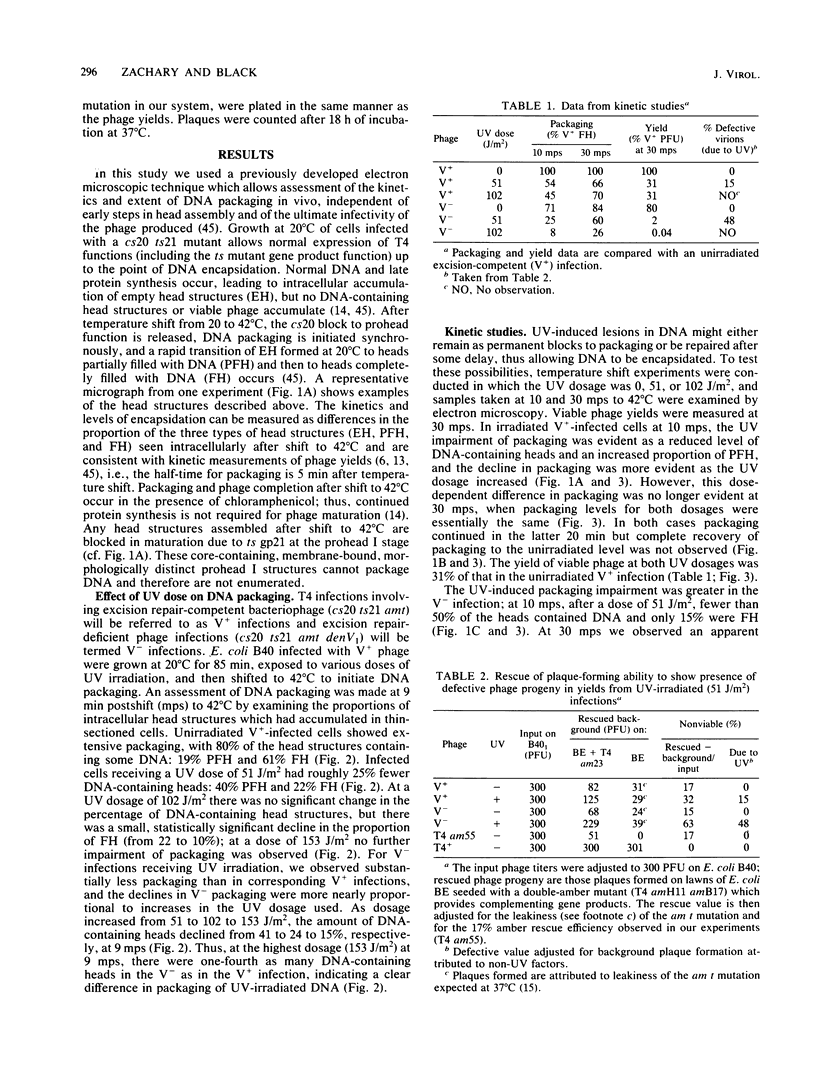
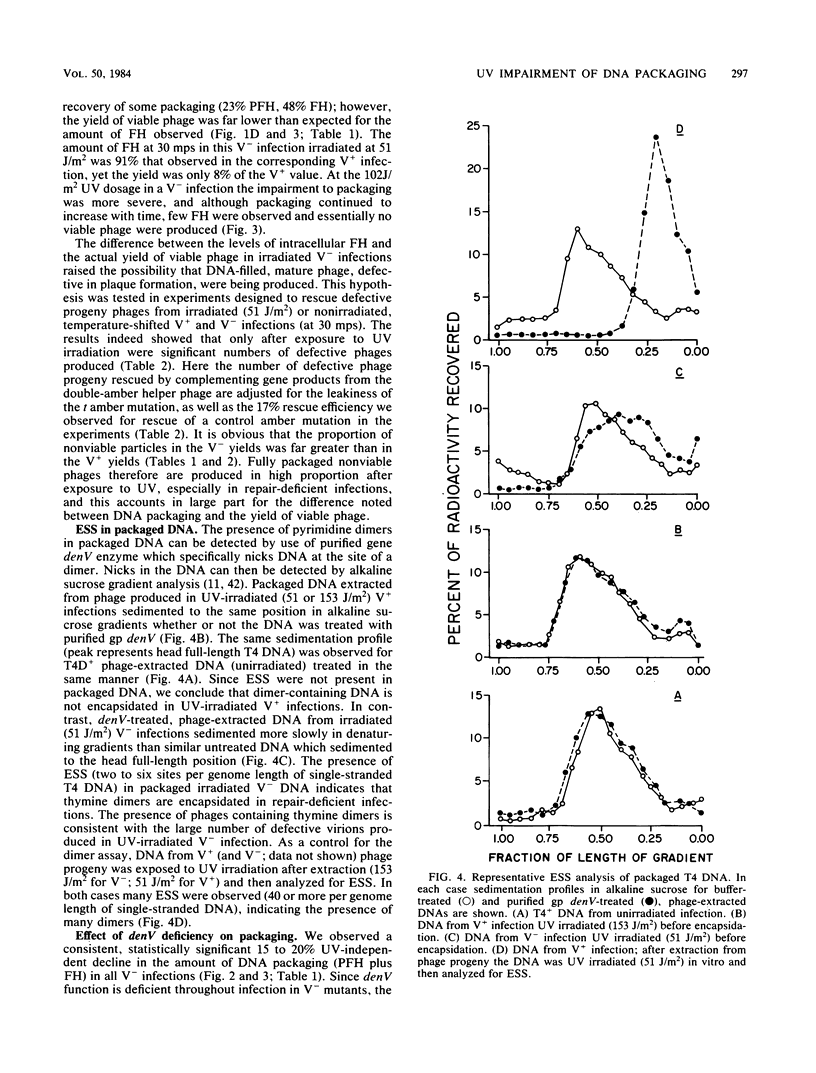
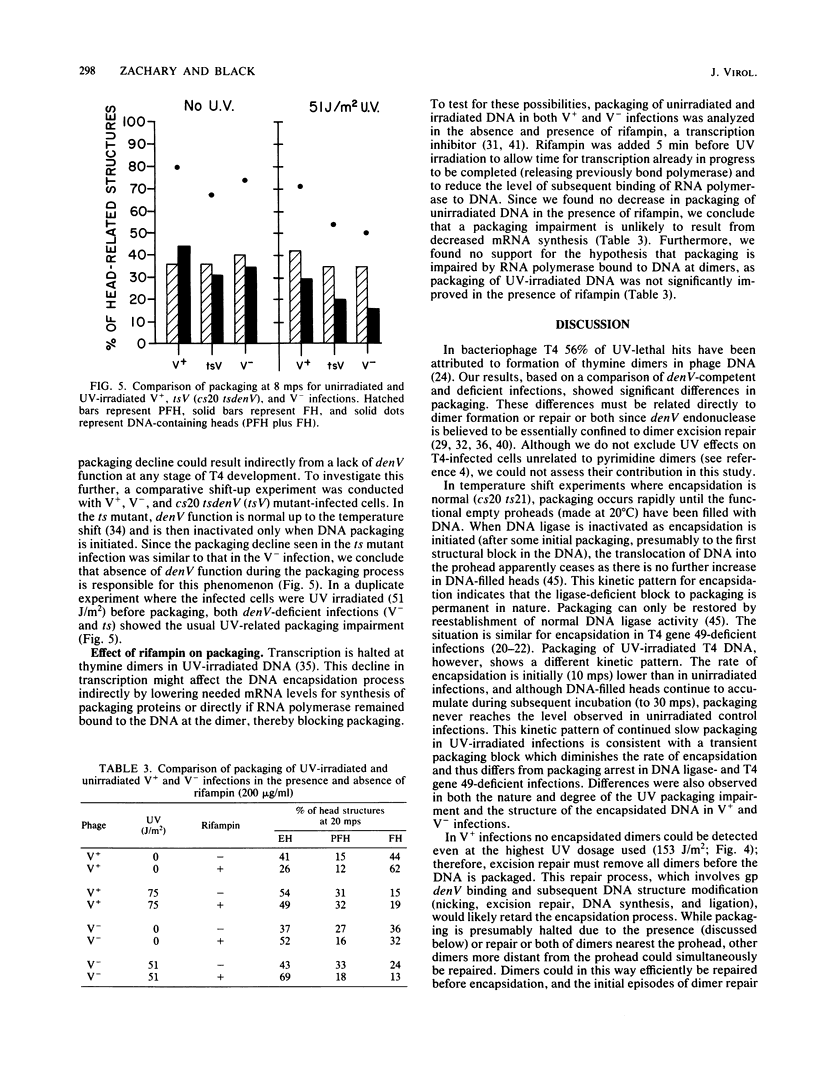
T4 DNA structural requirements for encapsidation in vivo were investigated, using thin-section electron microscopy to quantitate the kinetics and yields of head intermediates after synchronous DNA packaging into accumulated processed proheads. UV irradiation (254 nm) of T4-infected bacteria just before initiation of encapsidation resulted in a reduction in the rate of DNA packaged measured by electron microscopy and in the yield of viable phage progeny. In UV-irradiated infections with excision-deficient mutants (denV-), the extent of packaging decline was proportional to the UV dose and phage yields were lower than expected based on the packaging levels observed by microscopy. Rescue analysis of progeny from such infections revealed elevated levels of nonviable virions. Pyrimidine dimers were encapsidated in denV- infections, but in excision-competent infections (denV+) dimers were not packaged. A UV-independent, 15 to 20% packaging arrest was also observed when denV endonuclease was inactive during encapsidation, indicating a denV requirement to achieve normal T4 packaging levels. Pyrimidine dimers apparently represent or induce transient blockage of DNA encapsidation or both, causing a decline in the rate. This is in contrast to other DNA structural blocks to packaging induced by mutations in T4 genes 30 and 49, which appear to arrest the process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed F. E., Setlow R. B. DNA repair in V-79 cells treated with combinations of ultraviolet radiation and N-acetoxy-2-acetylaminofluorene. Cancer Res. 1977 Sep;37(9):3414–3419. [PubMed] [Google Scholar]
- Benz W. C., Berger H. Selective allele loss in mixed infections with T4 bacteriophage. Genetics. 1973 Jan;73(1):1–11. doi: 10.1093/genetics/73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Pardoll D. Evidence that mismatched bases in heteroduplex T4 bacteriophage are recognized in vivo. J Virol. 1976 Nov;20(2):441–445. doi: 10.1128/jvi.20.2.441-445.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C. Deoxyribonucleic acid repair in bacteriophage. Microbiol Rev. 1981 Mar;45(1):72–98. doi: 10.1128/mr.45.1.72-98.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W., Silverman D. J. Model for DNA packaging into bacteriophage T4 heads. J Virol. 1978 Nov;28(2):643–655. doi: 10.1128/jvi.28.2.643-655.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W. The mechanism of bacteriophage DNA encapsidation. Prog Clin Biol Res. 1981;64:97–110. [PubMed] [Google Scholar]
- Black L. W., Zachary A. L., Manne V. Studies of the mechanism of bacteriophage T4 DNA encapsidation. Prog Clin Biol Res. 1981;64:111–126. [PubMed] [Google Scholar]
- Castillo C. J., Hsiao C. L., Coon P., Black L. W. Identification and perperties of bacteriophage T4 capsid-formation gene products. J Mol Biol. 1977 Mar 5;110(3):585–601. doi: 10.1016/s0022-2836(77)80113-7. [DOI] [PubMed] [Google Scholar]
- Curtis M. J., Alberts B. Studies on the structure of intracellular bacteriophage T4 DNA. J Mol Biol. 1976 Apr 25;102(4):793–816. doi: 10.1016/0022-2836(76)90292-8. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K. A method for detecting pyrimidine dimers in the DNA of bacteria irradiated with low doses of ultraviolet light. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2753–2756. doi: 10.1073/pnas.70.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. L., Black L. W. DNA packaging and the pathway of bacteriophage T4 head assembly. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3652–3656. doi: 10.1073/pnas.74.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. L., Black L. W. Head morphogenesis of bacteriophage T4. III. The role of gene 20 in DNA packaging. Virology. 1978 Nov;91(1):26–38. doi: 10.1016/0042-6822(78)90352-5. [DOI] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Kemper B., Brown D. T. Function of gene 49 of bacteriophage T4. II. Analysis of intracellular development and the structure of very fast-sedimenting DNA. J Virol. 1976 Jun;18(3):1000–1015. doi: 10.1128/jvi.18.3.1000-1015.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Garabett M. Studies on T4-head maturation. 1. Purification and characterization of gene-49-controlled endonuclease. Eur J Biochem. 1981 Mar 16;115(1):123–131. [PubMed] [Google Scholar]
- Kemper B., Janz E. Function of gene 49 of bacteriophage T4. I. Isolation and biochemical characterization of very fast-sedimenting DNA. J Virol. 1976 Jun;18(3):992–999. doi: 10.1128/jvi.18.3.992-999.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Paulson J. R., Hitchins V. Maturation of the head of bacteriophage T4. V. A possible DNA packaging mechanism: in vitro cleavage of the head proteins and the structure of the core of the polyhead. J Supramol Struct. 1974;2(2-4):276–301. doi: 10.1002/jss.400020219. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- McMillan S., Edenberg H. J., Radany E. H., Friedberg R. C., Friedberg E. C. den V gene of bacteriophage T4 codes for both pyrimidine dimer-DNA glycosylase and apyrimidinic endonuclease activities. J Virol. 1981 Oct;40(1):211–223. doi: 10.1128/jvi.40.1.211-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich M. L. Contribution of thymine dimers to the ultraviolet light inactivation of mutants of bacteriophage T4. J Mol Biol. 1972 Apr 28;66(1):97–106. doi: 10.1016/s0022-2836(72)80008-1. [DOI] [PubMed] [Google Scholar]
- Minagawa T. Endonuclease of T4 ghosts. Virology. 1977 Jan;76(1):234–245. doi: 10.1016/0042-6822(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Minagawa T., Murakami A., Ryo Y., Yamagishi H. Structural features of very fast sedimenting DNA formed by gene 49 defective T4. Virology. 1983 Apr 15;126(1):183–193. doi: 10.1016/0042-6822(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Kemper B., Hays J., Weisberg R. A. T4 endonuclease VII cleaves holliday structures. Cell. 1982 Jun;29(2):357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Ghosal D., Bock S. Interactions between the maturation protein gp17 and the single-stranded DNA binding protein gp32 initiate DNA packaging and compete with initiation of secondary DNA replication forks in phage T4. Prog Clin Biol Res. 1981;64:139–150. [PubMed] [Google Scholar]
- Nakabeppu Y., Sekiguchi M. Physical association of pyrimidine dimer DNA glycosylase and apurinic/apyrimidinic DNA endonuclease essential for repair of ultraviolet-damaged DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):2742–2746. doi: 10.1073/pnas.78.5.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto H., Takayama M., Minagawa T. Purification and some properties of deoxyribonuclease whose synthesis is controlled by gene 49 of bacteriophage T4. Eur J Biochem. 1979 Oct 15;100(2):433–440. doi: 10.1111/j.1432-1033.1979.tb04186.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- Radany E. H., Friedberg E. C. A pyrimidine dimer-DNA glycosylase activity associated with the v gene product of bacterophage T4. Nature. 1980 Jul 10;286(5769):182–185. doi: 10.1038/286182a0. [DOI] [PubMed] [Google Scholar]
- Radany E. H., Friedberg E. C. Demonstration of pyrimidine dimer-DNA glycosylase activity in vivo: bacteriophage T4-infected Escherichia coli as a model system. J Virol. 1982 Jan;41(1):88–96. doi: 10.1128/jvi.41.1.88-96.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sekiguchi M. Studies on temperature-dependent ultraviolet light-sensitive mutants of bacteriophage T4: the structural gene for T4 endonuclease V. J Mol Biol. 1976 Mar 25;102(1):15–26. doi: 10.1016/0022-2836(76)90071-1. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Seawell P. C., Smith C. A., Ganesan A. K. den V gene of bacteriophage T4 determines a DNA glycosylase specific for pyrimidine dimers in DNA. J Virol. 1980 Sep;35(3):790–796. doi: 10.1128/jvi.35.3.790-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Christensen L. M., Persson M. L. Evidence that the UV endonuclease activity induced by bacteriophage T4 contains both pyrimidine dimer-DNA glycosylase and apyrimidinic/apurinic endonuclease activities in the enzyme molecule. J Virol. 1981 Oct;40(1):204–210. doi: 10.1128/jvi.40.1.204-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins R. J. Endonuclease-sensitive sites in the DNA of irradiated bacteria: a rapid and sensitive assay. Biochim Biophys Acta. 1973 Jun 8;312(1):33–37. doi: 10.1016/0005-2787(73)90049-x. [DOI] [PubMed] [Google Scholar]
- Wunderli H., vd Broek J., Kellenberger E. Studies related to the head-maturation pathway of bacteriophages T4 and T2:I. morphology and kinetics of intracellular particles produced by mutants in the maturation genes. J Supramol Struct. 1977;7(2):135–161. doi: 10.1002/jss.400070202. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B., Rosenstein B. S., Setlow R. B. Excision repair and patch size in UV-irradiated bacteriophage T4. J Virol. 1981 Nov;40(2):465–471. doi: 10.1128/jvi.40.2.465-471.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary A., Black L. W. DNA ligase is required for encapsidation of bacteriophage T4 DNA. J Mol Biol. 1981 Jul 15;149(4):641–658. doi: 10.1016/0022-2836(81)90351-x. [DOI] [PubMed] [Google Scholar]