We report 2 cases of eruptive melanocytic lesions associated with sorafenib, a multikinase inhibitor FDA-approved for the treatment of advanced renal cell carcinoma. Understanding the development of this previously unknown side effect may provide further insight into sorafenib’s mechanistic effects on tumors and normal tissues and into the development of pigmented lesions.
Report of Cases
Case 1
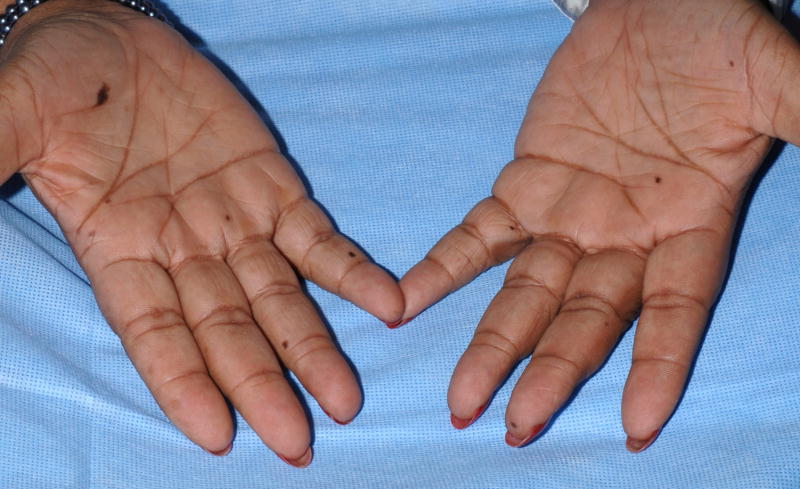
A 60-year-old black female with metastatic renal cell carcinoma received sorafenib 400 mg orally twice daily. After two months of sorafenib she noticed new dark macules on her palms and soles. Additional lesions developed over the ensuing 2 months. Dermatologic evaluation revealed multiple small dark brown macules on the palms (Figure 1A) and soles (Figure 1B). The plantar feet exhibited focal scaling consistent with her prior diagnosis of mild acral erythema (hand-foot skin reaction; hand-foot syndrome). Histologic examination of a palmar lesion demonstrated small nests of normal melanocytes at the dermal-epidermal junction (Figure 1C). A diagnosis of sorafenib-related eruptive benign melanocytic nevi was made.
Figure 1. Eruptive nevi and mild acral erythema (hand-foot skin reaction) in a patient on sorafenib.
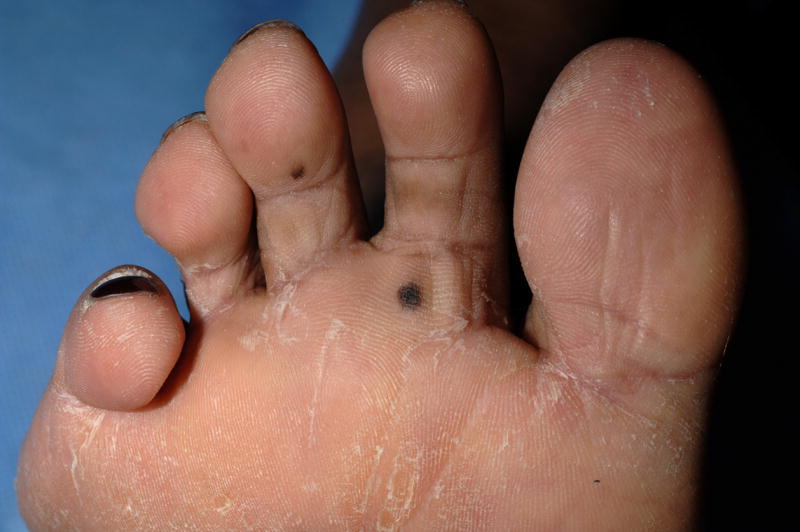
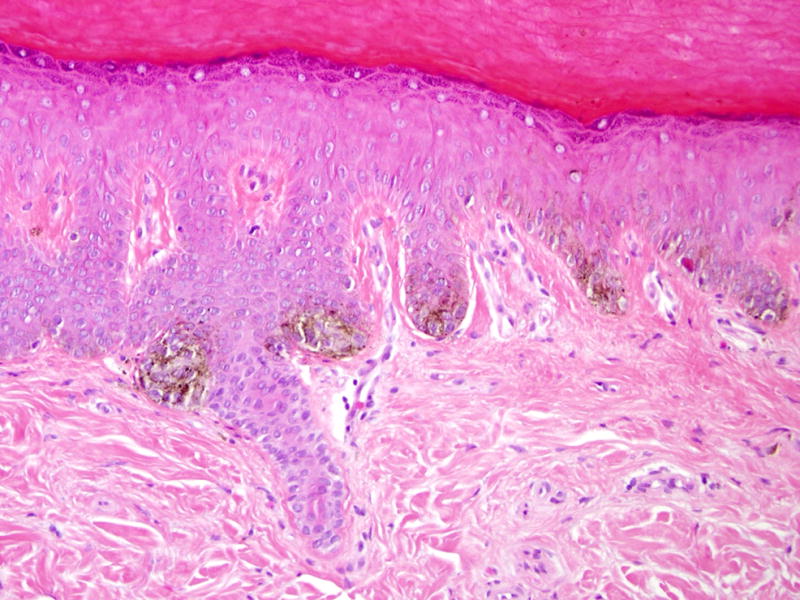
A, Multiple brown macules on the palms and fingers; B, Brown macules on the plantar foot with associated scaling; C, Palmar biopsy specimen shows small nests of normal melanocytes at the dermal-epidermal junction (hematoxylin-eosin, original magnification X200).
Case 2
A 59-year-old man with metastatic renal cell carcinoma began treatment with sorafenib 400mg twice daily. One month after initiation of therapy, he reported the sudden appearance of multiple pigmented skin lesions without preceding cutaneous inflammation or eruption. Examination revealed multiple monomorphic pigmented macules on the neck, trunk, anterior thighs (Figure 2A), palms, and soles. Histologic evaluation of a thigh macule demonstrated a proliferation of melanocytes at the basal layer of the epidermis with dermal melanophages (Figures 2B and C). The patient was diagnosed with sorafenib-associated lentigines.
Fig. 2.
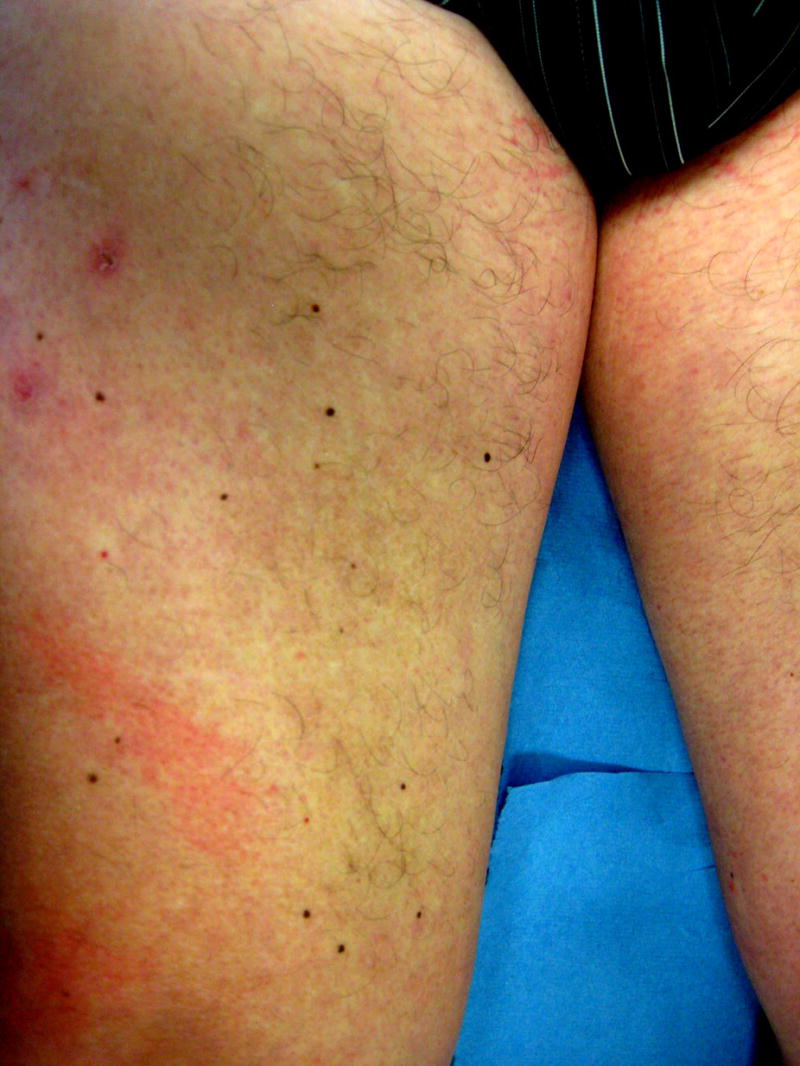
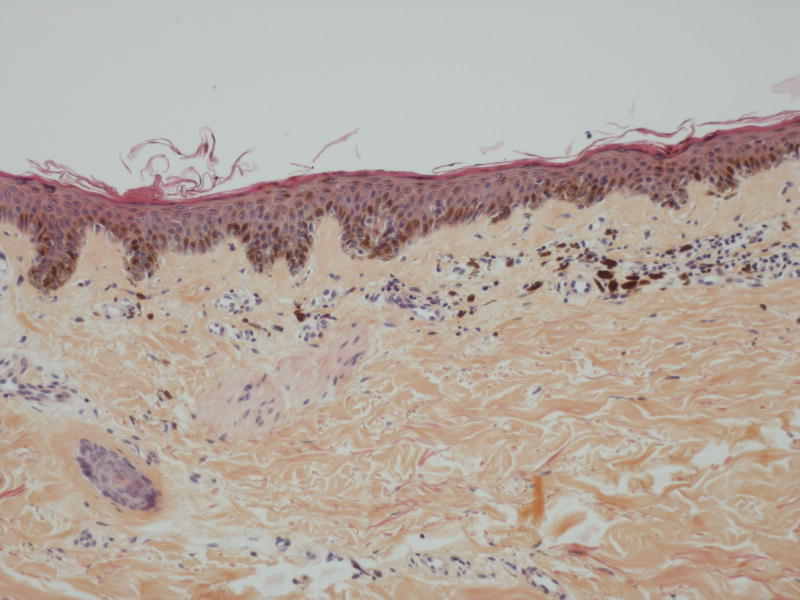
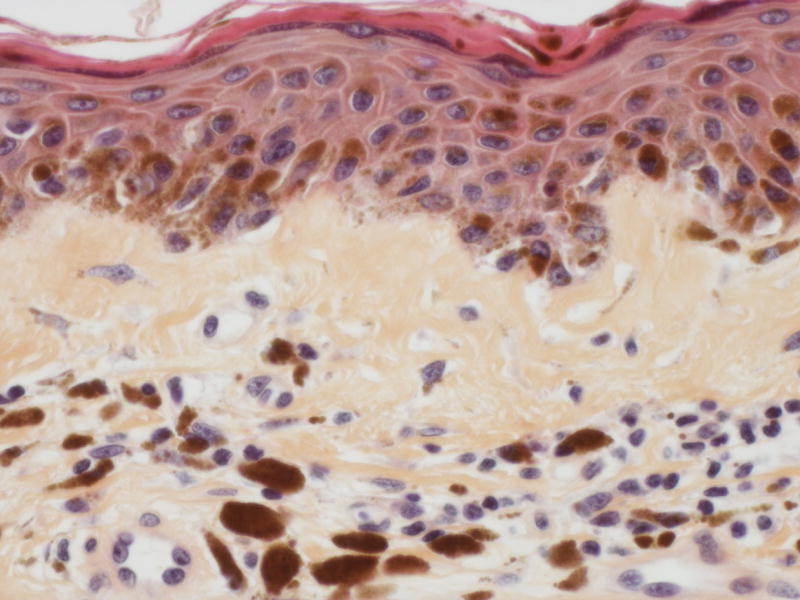
A, Multiple brown macules on the anterior thighs of a patient receiving sorafenib; B, Thigh biopsy specimen shows increased pigmentation in the epidermis and superficial dermis (hematoxylin-eosin, original magnification ×100); C, Higher magnification of the same biopsy specimen shows melanocytic proliferation at the basal epidermal layer and melanophages in the superficial dermis (hematoxylin-eosin, original magnification ×400).
Comment
Rapid development of pigmented lesions has been previously associated with chemotherapeutic agents, immunodeficiency, and blistering dermatoses.1 Commonly affected sites include the palms and soles, though eruptive pigmented lesions may be widespread. Sporadic nevi and lentigines are less frequent in acral locations.2 The significance of the acral predilection of eruptive nevi and lentigines is uncertain.
Development of eruptive pigmented lesions has been related to local skin inflammation or to immunosuppression, with potentially diminished immune surveillance allowing melanocyte growth factors to promote development of pigmented lesions in genetically predisposed individuals.1 In blistering skin disorders, eruptive nevi may localize to sites of prior cutaneous lesions. Inflammation from sorafenib-related acral erythema may similarly predispose to the development of nevi on palms and soles. Alternatively, preliminary data suggests that sorafenib inhibits the function of human dendritic cells and may be immunomodulatory.3 Interestingly, eruptive keratoacanthomas are also associated with states of immunosuppression and have been reported with sorafenib.4
Sorafenib inhibits a variety of serine/threonine and tyrosine kinases including wild-type and mutant BRAF (BRAFV600E), Raf-1, c-KIT, VEGFR-2, VEGFR-3, PDGFR-β, and Flt3.5 Activated RAF promotes melanocyte proliferation and induces oncogene-induced senescence in melanocytes6. Differential inhibition of these distinct activities of BRAF or BRAFV600E by sorafenib may promote the development of nevi and lentigines in unusual locations prior to onset of RAF-dependent senescence. The possibility that factors unrelated to the drug sorafenib, but related to the underlying malignancies contributed to the development of these melanocytic lesions also cannot be excluded.
The literature includes a solitary report of melanoma in situ diagnosed in a patient with chemotherapy-related eruptive nevi.7 The premalignant potential of sorafenib-associated melanocytic lesions is unknown. Close follow up may be warranted.
Acknowledgments
We thank Drs. Nilofer S. Azad and Edward W. Cowen for their assistance.
Research Support: none
Author contributions: Concept and design: Kong, Sibaud, Turner
Acquisition of data: Kong, Sibaud, Fojo
Analysis and interpretation of data: Kong, Sibaud, Turner, Hornyak, Chevreau
Drafting of the manuscript: Kong, Sibaud
Critical revision of the manuscript for important intellectual content: Kong, Sibaud, Turner, Fojo, Hornyak, Chevreau
Administrative, technical, and material support: Kong, Sibaud
Study supervision: Kong
Disclosures: None. All authors report no conflicts of interest.
Disclaimers: none
References
- 1.Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880–4. doi: 10.1111/j.1365-2133.2006.07189.x. [DOI] [PubMed] [Google Scholar]
- 2.Autier P, Boniol M, Severi G, et al. The body site distribution of melanocytic naevi in 6–7 year old European children. Melanoma Res. 2001;11:123–31. doi: 10.1097/00008390-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hipp MM, Hilf N, Walter S, et al. ASCO Annual Meeting Proceedings Part I. Vol. 25. Chicago, IL: Journal of Clinical Oncology; 2007. Sorafenib but not sunitinib affects the induction of immune responses; p. 3504. [Google Scholar]
- 4.Kong HH, Cowen EW, Azad NS, Dahut W, Gutierrez M, Turner ML. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–2. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12:2366s–2370s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- 6.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 7.Reutter JC, Long EM, Morrell DS, Thomas NE, Groben PA. Eruptive post-chemotherapy in situ melanomas and dysplastic nevi. Pediatr Dermatol. 2007;24:135–7. doi: 10.1111/j.1525-1470.2007.00359.x. [DOI] [PubMed] [Google Scholar]