Abstract
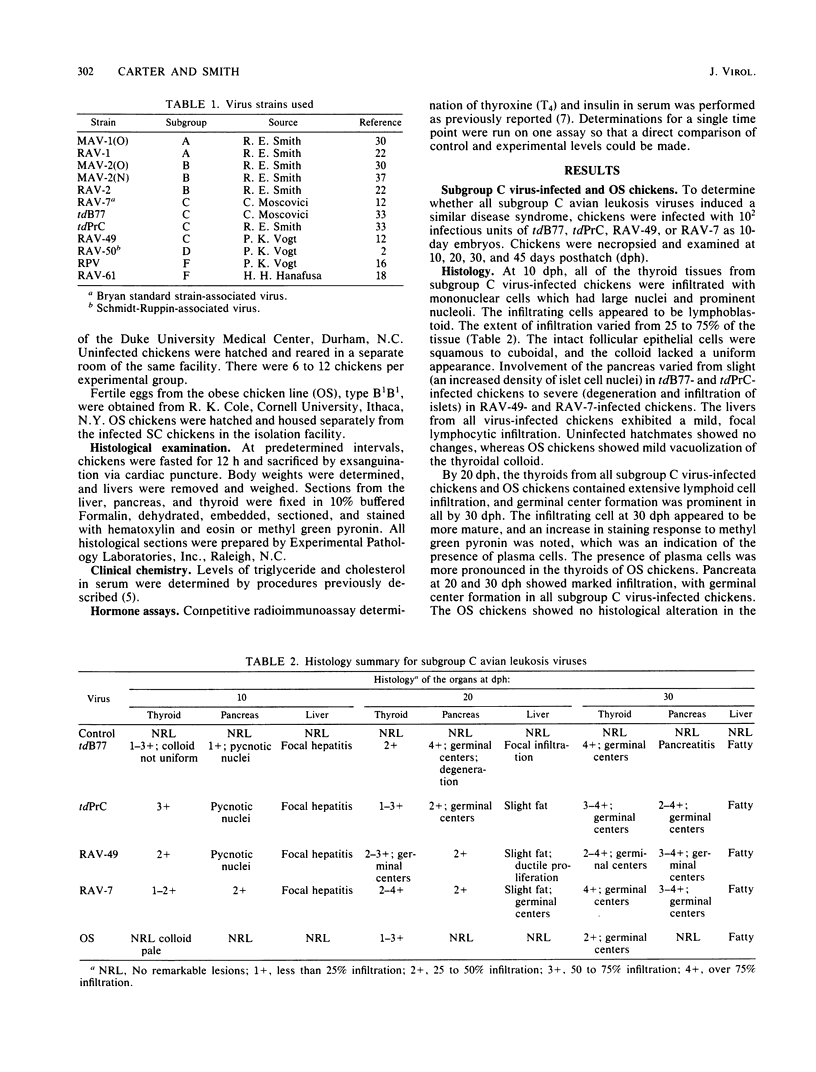
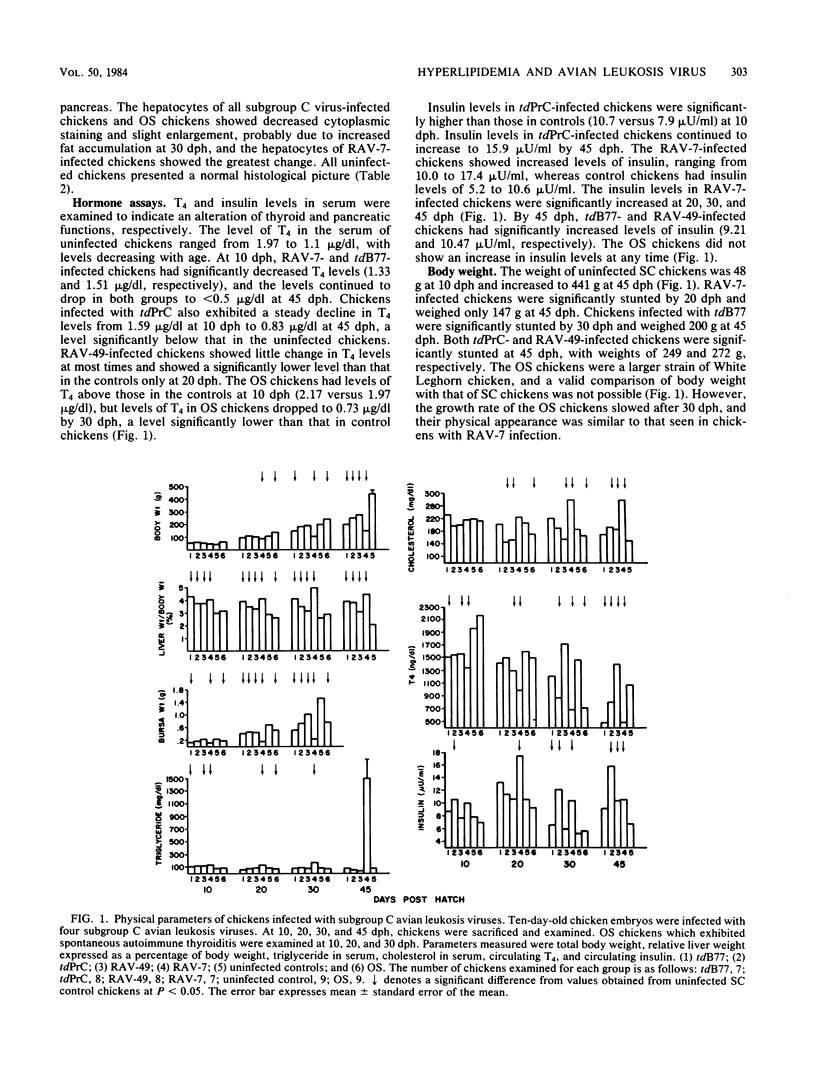
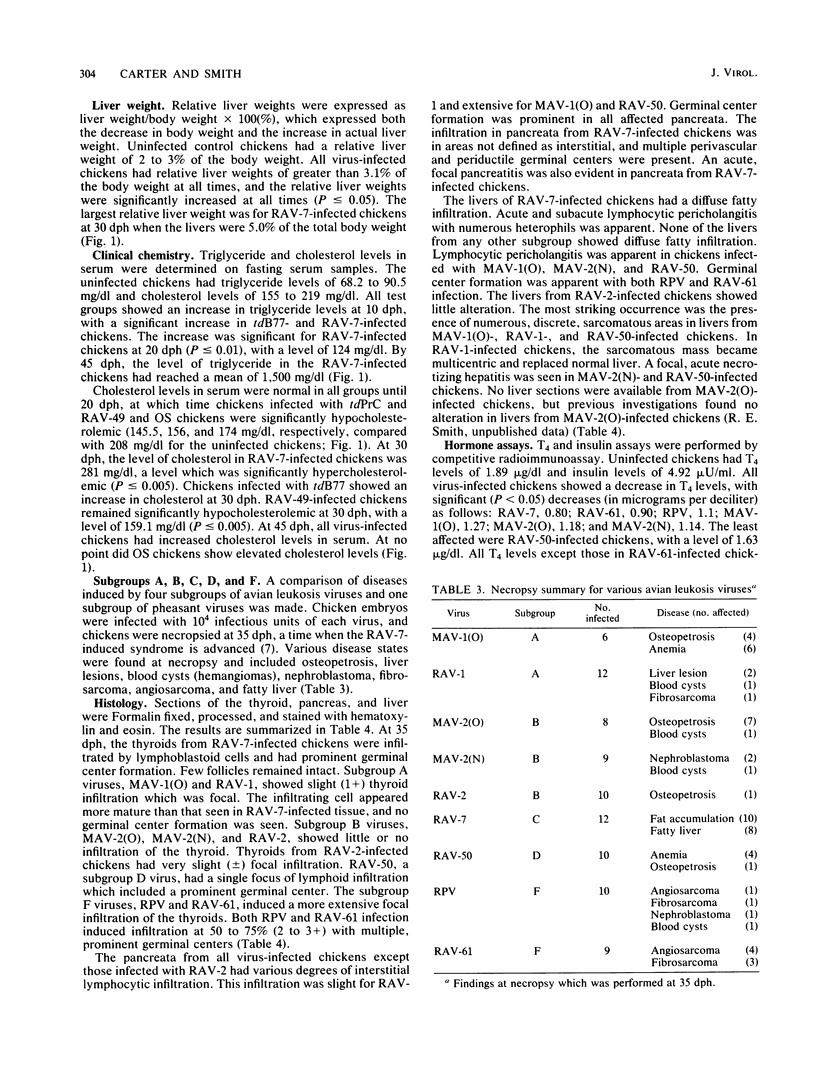
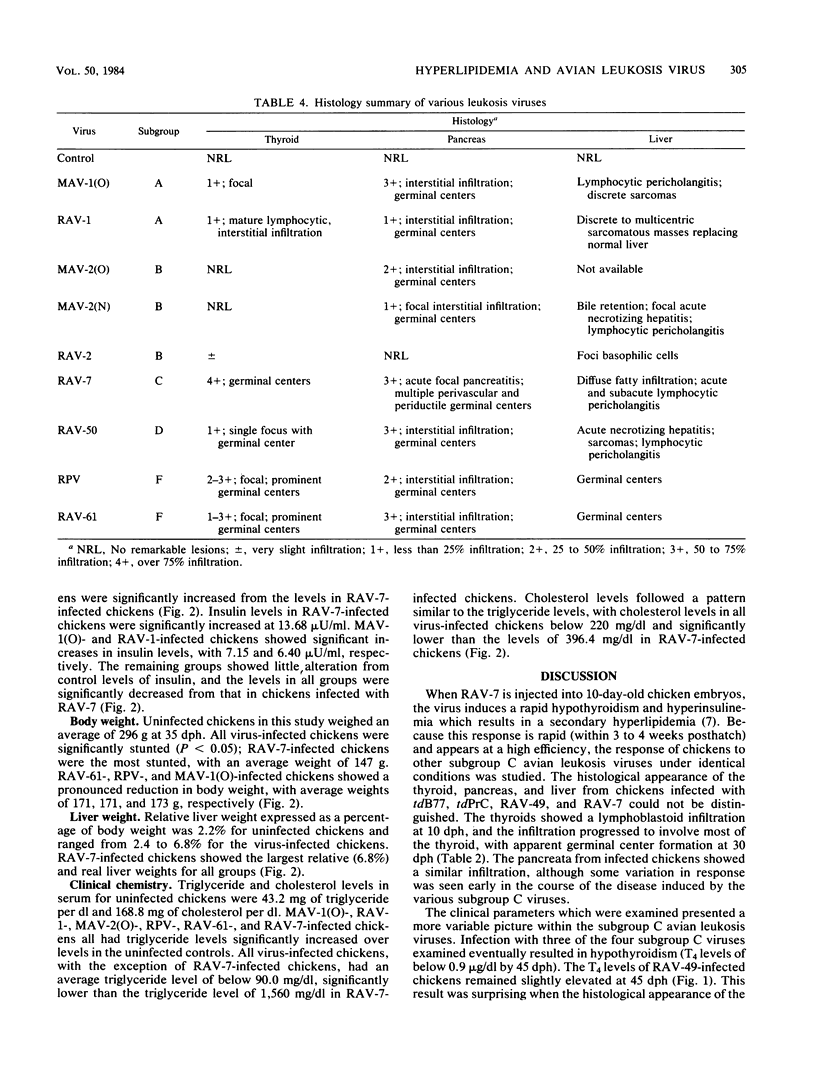
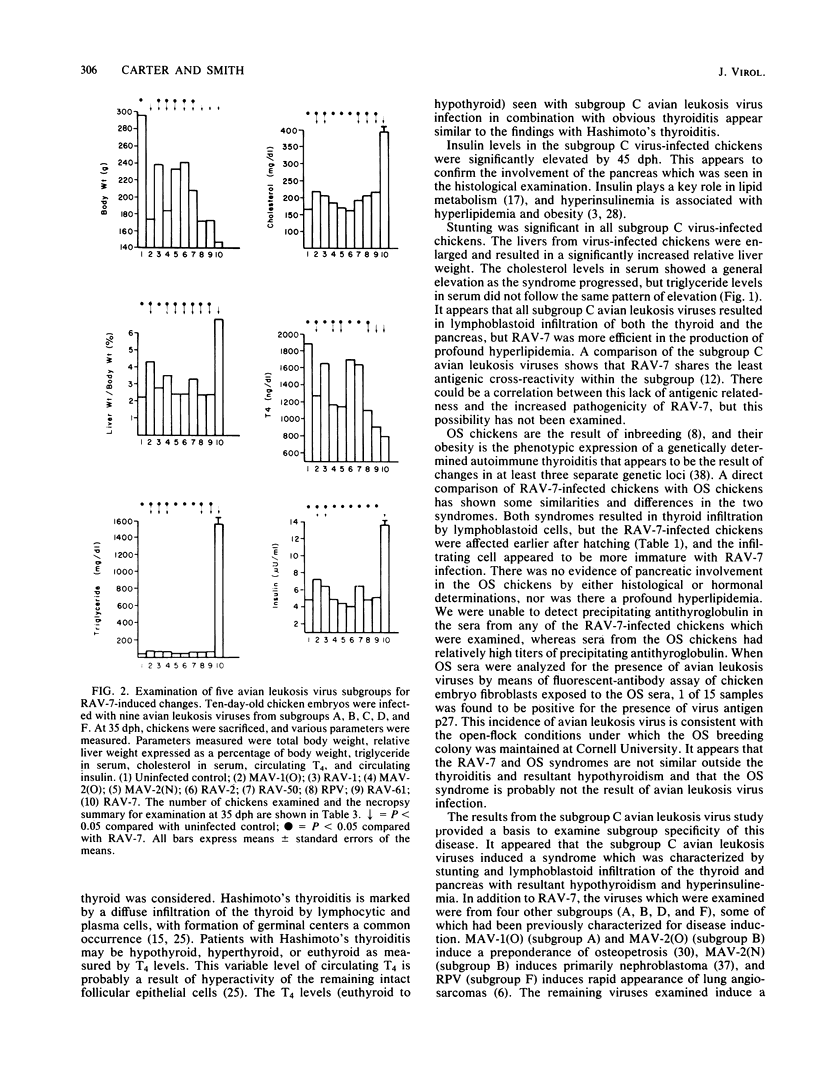
Rous-associated virus 7 (RAV-7) is a subgroup C avian leukosis virus which does not transform cells in vitro or carry an oncogene. When injected into 1-day-old hatched chicks, RAV-7 causes a low incidence of lymphoid leukosis after a latent period of several months. In contrast, infection of 10-day-old chicken embryos with RAV-7 leads to a disease syndrome characterized by stunting, obesity, atrophy of the bursa and the thymus, high triglyceride and cholesterol levels, reduced thyroxine levels, and increased insulin levels (Carter et al., Infect. Immun. 39:410-422, 1983; J.K. Carter and R.E. Smith, Infect. Immun. 40:795-805, 1983). Histopathological examination of tissues from affected chicks revealed an accumulation of lipid in the liver and an extensive infiltration of the thyroid and pancreas by lymphoblastoid cells. In the present investigation, the subgroup specificity of this syndrome was investigated. Other subgroup C avian leukosis viruses (transformation-defective B77, transformation-defective Prague C strain of Rous sarcoma virus, and RAV-49) caused stunting, infiltration of the thyroid and pancreas, increased liver weights, decreased thyroxine levels, and increased insulin levels, but they did not cause a uniform, profound increase in triglyceride and cholesterol levels. Avian leukosis viruses of subgroup A [myeloblastosis-associated virus 1 causing osteopetrosis [MAV-1(O)] and RAV-1], subgroup B [MAV-2(O), MAV-2 causing nephroblastoma [MAV-2(N)], and RAV-2], subgroup D (RAV-50), and subgroup F (ring-necked pheasant virus and RAV-61) did not cause a syndrome identical to that induced by RAV-7. All of the viruses examined induced some stunting and a reduction in thyroxine levels which correlated with the stunting. The two subgroup F viruses caused an infiltration of the thyroid which may have been secondary to severe lung involvement. We conclude that the RAV-7 syndrome is unique, particularly in the induction of a hyperlipidemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A., JAMIESON P. P. In vivo infectivity studies with avian mveloblastosis virus. Virology. 1961 May;14:33–45. doi: 10.1016/0042-6822(61)90129-5. [DOI] [PubMed] [Google Scholar]
- Biggs P. M., Milne B. S., Graf T., Bauer H. Oncogenicity of non-transforming mutants of avian sarcoma viruses. J Gen Virol. 1973 Mar;18(3):399–403. doi: 10.1099/0022-1317-18-3-399. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Genetically transmitted obesity in rodents. Physiol Rev. 1971 Jul;51(3):598–646. doi: 10.1152/physrev.1971.51.3.598. [DOI] [PubMed] [Google Scholar]
- Carter J. K., Ow C. L., Smith R. E. Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect Immun. 1983 Jan;39(1):410–422. doi: 10.1128/iai.39.1.410-422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. K., Proctor S. J., Smith R. E. Induction of angiosarcomas by ring-necked pheasant virus. Infect Immun. 1983 Apr;40(1):310–319. doi: 10.1128/iai.40.1.310-319.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. K., Smith R. E. Rapid induction of hypothyroidism by an avian leukosis virus. Infect Immun. 1983 May;40(2):795–805. doi: 10.1128/iai.40.2.795-805.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. K. Hereditary hypothyroidism in the domestic fowl. Genetics. 1966 Jun;53(6):1021–1033. doi: 10.1093/genetics/53.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano H. S., Dougherty R. M. Multiplication of avian leukosis virus in endocrine organs of congenitally infected chickens. J Natl Cancer Inst. 1969 Jan;42(1):147–154. [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Sites of avian leukosis virus multiplication in congenitally infected chickens. Cancer Res. 1967 Feb;27(2):322–332. [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Duran-Garcia S., Gomez-Nieto J., Fouchereau-Peron M., Padron V. F., Obregon M. J., Morreale de Escobar G., Escobar del Rey F. Effects of thyroid hormones on liver binding sites for human growth hormone, as studied in the rat. Clin Endocrinol (Oxf) 1979 Sep;11(3):275–290. doi: 10.1111/j.1365-2265.1979.tb03075.x. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Birnberg N. C., Rosenfeld M. G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D. J., Chen Y. C., Friis R. R., Vogt P. K. RNA tumor viruses of pheasants: characterization of avian leukosis subgroups F and G. Virology. 1974 Aug;60(2):558–571. doi: 10.1016/0042-6822(74)90350-x. [DOI] [PubMed] [Google Scholar]
- HEINE U., DE THE G., BEARD D., BEARD J. W. Multiplicity of cell response to the BAI strain A (myeloblastosis) avian tumor virus. V. Elaboration of virus by pancreas of chickens inoculated with the agent. J Natl Cancer Inst. 1963 Apr;30:817–835. [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H. Isolation of leukosis-type virus from pheasant embryo cells: possible presence of viral genes in cells. Virology. 1973 Jan;51(1):247–251. doi: 10.1016/0042-6822(73)90388-7. [DOI] [PubMed] [Google Scholar]
- Harvey S. Thyroid hormones inhibit growth hormone secretion in domestic fowl (Gallus domesticus). J Endocrinol. 1983 Feb;96(2):329–334. doi: 10.1677/joe.0.0960329. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Paterson R. W., Smith R. E. Characterization of anemia induced by avian osteopetrosis virus. Infect Immun. 1978 Dec;22(3):891–900. doi: 10.1128/iai.22.3.891-900.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Blais B. M., Tsichlis P. N., Coffin J. M. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1225–1229. doi: 10.1073/pnas.79.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Pearson M. N., DeSimone D. W., Tsichlis P. N., Coffin J. M. Subgroup-E avian-leukosis-virus-associated disease in chickens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1133–1141. doi: 10.1101/sqb.1980.044.01.122. [DOI] [PubMed] [Google Scholar]
- Schmidt E. V., Keene J. D., Linial M., Smith R. E. Association of 3' terminal RNA sequences with avian leukosis viruses causing a high incidence of osteopetrosis. Virology. 1982 Jan 15;116(1):163–180. doi: 10.1016/0042-6822(82)90411-1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Smith R. E., Schmidt E. V. Induction of anemia by avian leukosis viruses of five subgroups. Virology. 1982 Mar;117(2):516–518. doi: 10.1016/0042-6822(82)90492-5. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Van Eldik L. J. Characterization of the immunosuppression accompanying virus-induced avian osteopetrosis. Infect Immun. 1978 Nov;22(2):452–461. doi: 10.1128/iai.22.2.452-461.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. P., Smith R. E., Joklik W. K. 35S a and b RNA subunits of avian RNA tumor virus strains cloned and passaged in chick and duck cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):859–868. doi: 10.1101/sqb.1974.039.01.100. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Role of the C region in relative growth rates of endogenous and exogenous avian oncoviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1123–1132. doi: 10.1101/sqb.1980.044.01.121. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Conklin K. F., Coffin J. M. Mutant and recombinant avian retroviruses with extended host range. Proc Natl Acad Sci U S A. 1980 Jan;77(1):536–540. doi: 10.1073/pnas.77.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S. L., Smith R. E. Pathology of chickens infected with avian nephoblastoma virus MAV-2(N). Infect Immun. 1980 Feb;27(2):501–512. doi: 10.1128/iai.27.2.501-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Boyd R., Hala K., de Carvalho L., Kofler R., Müller P. U., Cole R. K. The obese strain (OS) of chickens with spontaneous autoimmune thyroiditis: review of recent data. Curr Top Microbiol Immunol. 1981;91:109–128. doi: 10.1007/978-3-642-68058-8_5. [DOI] [PubMed] [Google Scholar]
- Zeigel R. F., Barron A. L., Kite J. H., Witebsky E. Virological investigations of chickens with spontaneous autoimmune thyroiditis. II. Examination of tissues by electron microscopy. Avian Dis. 1970 Nov;14(4):617–619. [PubMed] [Google Scholar]


