Summary
A major challenge in understanding energy balance is deciphering the neural and molecular circuits that govern behavioral, physiological and metabolic responses of animals to fluctuating environmental conditions. The neurally expressed TGF-β ligand DAF-7 functions as a gauge of environmental conditions to modulate energy balance in C. elegans. We show that daf-7 signaling regulates fat metabolism and feeding behavior through a compact neural circuit that allows for integration of multiple inputs, and the flexibility for differential regulation of outputs. Perception of depleting food resources in daf-7 mutants causes fat accumulation despite reduced feeding rate. This fat accumulation is mediated, in part, through neural metabotropic glutamate signaling and upregulation of peripheral endogenous biosynthetic pathways that direct energetic resources into fat reservoirs. Thus, neural perception of adverse environmental conditions can promote fat accumulation without a concomitant increase in feeding rate.
Introduction
Animals respond to changes in food availability through coordinated behavioral, physiological, and metabolic responses. In mammals, the central nervous system (CNS) integrates internal and external signals of energy demand and availability and coordinates outputs ranging from feeding behavior and energy expenditure (Douglas et al., 2005; Morton et al., 2006; Saper et al., 2002). The identities and organization of neural circuits that regulate energy balance are just beginning to be elucidated (Elmquist et al., 1998; Morton et al., 2006; Sternson et al., 2005; Volkow and Wise, 2005). These circuits have largely been proposed based on a combination of gene expression studies, neuroanatomy, electrophysiology and imaging techniques. Recently, brain region specific gene activation and inactivation studies have begun to test and challenge the physiological significance of some these proposed neurocircuits (Balthasar et al., 2004; Balthasar et al., 2005; de Luca et al., 2005; Dhillon et al., 2006; Fulton et al., 2006; Hommel et al., 2006). However, the enormous complexity of the mammalian nervous system makes functional dissection of neural circuits a daunting task. In addition to internal signals of energy availability, visual, olfactory, gustatory, and other sensory cues also act on the CNS to modulate feeding behavior (Volkow and Wise, 2005). Little is known about the molecular and neural circuits that underlie perception of environmental conditions and their interplay with mechanisms that govern behavioral, physiological, and metabolic pathways.
The relative simplicity of the C. elegans nervous system offers the opportunity to functionally dissect the genetic and neural basis of food-evoked responses at great resolution. Several lines of evidence indicate that, as in mammals, changes in nutrient availability elicit myriad behavioral, physiological, and metabolic responses in C. elegans. For instance, removal of C. elegans from their food source, bacterial lawns, causes a ~50% reduction in feeding rate, which is, upregulated upon re-introduction to food (Avery and Horvitz, 1990). C. elegans feeding rate is measured by the number of contraction-relaxation cycles, pumps, exhibited by the pharyngeal muscle, the worm’s feeding organ (Avery, 1993b). When re-exposed to food, starved animals temporarily feed faster than well-fed animals (Avery and Horvitz, 1990). Thus, C. elegans feeding rate is not a simple reflex to food but can be entrained by the experience of starvation. Upon food depletion, hermaphrodites retain eggs (Trent, 1982), are less likely to mate with males (Lipton et al., 2004), and initiate various foraging behaviors (Hills et al., 2004; Sawin et al., 2000). C. elegans also respond to dietary change and starvation through alterations in expression of fat metabolic genes (Taubert et al., 2006; Van Gilst et al., 2005a; Van Gilst et al., 2005b) composition of fat stores (Brock et al., 2007; Van Gilst et al., 2005b), and depletion of fat reservoirs (McKay et al., 2003). How this wide range of responses are coordinated and molecularly relate to each other are poorly understood.
We investigated the signaling cascade initiated by the TGF-β like ligand DAF-7 as a potential neural sensory mechanism coordinating energy balance in response to fluctuating environmental conditions. DAF-7 and its downstream signaling cascade were first identified in the context of dauer formation, an alternative developmental stage akin to hibernation (Riddle, 1997; Wadsworth and Riddle, 1989). When faced with low food availability, high population density and high temperature, early larval stage animals halt development and enter the hibernating dauer stage. Like hibernating mammals, dauer larvae are generally non-motile, non-feeding, and contain large fat reservoirs (Riddle, 1997; Wadsworth and Riddle, 1989). Dauer stage animals display metabolic (O’Riordan and Burnell, 1989; Wadsworth and Riddle, 1989), and gene expression changes (Wang and Kim, 2003) that favor utilization of stored fats suggesting that fat reservoirs function as a nutrient source for these non-feeding animals.
daf-7 gene expression is only detected in one pair of sensory neurons named ASI (Ren et al., 1996; Schackwitz et al., 1996). Absence of food or excess daumone, a constitutively secreted pheromone (Butcher et al., 2007; Jeong et al., 2005; Ren et al., 1996) through which C. elegans gauge population density, reduce daf-7 expression leading to dauer formation (Ren et al., 1996; Schackwitz et al., 1996). Accordingly, daf-7 loss of function promotes constitutive dauer formation even under favorable food condition and low population density (Ren et al., 1996; Riddle, 1997). Exposure of dauers to food results in re-expression of daf-7, resumption of feeding and reproductive growth (Ren et al., 1996). Thus, the daf-7 pathway functions as a sensor of environmental conditions to determine whether larval stage animals grow reproductively or enter hibernation.
Here, we report that the daf-7 signaling pathway is not restricted to development but funcions generally as a neural sensory gauge of environmental conditions to modulate various energy balance pathways including feeding behavior, fat metabolism and reproduction in adult animals. Interestingly, down-regulation of daf-7, which reflects depleting nutrient conditions, causes build-up of fat reservoirs despite reduced feeding rate. To understand this inverse relationship between fat accumulation and feeding rate, we identified neurons and pathways that mediate daf-7 regulation of these processes. Although receptors for DAF-7 are broadly expressed, we found that signaling from the ASI sensory neurons to only two pairs of interneurons, RIM and RIC, was sufficient for wild-type growth, egg laying, fat, and feeding rate. In turn, we found that distinct signaling cascades are initiated in these interneurons to independently modulate fat and feeding. daf-7 inactivation leads to fat accumulation, in part, through enhanced endogenous fat biosynthesis. Therefore, despite reduced feeding rate, available energetic resources are preferentially targeted to fat reservoirs, an adaptive response consistent with perception of depleting nutrient resources. The organizational logic of the neurocircuitry revealed in our analyses allows for integration of multiple inputs and differential regulation of outputs indicating that fat and feeding are coordinated by independent outputs of the nervous system.
Results
Reduced DAF-7 Signaling Causes Excess Fat Despite Reduced Feeding
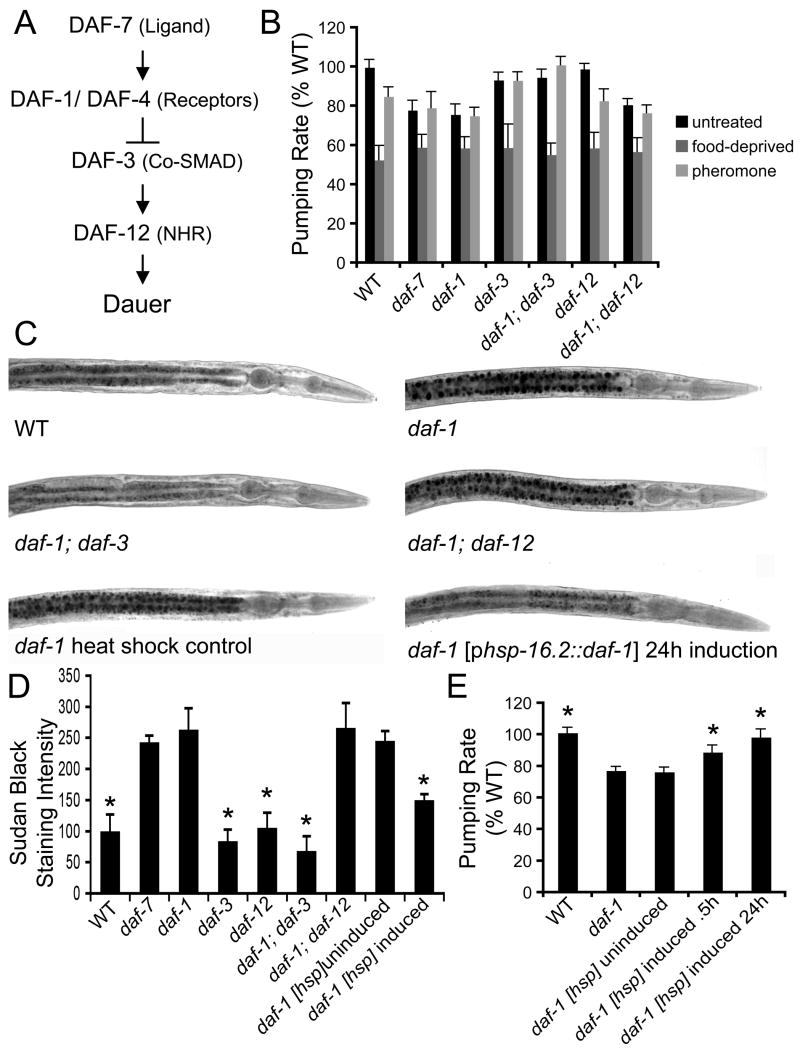
DAF-7/TGF-β ligand signals through the type I and type II TGF-β receptors DAF-1 and DAF-4 (Estevez et al., 1993; Georgi et al., 1990). Signaling through DAF-1/DAF-4 receptors inactivates DAF-3, a receptor associated co-SMAD, to block dauer entry and promote reproductive growth (Patterson et al., 1997). Dauer promoting activity of DAF-3 is, in turn, dependent on DAF-12, a nuclear hormone receptor (Antebi et al., 2000) (Figure 1A). At 25°C, loss of function mutations in daf-7, daf-1, and daf-4 cause constitutive dauer entry while loss of function mutations in daf-3 and daf-12 abrogate dauer entry (Riddle, 1997).
Figure 1. TGF-β Signaling Modulates Feeding Rate and Fat Storage in Adults.
(A) TGF-β signaling pathway as deduced from genetic analyses of dauer formation.
(B) Pharyngeal pumping rates of TGF-β pathway components under various conditions. Well-fed, pheromone untreated: daf-7(e1372) and daf-1(m40) pumped at 81% and 77% of wild-type rate, respectively. The reduced pumping rate of daf-1(m40) was suppressed by daf-3(mgDf90) but not by daf-12(m20). Food deprived, pheromone untreated: pumping rates of young adult wild type and all mutant genotypes were reduced to the same basal level. Well-fed, pheromone treated: treatment caused feeding reduction in well-fed wild type and daf-12 mutants. Similar treatment did not further reduce feeding rates of daf-7, daf-1, or daf-3 mutants. Standard deviation bars are shown.
(C) Examples of Sudan black fat staining. Adult daf-1(m40) mutants had increased fat relative to wild type. This excess fat was suppressed by daf-3(mgDf90) but not by daf-12(m20). Excess fat of daf-1(m40) animals was reduced by expression of daf-1(+) in late larval/adult stage animals using an hsp16.2 heat-shock promoter. These images are representative of numerous animals from each genotype observed in multiple, independent experiments (see Methods).
(D) Quantitation of relative amounts of Sudan black staining. Animals grown under identical conditions were fixed and stained using methods designed to minimize variation. For each genotype, average staining intensity of 10 animals is reported. Asterisks indicate statistical significance (p<0.001) as determined by t-test when comparing genotype of interest with daf-1(m40). Standard error bars are shown.
(E) Rescue of reduced pumping rate by expression of daf-1 in late larval/adult stage daf-1(m40) mutants. Pumping rate changes were noted 0.5h and 24h after induction. Asterisks indicate statistically significant change (p<0.001 determined by ANOVA with Bonferroni post-test) relative to daf-1(m40). Standard deviation bars are shown
When maintained at 20°C daf-7, daf-1, and daf-4 mutants grew into reproductive adults that displayed feeding rates that were reduced by ~20–25% relative to wild-type hermaphrodites (Figure 1B and Table S1). Similar to dauers (Kimura et al., 1997), late larval and early adult stage daf-7, daf-1 and daf-4 mutants accumulated ~2.5 fold more fat relative to wild type (Figures 1C–D, 5B–C, and data now shown). As previously reported (Trent, 1982), all of these mutants also retained eggs (examples shown in Figure S1). The high fat, egg retention and reduced feeding phenotypes daf-7 and daf-1 were dependent on daf-3 (Figure 1B–D and data not shown). By contrast, loss of daf-12 did not alter the excess fat, reduced feeding, and egg retention rates of these mutants (Figure 1B–D and data not shown). Thus, while both daf-3 and daf-12 are required for dauer entry when daf-7 or daf-1 are inactivated, fat, feeding, and egg retention phenotypes of these mutants only require daf-3. This suggests a molecular divergence in regulation of dauer entry from mechanisms that regulate fat, feeding, and egg retention.
daf-7 Functions as an Environmental Sensor in Adults
We sought to distinguish whether the excess fat and reduced feeding phenotypes of daf-7 and daf-1 mutants were due to altered development or reflected an adult sensory role for this pathway. Exposure of well-fed, wild-type adults to an acute 2 hr. dauer pheromone treatment caused a 15% reduction in feeding rate, mimicking the rate of untreated daf-7 mutants (Figure 1B). Under similar conditions, pheromone treatment did not alter the already reduced feeding rates of daf-7 and daf-1 mutants or change the wild-type feeding rate of daf-3 mutants (Figure 1B) suggesting that inhibition of DAF-7/DAF-1 signaling and subsequent activation of DAF-3 underlie the reduced feeding caused by acute pheromone treatment. Finally, daf-12 mutants were susceptible to pheromone-induced feeding reduction further supporting the notion that daf-12 is required for dauer formation but not other daf-7-regulated processes. This short pheromone treatment did not elicit detectable changes in fat content and egg retention in any of the genotypes tested (data not shown).
To examine the consequences of reactivating daf-1 in late larval/early adult stage daf-1(−) mutants, we generated transgenic daf-1(−) animals carrying full length daf-1 under the control of a ubiquitously expressed heat-shock activated promoter, hsp16.2 (Bacaj and Shaham, 2007; Stringham et al., 1992). Fat, feeding, and egg retention phenotypes of these transgenic animals grown to the young adult stage without heat-shock were indistinguishable from daf-1(−) mutants (Figure 1D–E and data not shown). By contrast, transgenic animals that were briefly heat-shocked at the L3/early L4 stage, displayed nearly wild-type fat, feeding, and egg retention phenotypes within 24 hours (Figure 1C–E and data not shown). Consistent with acute pheromone treatment results, heat-shocked transgenic animals displayed nearly wild-type feeding rates as early as 30 minutes post treatment (Figure 1E) while reversal of fat content and egg retention phenotypes took up to 24 hours.
We also examined feeding rates of food deprived adult-stage wild type and daf-7 pathway mutants. Within ~1 hr. of food deprivation, wild type and all mutant genotypes displayed similar feeding rates (~50% of rate of well-fed wild-type animals) (Figure 1B). Thus, inactivation of daf-7/daf-1 signaling does not shift basal feeding rates. Together, these findings suggest that the daf-7 pathway has an environmental sensory role in late larval and early adult stage animals and that phenotypes associated with inactivation of this pathway are not merely consequences of altered development.
Genetic Dissection of DAF-7 Neurocircuitry of Fat and Feeding
Since C. elegans reduce their feeding rate with decreasing food concentrations (Avery and Horvitz, 1990), the reduced feeding of daf-7 mutants is consistent with perception of increased population density and depleting food resources. Similar to fat accumulation in preparation for dauer entry (John, 2005; Kimura et al., 1997; Wadsworth and Riddle, 1989), adult stage build-up of fat reservoirs and retention of eggs is also consistent with preparations for periods of nutrient deprivation (John, 2005; Kimura et al., 1997; Wadsworth and Riddle, 1989). This, however, raises the perplexing question of how, despite a ~25% reduction in feeding rate, daf-7 and daf-1 mutants accumulate some of the greatest fat reservoirs observed in C. elegans (Kimura et al., 1997; Sze et al., 2000). To understand the perplexing relationship between fat and feeding in daf-7 and daf-1 mutants, we next sought to determine cellular sites through which daf-7 signaling modulates fat and feeding.
While daf-7 expression is restricted to the pair of ASI sensory neurons, its receptors, daf-1 and daf-4, are broadly expressed (Gunther et al., 2000). daf-1 is expressed in pharyngeal neurons, ciliated sensory neurons, interneurons, ventral nerve cord neurons, and in distal tip cells of the gonad. daf-4 expression overlaps with daf-1 expression but also extends to other cells and tissues (Gunther et al., 2000). We focused on cells that express the DAF-1 receptor for three reasons. First, daf-1(−) fat, feeding, egg retention, and growth phenotype are indistinguishable from corresponding daf-7(−) phenotypes. Second, although the C. elegans genome expresses several TGF-β type ligands, thus far, the DAF-1 receptor has only been implicated in DAF-7 signaling (Krishna et al., 1999). Third, inactivation of sma-6, encoding the only other type I receptor identified in the C. elegans genome, causes small body size but is not associated with changes in feeding rate and fat (Table S1 and data not shown).
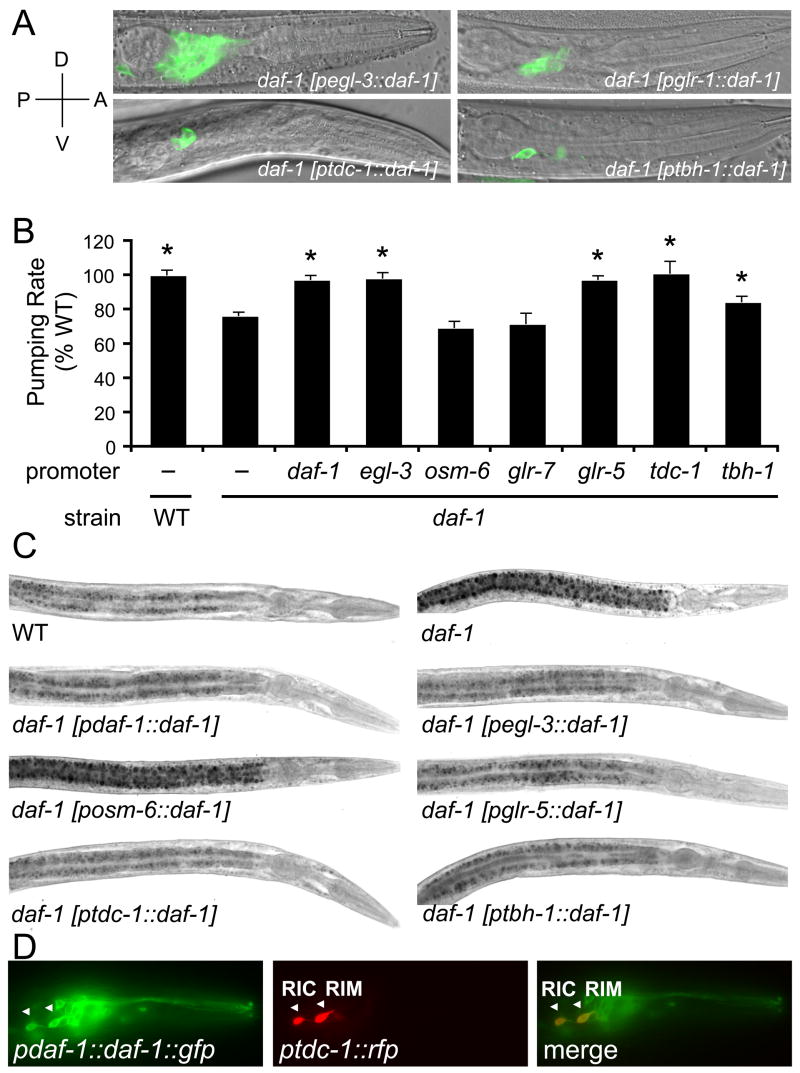
To identify cells that require daf-1 signaling for wild-type regulation of feeding, fat storage, egg retention and dauer formation, we expressed full-length daf-1 using 20 different neuron-specific promoters in daf-1(−) mutant animals. For each promoter, we generated a full length daf-1::gfp fusion and a corresponding promoter::gfp reporter. For each construct, we generated multiple independent transgenic lines (3 on average for each promoter::daf-1::gfp). Gene expression was confirmed by observing appearance of GFP in target cells (examples in Figure 2A). The results described below and summarized in Table 1 were consistent across multiple independent lines.
Figure 2. Reconstitution of daf-1 in RIM and RIC Interneurons Restores Wild-type Feeding Rate and Fat Storage to daf-1(m40) Mutants.
Tissue-specific promoters listed in Table 1 were used to express full-length daf-1::gfp in various cell types of daf-1(m40) animals.
(A) Examples of neuronal expression patterns of promoters used to target daf-1::gfp. Axis indicates P, posterior, A, anterior, D, dorsal, V, ventral.
(B) Effects of tissue selective reconstitution of daf-1 on feeding rate. Asterisks indicate statistical significance relative to daf-1(m40) (p<0.001 as determined by ANOVA with Bonferroni post-test). Standard deviation bars are shown.
(C) Effects of tissue selective reconstitution of daf-1 on fat. Quantitations for representative fat phenotypes shown here are reported in Figure S3.
(D) Pdaf-1::daf-1::gfp co-localized with Ptdc-1::RFP in RIM and RIC (white arrowheads).
Table 1. Expression of daf-1 in Interneurons Is Sufficient to Rescue the Dauer Formation, Feeding Rate, and Fat Storage Phenotypes of daf-1(m40) Mutants.
daf-1::gfp was expressed under various promoters in daf-1(m40) mutants. Larval dauer formation, adult feeding rate and fat content were measured in each of the transgenic lines. Indicated results were consistent for at least three independent transgenic lines. Promoters used to drive daf-1::gfp are grouped based on anatomical expressions.
| Genetic background | Promoter driving daf-1::gfp | No. of Cells expressing daf-1::gfp | % Dauer (n) a | % WT pumping rate b | Eggs retained c | |
|---|---|---|---|---|---|---|
| Control | N2 (WT) | none | 0 (150) | 100 ± 2.8 e | + | |
|
|
||||||
| daf-1(m40) | none | 98 (480) | 76 ± 2.7 | +++ | ||
|
|
||||||
| daf-1(m40) | daf-1 d | many (>80) neurons | 2 (179) | 97 ± 2.1 e | + | |
|
| ||||||
| Pan-Neuronal | daf-1(m40) | egl-3 d | many (>80) neurons | 3 (112) | 98.2 ± 3 e | + |
|
| ||||||
| Sensory Neurons | daf-1(m40) | bbs-1 | 60 ciliated neurons | 99 (142) | 75 ± 6.7 | +++ |
|
|
||||||
| osm-6 | 56 ciliated neurons | 100 (140) | 69 ± 3.5 | +++ | ||
|
|
||||||
| daf-7 | 2 (ASI) | 100 (79) | N/D | +++ | ||
|
| ||||||
| Pharynx | daf-1(m40) | glr-8 | 25 pharyngeal neurons and interneurons | 56 (147) | 81.7 ± 4 | +++ |
|
|
||||||
| glr-7 | 9 pharyngeal neurons | 98 (249) | 71 ± 6.0 | +++ | ||
|
|
||||||
| B0280.7 | 5 pharyngeal gland | 98 (181) | 74 ± 5.4 | +++ | ||
|
| ||||||
| Interneurons | daf-1(m40) | glr-2 | 24 | 94 (332) | 74 ± 2.3 | +++ |
|
|
||||||
| unc-47 | 29 | 100 (102) | 72 ± 4.7 | +++ | ||
|
|
||||||
| unc-17 | 80 | 37 (174) | 76 ± 6.3 | +++ | ||
|
|
||||||
| glr-5 d | 56 | 6 (175) | 97 ± 1.9 e | ++ | ||
|
|
||||||
| glr-4 d | 38 | 3 (248) | 96 ± 2.9 e | ++ | ||
|
|
||||||
| glr-1 d | 24 | 14 (170) | 90 ± 4.6 e | ++ | ||
|
|
||||||
| ggr-1 | 10 | 94(119) | 77 ± 7.1 | +++ | ||
|
|
||||||
| flp-1 | 2 | 100 (216) | 76 ± 2.6 | +++ | ||
|
|
||||||
| dop-1 | 12 | 100 (231) | 76 ± 6.1 | ++ | ||
|
|
||||||
| nmr-2 d | 12 | 2 (221) | 95 ± 3.2 e | ++ | ||
|
|
||||||
| tdc-1 d | 4 interneurons and UV1 cells | 1 (188) | 98 ± 3.1 e | ++ | ||
|
|
||||||
| tbh-1 d | 2 | 57 (178) | 84 ± 3.1 e | ++ | ||
% animals that entered dauer when placed on food plates as L1s at 25°C. Phenotypic example shown in Figure S1
Average pumping rate of well-fed, young gravid adults. Standard deviations are shown.
Young, well-fed wild-type animals retained ~10 eggs on average. This phenotype is denoted as (+). Mutants such as daf-1(m40) retained excess eggs (~24), denoted as (+++). Intermediate egg retention is denoted as (++). Phenotypic examples are shown in Figure S1.
Expression of daf-1(+) by these promoters reduced the excess fat of daf-1(m40) to wild-type levels. Phenotypic examples shown in Figure 1B.
Statistically significant (p <0.001, determined by ANOVA with Bonferroni post-test) increases in feeding rate relative to daf-1(m40).
Expression of daf-1::gfp under its own promoter or the egl-3 pan-neural promoter fully rescued the adult feeding rate (Table 1, Figure 2B), fat storage (Figures 2C and S2), egg retention, and larval dauer phenotypes of daf-1(−) animals (Table 1, examples of dauers and egg retention phenotypes are shown in Figure S2). We then systematically targeted daf-1::gfp to sensory, inter-, and pharyngeal neurons. Reconstitution of daf-1 in ASI neurons alone, site of daf-7 expression, or in almost all 60 ciliated sensory neurons using the bbs-1 and osm-6 promoters (Ansley et al., 2003; Collet et al., 1998), failed to rescue any of the daf-1(−) phenotypes (Table 1, Figures 2B–C and S2, and data not shown). Reconstitution of daf-1 in pharyngeal neurons or gland cells using glr-7, glr-8 (Brockie et al., 2001a) and B0280.7 promoters also failed to rescue any of the daf-1(−) phenotypes (Table 1, Figure 2B, and data not shown). By contrast, directing daf-1::gfp expression by glr-1, glr-4, and glr-5 promoters (Brockie et al., 2001a), which target interneurons, fully reverted the dauer formation, egg-laying, feeding rate and fat storage phenotypes of daf-1(−) mutants back to wild-type levels (Table 1, Figures 2B–C and S2). However, expression of daf-1::gfp under the glr-2 promoter, which targets 24 interneurons, or the unc-47 and unc-17 promoters (Alfonso et al., 1993; Eastman et al., 1999), which target the 29 GABAergic and the 80 acetylcholinergic motor neurons, respectively, did not alter various daf-1(−) mutant phenotypes (Table 1 and data not shown). Thus, daf-1 expression in a specific subset of interneurons is sufficient to allow for normal development, egg laying, pumping rate, and fat storage. This is consistent with the role of interneurons as integrators of sensory information.
DAF-7 Signals to RIM and RIC Interneurons To Regulate Fat and Feeding
We asked whether reconstitution of daf-1 in only a subset of interneurons targeted by the rescuing glr-1, glr-4, and glr-5 promoters would be sufficient to restore wild-type fat, feeding, growth, and egg-retention to daf-1 mutants. We reconstituted daf-1::gfp expression under each of glr-2, ggr-1, dop-1, nmr-2, and flp-1 promoters (Brockie et al., 2001a; Tsalik et al., 2003) which direct expression to various subsets of cells also targeted by glr-5, glr-4, and glr-1 rescuing constructs (Table S2). daf-1(−) phenotypes were fully rescued when daf-1::gfp expression was directed by nmr-2 but not by glr-2, ggr-1, flp-1, or dop-1 promoters (Table 1 and data not shown). Expression of glr-5, glr-4, glr-1, and nmr-2 promoters compared to all other tested promoters pointed to the RIM pair of interneuron as the only cells commonly targeted by these rescuing promoters and absent in all non-rescuing promoters (Table S2). This was possible since we could verify expression patterns of various promoters in anatomically identifiable neurons (Table S2).
To test the role of RIM in daf-1 signaling, we used a tdc-1 promoter targeting gene expression to only two pairs of interneurons, RIM and RIC, as well as the UV-1 uterine–vulval cells (Alkema et al., 2005). All daf-1(−) phenotypes were fully rescued when daf-1::gfp expression was directed by this promoter (Table 1, Figures 2B–C, S2 and data not shown). Exclusive expression of daf-1::gfp in RIC interneurons by a tbh-1 promoter also restored wild-type fat but only partially rescued the reduced feeding and dauer constitutive phenotypes of daf-1(−) (Table 1, Figures 2B–C and S2). Unlike the tdc-1 promoter, this tbh-1 promoter did not rescue egg retention phenotype of daf-1(−) animals (data not shown). To demonstrate that RIM and RIC are normally recipients of the DAF-7 signal, we expressed Ptdc-1::RFP and a functional Pdaf-1::daf-1::gfp and noted a clear overlap between these reporter-fusions in RIM and RIC (Figure 2D). Consistent with a previous report (Gunther et al., 2000), daf-1 expression was not detectable in UV-1 cells suggesting that these cells are not physiological targets of DAF-7.
These data strongly suggest that the site of daf-1 expression, specifically in RIM and RIC, rather than extent of daf-1 expression determine whether wild-type fat, feeding, and reproduction levels are restored to daf-1(−) animals. Moreover, since ASI does not synapse onto either RIM or RIC, our data indicate that DAF-7 peptidergic signaling is not confined by direct synaptic connections.
Selective Activation of daf-3 in RIM and RIC Interneurons
Since DAF-1 signaling antagonizes activity of the co-SMAD DAF-3, we asked whether selective activation of DAF-3 in RIM and RIC interneurons could lead to growth, fat, feeding, or egg retention phenotypes. Full length daf-3 was expressed using daf-1, osm-6, glr-7, tdc-1, and tbh-1 promoters in daf-1(−); daf-3(−) double mutants. In this background, targeted cells would gain DAF-3 activity since the inhibitory function of DAF-1 is eliminated. Selective reconstitution of daf-3 in ciliated sensory and pharyngeal neurons using the osm-6 and glr-7 promoters, respectively, did not alter the fat and feeding rates of daf-1(m40); daf-3(mgDf90) animals (Figure 3A–C). By contrast, reconstitution using the tdc-1 promoter resulted in reduced feeding rate and increased fat levels similar in magnitude to those seen when daf-3 was broadly expressed using the daf-1 promoter (Figure 3A–C). Furthermore, up to ~20% of animals in which daf-3 was reconstituted in only RIM and RIC formed dauers (Table S3). This is a significant percentage since broad neuronal expression of daf-3 caused dauer formation in only ~40% of transgenic animals. Finally, selective reconstitution of daf-3 in RIC interneuron alone was insufficient to alter the fat and feeding phenotypes of daf-1(−); daf-3(−) animals. These findings supported the daf-1 reconstitution studies in showing that regulation of fat and feeding in response to the environmentally-sensitive DAF-7 signal is funneled through RIM and RIC.
Figure 3. Activation of daf-3 in RIM and RIC Causes Fat Accumulation despite Feeding Reduction.
(A) Feeding rate. Asterisks indicate statistical significance (p<0.001 determined by ANOVA with Bonferroni post-test) relative to daf-1(m40); daf-3(mgDf90). Standard deviation bars are shown.
(B–C) Fat content. Asterisks indicate statistical significance (p<0.001) as determined by t-test comparing genotype of interest with daf-1(m40); daf-3(mgDf90). Standard error bars are shown.
Inactivation of daf-7 Reduces Feeding Via Neurotransmitters Tyramine and Octopamine
RIM and RIC are somatic nerve interneurons located in the lateral ganglion (White et al., 1986). RIM functions as a motor neuron and links the C. elegans tactile-responsive locomotory neural circuit to the neural circuit that regulates head movements. RIC is the sole cellular site for synthesis of the monoamine neurotransmitter octopamine (Alkema et al., 2005). Synthesis of tyramine and octopamine requires conversion of tyrosine to tyramine by tyrosine decarboxylase (TDC-1) and subsequent conversion of tyramine to octopamine by tyramine β-hydroxylase (TBH-1). RIM synthesizes tyramine but not octopamine while RIC can synthesize both tyramine and octopamine (Alkema et al., 2005). Other neurons do not synthesize either of these neurotransmitters.
Exogenous tyramine or octopamine were previously shown to reduce pharyngeal pumping rates (Horvitz et al., 1982). Treatment of wild-type animals with exogenous tyramine or octopamine caused reduced feeding reminiscent of rates of daf-7 or daf-1 mutants while loss of function of either tdc-1 or tbh-1 restored nearly wild-type feeding rate to daf-1 and daf-7 mutants (Figure 4A). These data suggested that active daf-7/daf-1 signaling regulates feeding by blocking tyraminergic and octopaminergic transmissions that originate in RIM and RIC.
Figure 4. Tyramine and Octopamine Are Required for the Reduced Feeding Rate but not the Excess Fat of daf-1(m40) Mutants.
(A) Effects of 4mM tyramine or octopamine on pumping rates of well-fed adult stage animals. Rescue of daf-1(m40) reduced feeding rate by each of tdc-1(ok914), tbh-1(ok1196), and ser-2(pk1357). Asterisks indicate statistical significance (p<0.001 as determined by ANOVA with Bonferroni post-test) for indicated comparisons. Standard deviation bars are shown.
(B–C) tdc-1(ok914), tbh-1(ok1196), and daf-12(m20) mutations did not suppress the excess fat of daf-1(m40). Asterisks indicate statistical significance (p<0.001) as determined by t-test comparing genotype of interest and daf-1(m40). Standard error bars are shown.
(D) Reconstitution of SER-2 tyraminergic receptor using tissue specific promoters in daf-(m40); ser-2(pk1357) mutants. Asterisks indicate statistical significance (p<0.001 determined by ANOVA with Bonferroni post-test) relative to non-transgenic daf-1(m40); ser-2(pk1357). Standard deviation bars are shown.
To further delineate the daf-7 feeding regulatory pathway, we examined feeding rates of ser-2 and tyra-2 mutants. ser-2 and tyra-2 encode two C. elegans biogenic G-protein coupled receptors that exhibit greatest in vitro binding affinity for tyramine (Rex et al., 2005; Rex et al., 2004). ser-2(−) and tyra-2(−) mutants had wild-type feeding rates, however, inactivation of ser-2 but not tyra-2 restored wild-type feeding rate to daf-1 mutants (Figure 4A and Figure S3). This was consistent with previous characterization of ser-2 as a tyraminergic receptor that modulates feeding (Rex and Komuniecki, 2002; Rex et al., 2004). To demonstrate that tyramine signaling through the SER-2 receptor modulates feeding in response to environmental cues, tdc-1(−) and ser-2(−) adults were acutely treated with pheromone. Unlike wild-type animals, pheromone treatment did not alter the feeding rates of these mutants (Figure S3). Additionally, in response to starvation, tdc-1(−) and ser-2(−) mutants did not exhibit the full extent of feeding reduction seen in wild-type animals (Figure S3).
To determine cellular sites where ser-2 signaling elicits feeding rate reduction, we selectively reconstituted ser-2 in daf-1(−);ser-2(−) double mutants. ser-2 expression has been reported in ~10% of head neurons, head muscle, and pharyngeal neurons (Tsalik et al., 2003). Selective reconstitution of ser-2 in pharyngeal muscle using the myo-2 promoter (Okkema PG et al., 1993) did not alter the feeding rate of daf-1(−); ser-2(−) (Figure 4F). Only a modest reduction in feeding rate was obtained when an egl-3 promoter was used to broadly express ser-2 in head neurons but only weakly in pharyngeal neurons (Figure 4D). By contrast, ser-2 reconstitution using a glr-7 promoter was sufficient to reduce feeding rate of daf-1(−); ser-2(−) double mutants down to the rate of daf-1(−) (Figure 4D). glr-7 promoter targets expression to I1, I2, I3, and I6 pharyngeal interneurons, M1 pharyngeal neuron, and NSM neurosecretory motoneurons (Brockie et al., 2001a). Some of these neurons have previously been implicated in regulation of pharyngeal muscle pumping (Avery, 1993a; Avery and Horvitz, 1989; Niacaris and Avery, 2003).
Together, these findings suggest a feeding circuit in which daf-7 signaling from ASI sensory neurons acts on RIM and RIC interneurons to inhibit tyraminergic and octopaminergic neurotransmissions from these interneurons. When adverse environmental conditions inactivate daf-7, its inhibitory effects on tyramine and octopamine are relieved, and in turn, tyraminergic signaling reduces feeding rate largely via signaling to the SER-2 receptor on a small subset of pharyngeal neurons that regulate activity of the pharyngeal muscle.
daf-7 Regulation of Feeding Is Distinct From Fat Accumulation and Dauer Formation
Elimination of tyraminergic and octopaminergic signaling by each of tdc-1, tbh-1, and ser-2 mutations restored nearly wild-type feeding rate to daf-1(−) animals without altering larval constitutive dauer formation, adult egg retention and excess fat phenotypes of this mutant. This was reminiscent of the finding that inactivation of the daf-12 abrogated the dauer constitutive but not the fat, feeding, and egg retention phenotypes caused by daf-7 inactivation. Accordingly, daf-1(−); tdc-1(−); daf-12(−) and daf-1(−); tbh-1(−); daf-12(−) triple mutants bypassed dauer entry and, as adults, displayed nearly wild-type feeding rates yet accumulated excess fat and retained eggs (Figure 4B–C and data not shown). Together, these findings suggested that, upon DAF-3 activation in RIM and RIC, distinct molecular pathways are engaged to regulate food intake behavior, growth, and metabolism: the effects on growth are eventually mediated by the DAF-12 nuclear hormone receptor while the effects on feeding behavior are mediated by RIM/RIC synthesized neurotransmitters, tyramine and octopamine.
daf-7 Inactivation Causes Fat Accumulation through GPCR Signaling
The FOXO transcription factor encoded by daf-16 is required for the excess fat, long lifespan, stress resistance, and dauer constitutive phenotypes associated with inhibition of C. elegans insulin signaling (Lin et al., 1997; Ogg et al., 1997). However, dauer constitutive or excess fat phenotypes of daf-7 mutants were independent of this insulin-signaling regulated transcription factor (Ogg et al., 1997).
To identify mechanisms that underlie excess fat accumulation of daf-7 mutants, we conducted a mutagenesis screen. If the fat, feeding, and dauer responses elicited by daf-7 inactivation are mediated through distinct mechanisms, we asked whether we could identify mutations that specifically abrogate daf-1(−) fat increase without concomitant abrogation of other phenotypes of this mutant. Among several such mutants identified, one line, daf-1(m40 ft9), was selected for further analysis. When dauer constitutive daf-1(m40 ft9) mutants were allowed to bypass the dauer stage, they grew to adults that had wild-type fat levels (Figure S4A), were hyperactive, egg laying constitutive and displayed loopy movement and vulval protrusion (data not shown). Similar results were obtained for daf-7(e1372 ft9) mutants. The ft9 suppressor was mapped to egl-30 (Figure S4 data not shown), which encodes a Gq class α subunit of heterotimeric G proteins (Bastiani et al., 2003; Schade et al., 2005). The G to T nucleotide base change in egl-30 sequence obtained from daf-7(e1372); egl-30(ft9) but absent in wild-type or daf-7(e1372) is predicted to result in a glutamic to aspartic acid substitution in a highly conserved residue within a phylogenetically conserved domain (Figure S4B–D). The semi-dominant segregation as well as movement and egg-laying phenotypes of egl-30(ft9) are consistent with previously described egl-30 gain-of-function (gof) mutations (Bastiani et al., 2003; Schade et al., 2005).
EGL-30/Gqα signaling through phospholipase C-β generates the second messenger diacylglycerol while G0α signaling inhibits its accumulation through activity of DGK-1 kinase (Miller et al., 1999). Thus, phenotypes caused by gain of function mutations in egl-30 can be mimicked by loss of function mutations in goa-1 and dgk-1 (Hajdu-Cronin et al., 1999; Miller et al., 1999; Segalat et al., 1995). goa-1(−) or dgk-1(−) did not significantly alter fat levels of wild-type animals but reduced the excess fat of daf-7(−) mutants to nearly wild-type levels (Figure 5B–C and data not shown). daf-7(−); egl-30(gof) as well as daf-7(−); goa-1(−), and daf-7(−); dgk-1(−) double mutants remained dauer constitutive and, as adults, displayed reduced feeding rates (Figure 5A and data not shown). While these findings supported the notion that fat and feeding could be regulated through distinct signaling pathways, interpretation of the results were confounded by the fact that numerous processes including pharyngeal pumping, egg laying, and movement involve G-protein signaling.
Figure 5. Mutations in GPCR Signaling and Metabotropic Glutamate Receptors Abrogate the Excess Fat but not Reduced Feeding of daf-7(.
−).
Increased fat but not reduced feeding rate of daf-7(e1372) was suppressed by mutations in goa-1, dgk-1, eat-4, mgl-1, and mgl-3 as well as a predicted gain of function mutation in egl-30.
(A) Feeding rate. Asterisks indicate statistical significance relative to daf-7(e1372) (p<0.001 determined by ANOVA with Bonferroni post-test). Standard deviation bars are shown.
(B) Representative examples of fat content in various mutants.
(C) Fat quantitations. Asterisks indicate statistical significance (p<0.001) as determined by t-test when comparing genotype of interest with daf-7(e1372). Standard error bars are shown.
(D) Expression sites of mgl-1 and mgl-3. Pmgl-1::mCherry was expressed in AIA amphid interneurons, RMDV and RMDD ring interneurons/motoneurons, and pharyngeal NSM serotonergic neurons. Pmgl-3::gfp was expressed in NSM, ADF, ASE, and AWC amphid sensory neurons, and the RIB and RIC interneurons. Occasional expression in BAG ciliated neurons was also noted.
daf-7 Inactivation Causes Excess Fat Via Metabotropic Glutamate Signaling
To identify signals that may be specifically required for excess fat in daf-7(−), we analyzed neurotransmitter and neuropeptide pathways that utilize GPCR signaling. The C. elegans genome encodes ~250 predicted neuropeptides, a subset of which are processed by the subtilisin-family proprotein convertase EGL-3 (Husson et al., 2006). Wild-type processing of at least 25% of predicted neuropeptides is altered in egl-3 mutants, one consequence of which is reduced fat content (Husson et al., 2007). However, EGL-3 activity was dispensable for excess fat of daf-7(−) (data not shown). Similarly, mutations in unc-17 (Alfonso et al., 1993) and cat-2 (Lints and Emmons, 1999) which abrogate acetylcholine and dopamine signaling pathways, respectively, did not alter fat accumulation in daf-7 mutants (data not shown). By contrast, inactivation of the vesicular glutamate transporter encoded by eat-4 (Lee et al., 1999) partially abrogated excess fat of daf-7(−) without suppressing the reduced feeding or dauer constitutive phenotypes of this mutant (Figure 5A–C and data now shown). Unambiguous dissociation of fat and feeding regulation was again confounded since glutamate signaling also regulates feeding rate (Avery, 1993b; Lee et al., 1999).
Given requirements of GPCR and glutamate pathways, we tested whether metabotropic glutamate signaling promotes fat accumulation upon daf-7 inhibition. The C. elegans genome encodes for three metabotropic glutamate receptors, mgl-1, -2, and -3 (Dillon et al., 2006). Loss of either mgl-1 or mgl-3 but not mgl-2 reduced the excess fat of daf-7 mutants from ~245% to ~170% of wild-type levels (p<0.001) (Figures 5C and S5). Combination of mgl-1(−) and mgl-3(−) further reduced excess fat accumulation of daf-7 mutants to ~135% of wild-type levels (Figure 5B–C). Despite nearly normalized fat levels, these triple mutants remained dauer constitutive, retained eggs, and displayed reduced feeding rates that were indistinguishable from daf-7(−) (Figure 5A, Table S1 and data not shown). Importantly, mgl-1(−) and mgl-3(−) mutants had wild-type feeding rates allowing for unambiguous dissociation of fat and feeding mechanisms elicited by daf-7 inactivation (Figure 5A, Table S1). Finally, to investigate the specificity of metabotropic glutamate receptors in suppressing excess fat levels of daf-7(−) mutants, we investigated the consequences of inactivation in nmr-1 and glr-1, which encode for an N-methyl-D-aspartate (NMDA) and a non-NMDA class of ionotropic glutamate receptors, respectively (Brockie et al., 2001; Hart et al., 1995). nmr-1(−) and glr-1(−) mutants had wild-type fat accumulation and did not alter the excess fat of daf-7(−) (data not shown). This supported the notion that the excess fat of daf-7(−) is dependent on activation of specific glutamergic receptors.
To determine potential sites of mgl-1 and mgl-3 function, we generated reporter fusions to each of their predicted promoters. These promoter fusions were expressed in a very limited number of neurons and only overlapped in the serotonergic pharyngeal NSM neurons (Figure 5D). The largely non-overlapping expression patterns of these reporter fusions were consistent with redundant functions of mgl-1 and mgl-3 in mediating excess fat of daf-7 mutants.
daf-7 mutants have increased de novo fat synthesis
Given that excess fat accumulation in daf-7 and daf-1 mutants could not be attributed to molecular mechanisms mediating the consequences of inactivation of this pathway on feeding, egg laying and growth, we sought to determine the effects of daf-7(−) on metabolic pathways. As an initial characterization, we examined de novo rates of fat synthesis. By following the incorporation of 13C labeled carbons into palmitate, stearate, and vaccenic acids extracted from C. elegans lysates, we obtained estimates for the fraction of total lipids that were synthesized de novo. Relative to wild type, fat synthesis was upregulated nearly ~3 fold in daf-7 and daf-1 mutants and this upregulation was dependent on daf-3 (Figure S6A and data not shown).
To further investigate the role of de novo fat synthesis in excess fat of daf-7 and daf-1 mutants, we used RNAi to inactivate fasn-1, encoding fatty acid synthase. Fatty acid synthase carries out the cyclical reactions that generate fats from malonyl-CoA (Asturias et al., 2005). Exposure of wild-type, daf-7(−) and daf-1(−) animals to fasn-1 RNAi resulted in larval growth arrest (example in Figure S6B). Growth arrested daf-7 and daf-1 mutants had reduced fat levels indistinguishable from growth arrested wild-type animals (Figure S6B-C and data not shown). Finally, we determined that a ~6kb predicted fasn-1 promoter-reporter fusion was expressed in epidermal skin-like tissue (hypodermis) (Figure S6D). Epidermal expression is consistent with the metabolic role of fasn-1 since epidermal and intestinal cells are major sites of fat storage and metabolism in C. elegans (Kimura et al., 1997; Mak et al., 2006; McKay et al., 2003). Together, these results indicate that fasn-1 is critical for fat synthesis and growth and that inactivation of daf-7 initiates a signaling cascade that ultimately promotes de novo fat synthesis in the periphery providing one explanation for the excess fat despite reduced feeding rate.
Discussion
Independent Neural Signaling Pathways Regulate Fat and Feeding
We delineated a genetic and corresponding neuronal circuit that links an environmental sensory mechanism to the regulation of fat content and food intake behavior in C. elegans. Adverse environmental conditions such as increased population density and low food availability inactivate the neurally expressed DAF-7 TGF-β like ligand leading to reduced feeding rate, retention of eggs, and shift of energetic resources into fat reservoirs. Inhibition of daf-7 elicits these responses even in the presence of plentiful food and uncrowded conditions suggesting that perception of unfavorable conditions promotes fat storage. Since daf-7 is exclusively expressed in the ASI neurons and these neurons have been implicated in the regulation of lifespan by dietary restriction (Bishop and Guarente, 2007), it is possible that, as a gauge of conditions, daf-7 may be subject to regulation by both external and internal cues of nutrient status.
Our analyses provide a solution to the paradoxical increase in fat despite reduced feeding rate by revealing that daf-7 regulation of fat is molecularly independent from daf-7 regulation of feeding. This molecular divergence abrogates the requirement of increased feeding rate as the sole basis of increased fat accumulation. Excess fat accumulation in daf-7 mutants is associated with increased rate of de novo fat synthesis in peripheral tissues. Therefore, while the rate of food intake is reduced, energetic resources are preferentially directed into build up of fat reservoirs, an adaptive response in preparation for periods of nutrient deprivation (Wang et al., 2006).
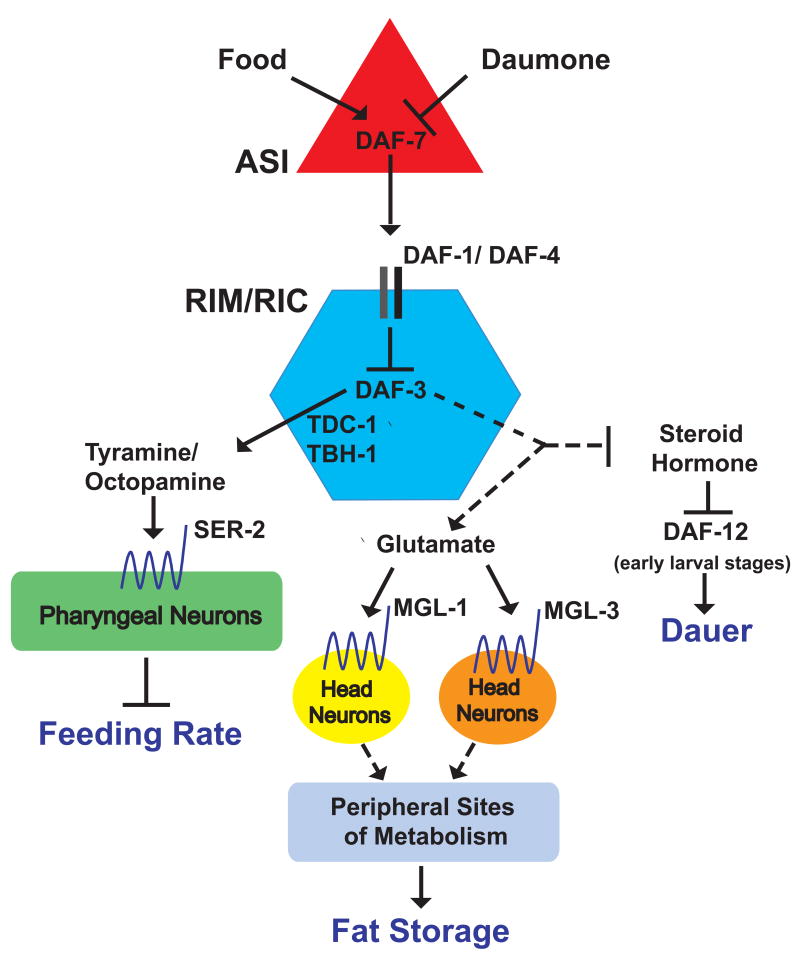
The combination of tissue specific expression studies, pharmacology, and genetic epistasis analyses presented here strongly support a model whereby under favorable environmental conditions, DAF-7 signaling from the ASI sensory neurons to its receptors on RIM and RIC interneurons inhibits DAF-3 activation allowing for wild-type fat content, feeding rate and reproduction. Under poor environmental conditions, DAF-3 activation in RIM and RIC leads to tyraminergic regulation of feeding via the SER-2 receptor on pharyngeal neurons and glutamatergic regulation of fat through MGL-1 and MGL-3 metabotropic receptors (Figure 6). Since mgl-1 and mgl-3 reporter fusions localize to only a few neurons, activation of metabotropic glutamate signaling in these neurons must initiate other neural or hormonal signals that ultimately signal to sites of fat regulation to shift the balance between storage and utilization pathways.
Figure 6. Model for the TGF-β Neural Circuit of Fat and Feeding Regulation.
The DAF-7 TGF-β ligand expression is only detected in the ASI pair of sensory neurons while its receptors, DAF-1 and DAF-4 are broadly expressed in the nervous system. During favorable environmental conditions DAF-7 is secreted from ASI and signaling to two pairs of synaptically distant interneurons, RIM and RIC, to inhibit the DAF-3 co-SMAD. This promotes wild-type growth, egg laying, food intake behavior, and fat accumulation. Adverse environmental conditions such as increased population density combined with reduced food availability inactivate daf-7, relieving inhibition of DAF-3. During early larval stage, this leads to dauer formation and requires the nuclear hormone receptor DAF-12. In adults, DAF-3 activation in RIM and RIC causes reduced feeding rate, egg retention, and increased fat. These processes are regulated through distinct signals. Subsequent to DAF-3 activation, tyramine, synthesized in RIM and RIC, and octopamine, synthesized in RIC, cause feeding reduction. Tyraminergic feeding reduction is mediated through activation of the SER-2 GPCR on a subset of pharyngeal neurons. Glutamate signaling through neuronally expressed metabotropic glutamate receptors, MGL-1 and MGL-3, mediates fat increasing effects of daf-7(−). Fat increase is ultimately associated with increased de novo fat synthesis in the periphery.
RIM/RIC activation of DAF-3 also regulates the decision for larval stage animals to grow reproductively or enter hibernation. Genetic and biochemical analyses have placed the DAF-9 cytochrome P450 enzyme downstream of daf-3 and upstream of the daf-12 nuclear hormone receptor in the dauer entry pathway (Albert and Riddle, 1988; Gerisch and Antebi, 2004; Gerisch et al., 2001; Jia et al., 2002; Thomas et al., 1993). DAF-9 synthesizes steroid hormone ligands that bind to DAF-12 to inhibit dauer formation and induce reproductive growth (Motola et al., 2006). daf-9 is expressed in epidermal cells, the pair XXX head cells that are associated with the epidermis, and hermaphrodite spermathecae (Gerisch and Antebi, 2004; Mak and Ruvkun, 2004). Selective reconstitution of daf-9 in epidermal cells is sufficient to rescue the dauer constitutive but not the egg-laying defect of daf-7(−) (Gerisch and Antebi, 2004; Mak and Ruvkun, 2004). Combining these findings with our results, we speculate that signaling from RIM/RIC to epidermal cells regulates DAF-9 during the dauer entry program. Epidermal expressions of fasn-1 and daf-9 suggest that this tissue is a major peripheral recipient of neural signals that alter fat and sterol pathways in C. elegans.
The only signals that unambiguously originate in RIM and RIC are tyramine and octopamine. Thus, it is possible that an as of yet unidentified signal originates in RIM and RIC that commonly regulates fat, dauer, and egg retention. Upon release from RIM and RIC, this signal must then diverge into glutamergic regulation of fat and other molecular pathways that regulate egg retention and dauer formation (Figure 6).
Neuronal Circuitry of Energy Balance
Why should behavioral, physiological, and metabolic responses regulated by daf-7 signaling be funneled through very specific pairs of interneurons and subsequently diverge into distinct molecular pathways? As interneurons, RIM and RIC are recipients of multiple neural inputs (White et al., 1986). This allows for integration of various cues regarding energy availability and demand. In turn, regulation of diverse behavioral, physiological, and metabolic responses through distinct molecular outputs allows for fine-tuning and even short-circuiting of individual outputs. Such a differential regulation may be required under different enviromental conditions. For instance, food sources that range in their nutrient qualities or presence of conflicting environmental cues could require differential activation of food-related responses. The daf-7 circuitry described here provides an efficient solution for simultaneously allowing for coordination while maintaining the flexibility for differential modulation of responses.
The daf-7 circuit from ASI sensory neurons to RIM and RIC interneurons would not be predicted from the C. elegans neural wiring diagram since there are no direct synaptic connections from ASI onto RIM or RIC. Both C. elegans and mammalian neurons secrete a large number of peptides associated with profound behavioral and physiological effects (Ludwig and Leng, 2006). Since peptidergic circuits are not confined by synaptic connections, elucidation of such circuits poses a major challenge that is particularly relevant to mammalian hypothalamus as it is enriched in neurons that secrete a plethora of peptides (Seeley and Woods, 2003).
Conserved Mechanisms of Energy Balance in C. elegans and Mammals
Since energy balance is fundamental to organismal survival, we speculate that the basic organizational logic as well as molecular components of energy balance identified in C. elegans are likely conserved in mammals. Given identification of metabotropic glutamate signaling in neural regulation of fat in C. elegans, it is intriguing that adult mice mutant in metabotropic glutamate receptor subtype 5 weigh significantly less than controls while having a similar ad libitum food intake (Bradbury et al., 2005). Furthermore, we identified tyramine, functional counterpart of vertebrate noradrenaline (Roeder, 2005), as a modulator of C. elegans feeding. Insertion of crystalline noradrenaline in the lateral hypothalamus enhances feeding while pharmacological inhibition of its synthesis decreases meal size in satiated rats (Ramos et al., 2005). Thus, excess noradrenaline signaling mimics perception of hunger just as C. elegans tyramine signaling elicits a food intake behavior normally caused by food deprivation. Moreover, genetic dissection of mammalian energy regulatory neurocircuits already point to functional and anatomical divergence of brain areas that independently regulate feeding behavior and energy expenditure. For instance, inactivation of melanocortin-4-receptor (MC4R) causes severe obesity marked by hyperphagia and reduced energy expenditure in rodents and humans (Huszar et al., 1997; Vaisse et al., 1998). By genetically reactivating MC4R expression in subsets of rodent neurons, Balthasar and colleagues showed that functional reconstitution of MC4R in the paraventricular nucleus of the hypothalamus and amygdala fully corrects the hyperphagia of MC4R null mice (Balthasar et al., 2005). These mice still develop obesity because of reduced energy expenditure suggesting that MC4R regulates feeding and energy expenditure through distinct neural sites (Balthasar et al., 2005).
In summary, we functionally characterized a C. elegans molecular and neural circuit that regulates fat and feeding in response to sensory cues. Rather than feeding as the primary determinant of fat regulation, we found that these pathways are coordinated but independent outputs of the nervous system. Aberrant activation of neural fat promoting pathways can therefore lead to excess fat accumulation without concomitant increases in feeding rate.
Experimental Procedures
C. elegans Maintenance and Strain Constructions
Nematodes were grown and maintained on NGM plates using OP50 E. coli bacteria as food source as described (Brenner, 1974). Dauer constitutive strains were maintained at the permissive temperature of 20°C. Although daf-1(m40) and daf-7(e1372) dauer phenotypes are temperature sensitive, neither the pumping rate nor the fat phenotypes depended on temperature.
Strains were obtained from the CGC (www.cbs.umn.edu/CGC/), OMRF Knockout Consortium (www.mutantfactory.ouhsc.edu/), and the National Bio-resources Project (shigen.lab.nig.ac.jp/c.elegans/) (Table S1). When generating double and triple mutants (Table S1), animals with desired combination of mutations were confirmed by PCR and sequencing.
Sudan Black Fat Staining and Quantitation
All Sudan black assays were performed at room temperature (22°C) as previously described (Kimura et al., 1997) with the following modification to minimize staining variability which allowed for quantitative comparisons between various genotypes: animals from one genotype were labeled with FITC and then fixed and stained in the same tube as unlabeled animals from another genotype. For transgenic lines, fat phenotype was assayed by comparing transgenic animals marked with Podr-1::dsRED and non-transgenic animals, which were fixed and stained in the same tube. Genotype combinations that caused a significant change in Sudan black stainings of daf-7(e1372) or daf-1(m40) were tested at least 5 independent times giving consistent results. Genotype combinations that did not significantly alter the excess fat of daf-7(e1372) or daf-1(m40) strains were tested in at least two independent experiments. For quantitation, Sudan black images were collected on a Zeiss Axioplan 2 microscope fitted with a Hamamatsu Orca-AG camera. Staining intensities were quantitated using Improvision Openlab software. Mean pixel intensity was calculated for staining in the region from the first intestinal cells adjacent to the pharynx midway through the animal to the vulva. Background was determined based on pixel intensity of nonspecific staining in the pharynx. Values are reported as mean pixel intensity minus background for at least ten randomly selected test and control animals fixed and stained in the same tube.
Pharyngeal Pumping Assay
Well-fed condition: synchronized L1 stage larvae were grown on OP50 E. coli at low density at 20°C. Pharyngeal pumping rate was counted at room temperature for individual well-fed, gravid adults using a Zeiss M2 BIO microscope and an electronic counter (Diffcount). Number of pumps of individual animals was counted in 15-second intervals. Normally, pumping rates of ~20 animals were counted per genotype per day. These counts were repeated on at least 3 independent assays performed on separate days.
Food deprived condition: well-fed young animals were washed off plates and rinsed twice with S-basal buffer (Brenner, 1974) and maintained in S-basal in a rotating 15ml conical tube for 30 minutes. Animals were then spun into a pellet, rinsed, and plated on unseeded plates. Pumping rates were measured for ~20 animals per genotype 30 minutes post plating. These counts were repeated at least 3 independent times.
Dauer pheromone treatment: 30 animals grown to the desired stages (late larval stage/young adult) under well-fed conditions were transferred to pheromone plates containing 30 μl/ml of a dauer pheromone prep (Golden and Riddle, 1982) and 30 μl of OP50 E. coli food. These conditions caused dauer entry in ~75% of early larval stage animals. The reported values are averages of 20 animals per genotype per assay. Assays were repeated three independent times.
Dauer and Egg-retention Assays
Synchronized L1 animals were seeded at low density on plates with plentiful OP50 E. coli at 25°C. The plates were maintained at 25°C and numbers of adults and dauer larvae were counted three days after plating. Dauers were identified based on their dramatic visual characteristics (example in Figure S1). To confirm this visual inspection, in select cases, animals were washed off plates and subjected to 1% SDS treatment and surviving animals were counted (Cassada and Russell, 1975). To assess egg retention phenotype, animals were synchronized and grown under well-fed conditions at low density. Egg retention was scored in 2-day-old adults.
Plasmid and GFP-Reporter Fusion Constructs
Plasmids for tissue reconstitution rescue lines were constructed using Gateway Technology (www.invitrogen.com). The daf-1 ORF was obtained from the C. elegans ORFeome (www.openbiosystems.org). The daf-3 genomic DNA was cloned using C. elegans ORFeome primers and the pDONR-221 vector (Deplancke et al., 2004; Dupuy et al., 2004). Promoters for egl-3, bbs-1, osm-6, glr-8, B0280.7, glr-1, glr-5, unc-17, glr-2, glr-4, unc-47, ggr-2, flp-1, dop-1, nmr-2, myo-2 and flp-18 were constructed using Promoterome primers and the pDONR-P4-P1R vector (Deplancke et al., 2004; Dupuy et al., 2004). These promoters generally contained 2 kb of genomic sequence upstream of the corresponding gene’s ATG start site. Promoters for daf-1, daf-7, glr-7, tbh-1, tdc-1, and hsp16.2 were generated using primers to amplify published promoter lengths (Alkema et al., 2005; Brockie et al., 2001a; Gunther et al., 2000; Ren et al., 1996). Rescue constructs were generated using the pDEST-MB14 (daf-1 and daf-1;daf-3 rescue) or modified plasmid pKA453 (daf-1;ser-2 rescue) which lacks the unc-119 rescue region and contains a polycistronic GFP fusion. Rescue constructs were generated using the pDEST-MB14 plasmid to obtain promoter::daf-1-ORF::gfp or promoter::daf-3::gfp translational fusions (Vaglio et al., 2003) such that gfp was fused in frame to the C-terminal end of daf-1 ORF where the daf-1 stop codon had been eliminated. Expression patterns of these translational full-length fusions were compared to expression patterns of promoter::gfp transcriptional fusions constructed by using pDEST-DD04 vector.
Transgenic Animals
For tissue-specific rescue experiments, daf-1(m40), daf-1(m40); daf-3(mgDf90), and daf-1(m40); ser-2(pk1357) animals were injected with plasmid of interest at a concentration of 20ng/μl and co-injection marker Podr-1::dsRED at a concentration of 30ng/μl. At least three independent lines were generated for each transgene.
Heat Shock Activation of Phsp16.2::daf-1 Transgene
Synchronized L1 stage control and test animals were grown to L3/L4 or the adult stage at 20°C, then subjected to 34°C heat shock for 45 minutes. Heat shocked animals were allowed to recover at room temperature for 1 hr. Fat, feeding, and egg retention phenotypes were assayed 30 minutes to 24 hrs post recovery.
Genetic Screening and Molecular Mapping
~2500 daf-1(m40) animals were EMS mutagenized (Brenner, 1974). ~24,000 resulting F1 progeny were grown at 20°C and equally divided into 15 pools. These pools were allowed to self fertilize giving rise to ~125,000 F2 progeny. These animals were visually inspected for dark intestine, egg retention, and Nile Red staining at 20°C (Ashrafi et al. 2003). Hermaphrodites with the desired phenotypes were singled onto plates and allowed to reproduce. F1 progeny of these hermaphrodites were then re-examined for the desired phenotypes and F2 progeny were divided into two groups, one of which was examined for constitutive dauer entry at 26.5°C and the other was assayed for Sudan black staining.
The ft9 suppressor was crossed into daf-7(e1372) animals and backcrossed four times. For mapping purposes, we generated a CB4856 strain carrying the Bristol daf-7(e1372) locus. The ft9 suppressor was mapped using standard single nucleotide polymorphism techniques (Jakubowski and Kornfeld, 1999).
Biochemical Assessment of Fat Synthesis
Synchronized C. elegans were grown on a mixture of unlabeled bacteria and 13C labeled bacteria. Total lipid was purified, converted to fatty acid methyl esters and analyzed by GC/MS (Watts and Browse, 2002). The fraction of palmitic, stearic, and vaccenic fatty acid produced via de novo fatty acid synthesis, as opposed to fatty acid obtained from direct dietary absorption, was calculated based on the relative isotope distribution.
Anatomical Methods
Identities of cells expressing gene-reporter fusions were determined by standard anatomical methods including dye filling of amphid neurons to help orient positions of various neurons.
Supplementary Material
Acknowledgments
E.R.G was supported by a predoctoral fellowship from the American Heart Association. This work was supported by an RO1 grant from the NIH/NIDDK (DK070149), The Sandler Family Fund, The Searle Scholars Program and The Burroughs Wellcome Fund. We thank the Bargmann and Vidal labs for plasmids. We are grateful to members of the Ashrafi lab, Henry Bourne, Holly Ingraham, Roland Bainton, and Nils Faergeman for discussions and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert PS, Riddle DL. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol. 1988;126:270–293. doi: 10.1016/0012-1606(88)90138-8. [DOI] [PubMed] [Google Scholar]
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Asturias FJ, Chadick JZ, Cheung IK, Stark H, Witkowski A, Joshi AK, Smith S. Structure and molecular organization of mammalian fatty acid synthase. Nat Struct Mol Biol. 2005;12:225–232. doi: 10.1038/nsmb899. [DOI] [PubMed] [Google Scholar]
- Avery L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J Exp Biol. 1993a;175:283–297. doi: 10.1242/jeb.175.1.283. [DOI] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993b;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Shaham S. Temporal control of cell-specific transgene expression in Caenorhabditis elegans. Genetics. 2007;176:2651–2655. doi: 10.1534/genetics.107.074369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLC beta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford ND, Anderson J, Varney MA, Strack AM. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TJ, Browse J, Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 2007;176:865–875. doi: 10.1534/genetics.107.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci. 2001a;21:1510–1522. doi: 10.1523/JNEUROSCI.21-05-01510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron. 2001b;31:617–630. doi: 10.1016/s0896-6273(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B, Dupuy D, Vidal M, Walhout AJ. A gateway-compatible yeast one-hybrid system. Genome Res. 2004;14:2093–2101. doi: 10.1101/gr.2445504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dillon J, Hopper NA, Holden-Dye L, O’Connor V. Molecular characterization of the metabotropic glutamate receptor family in Caenorhabditis elegans. Biochem Soc Trans. 2006;34:942–948. doi: 10.1042/BST0340942. [DOI] [PubMed] [Google Scholar]
- Douglas SJ, Dawson-Scully K, Sokolowski MB. The neurogenetics and evolution of food-related behaviour. Trends Neurosci. 2005;28:644–652. doi: 10.1016/j.tins.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dupuy D, Li QR, Deplancke B, Boxem M, Hao T, Lamesch P, Sequerra R, Bosak S, Doucette-Stamm L, Hope IA, Hill DE, Walhout AJ, Vidal M. A first version of the Caenorhabditis elegans Promoterome. Genome Res. 2004;14:2169–2175. doi: 10.1101/gr.2497604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman C, Horvitz HR, Jin Y. Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J Neurosci. 1999;19:6225–6234. doi: 10.1523/JNEUROSCI.19-15-06225.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- Estevez M, Attisano L, Wrana JL, Albert PS, Massague J, Riddle DL. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Gunther CV, Georgi LL, Riddle DL. A Caenorhabditis elegans type I TGF beta receptor can function in the absence of type II kinase to promote larval development. Development. 2000;127:3337–3347. doi: 10.1242/dev.127.15.3337. [DOI] [PubMed] [Google Scholar]
- Hajdu-Cronin YM, Chen WJ, Patikoglou G, Koelle MR, Sternberg PW. Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G(o)alpha signaling and regulates G(q)alpha activity. Genes Dev. 1999;13:1780–1793. doi: 10.1101/gad.13.14.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Janssen T, Baggerman G, Bogert B, Kahn-Kirby AH, Ashrafi K, Schoofs L. Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analyzed by mass spectrometry. J Neurochem. 2007;102:246–260. doi: 10.1111/j.1471-4159.2007.04474.x. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jakubowski J, Kornfeld K. A local, high-density, single-nucleotide polymorphism map used to clone Caenorhabditis elegans cdf-1. Genetics. 1999;153:743–752. doi: 10.1093/genetics/153.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- John D. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–497. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Krishna S, Maduzia LL, Padgett RW. Specificity of TGFbeta signaling is conferred by distinct type I receptors and their associated SMAD proteins in Caenorhabditis elegans. Development. 1999;126:251–260. doi: 10.1242/dev.126.2.251. [DOI] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in caenorhabditis elegans. J Neurosci. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development. 1999;126:5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci. 2004;24:7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G. Polygenic control of Caenorhabditis elegans fat storage. Nat Genet. 2006;38:363–368. doi: 10.1038/ng1739. [DOI] [PubMed] [Google Scholar]
- Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- McKay RM, McKay JP, Avery L, Graff JM. C elegans: a model for exploring the genetics of fat storage. Dev Cell. 2003;4:131–142. doi: 10.1016/s1534-5807(02)00411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Niacaris T, Avery L. Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J Exp Biol. 2003;206:223–231. doi: 10.1242/jeb.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan V, Burnell AM. Intermediary metabolism in the dauer larva of the nematode C. elegans. I. Glycolysis gluconeogenesis, oxidative phosphorylation and the tricarboxylic acid cycle. Comp Biochem Physiol. 1989;92B:233–238. [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana AAF. Sequence requiremetns for myosin gene expression and regulation in Caenorhabditis elegans. Genetics. 1993;135:385–404. doi: 10.1093/genetics/135.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EJ, Meguid MM, Campos AC, Coelho JC. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition. 2005;21:269–279. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Rex E, Hapiak V, Hobson R, Smith K, Xiao H, Komuniecki R. TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J Neurochem. 2005;94:181–191. doi: 10.1111/j.1471-4159.2005.03180.x. [DOI] [PubMed] [Google Scholar]
- Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. J Neurochem. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Rex E, Molitor SC, Hapiak V, Xiao H, Henderson M, Komuniecki R. Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J Neurochem. 2004;91:1104–1115. doi: 10.1111/j.1471-4159.2004.02787.x. [DOI] [PubMed] [Google Scholar]
- Riddle DBT, Meyber B, Priess J. C elegans II. 1997. [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Schade MA, Reynolds NK, Dollins CM, Miller KG. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics. 2005;169:631–649. doi: 10.1534/genetics.104.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- Segalat L, Elkes DA, Kaplan JM. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995;267:1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006;20:1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent C. PhD thesis. Cambridge, MA: Massachusetts Institute of Technology; 1982. Genetics and behavioral studies of the egg-laying system of Canorhabditis elegans. [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglio P, Lamesch P, Reboul J, Rual JF, Martinez M, Hill D, Vidal M. WorfDB: the Caenorhabditis elegans ORFeome Database. Nucleic Acids Res. 2003;31:237–240. doi: 10.1093/nar/gkg092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005a;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005b;102:13496–13501. doi: 10.1073/pnas.0506234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wadsworth WG, Riddle DL. Developmental regulation of energy metabolism in Caenorhabditis elegans. Dev Biol. 1989;132:167–173. doi: 10.1016/0012-1606(89)90214-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol. 2006;68:223–251. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate Thomson E, Brenner JNS. The Structure of the Nervous System of the Nematode Caenorhabditis Elegans. Philosophical Transactions of the Royal Society of London. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.