Abstract
The flux enhancing mechanisms of transscleral iontophoresis are not well understood. The objective of the present study was to investigate the ocular barrier and barrier alterations in transscleral iontophoretic delivery with magnetic resonance imaging (MRI). Experiments involving constant current transscleral iontophoresis of 2 mA (current density 10 mA/cm2) and subconjunctival injection were conducted with rabbits in vivo and postmortem and with excised sclera in side-by-side diffusion cells in vitro. The postmortem and in vitro experiments were expected to be helpful in clarifying the importance of vascular clearance and other transport barriers in transscleral iontophoresis. Manganese ion (Mn2+) and manganese ethylenediaminetetraacetic acid complex (MnEDTA2−) were the model permeants. The results show that pretreatment of the eye with an electric field by iontophoresis enhanced subconjunctival delivery of the permeants to the anterior segment of the eye in vivo. This suggests that electric field-induced barrier alterations can be an important absorption enhancing mechanism of ocular iontophoresis. Penetration enhancement was magnified in the postmortem experiments with larger amounts of the permeants delivered into the eye and to the back of the eye. The different results observed in the in vivo and postmortem studies can be attributed to ocular clearance in ocular delivery.
Keywords: ocular, iontophoresis, transscleral, drug delivery, MRI
INTRODUCTION
Ocular iontophoresis has been examined for the delivery of drug compounds in the treatments of eye diseases.1-3 It has been suggested that this technique is relatively safe at low electric current (density)5-8 and allows drug delivery to the back of the eye.4-6 In ocular iontophoresis, a donor electrode is placed in the eye and another electrode is placed on another body surface to complete the electrical circuit. The drug to be delivered into the eye is loaded in the donor electrode. An electric field is applied across the eye to enhance the delivery of the drug into the eye. Iontophoresis operates involving the mechanisms of electrophoresis, electroosmosis, and electroporation.9,10 Electrophoresis is the enhanced movement of an ionic species by an applied electric field.11 Electroosmosis is the transport of both neutral and charged species by an electric field-induced convective solvent flow.12,13 Electropermeabilization or electroporation is the alteration of the tissue barrier under an electric field; this increases the intrinsic permeability of tissues and enhances transport.14,15 The mechanisms of electroporation include the formation of aqueous channels in lipid bilayer that can be transient and reversible or irreversible, depending on the magnitude of the applied electric field and the duration of the electric field application.16,17 The term electroporation is used in this paper to describe any alterations or disruption of the tissue barrier induced by an electric field. Although the mechanisms of iontophoresis have been well characterized in the field of transdermal delivery, the mechanisms of ocular iontophoretic delivery are not well understood. For example, it is well known that electrophoresis is a mechanism of ocular iontophoresis, but iontophoretic delivery of uncharged compounds has been investigated and found to be satisfactory.3,5 Little is mentioned in the literature about electroosmosis and electroporation in ocular iontophoresis. In a previous study, electroosmotic transport was observed from the anode to cathode during transscleral iontophoresis under physiological pH, suggesting that the sclera is net negatively charged under normal physiological conditions.10 However, in this study the contribution of transscleral electroosmosis to iontophoretic delivery of small ions was found not to be significant. To understand tissue alteration during ocular iontophoresis, histological and microscopy methods have been employed.18,19 These methods are suitable to assess gross tissue alteration in the eye caused by iontophoresis. It has been concluded based on these methods that transscleral iontophoresis is not harmful to the tissues at low current densities.8 Nevertheless, possible barrier alteration of the tissue as a mechanism of iontophoretically enhanced transport has not been fully investigated. This is mainly because histological and microscopy techniques may not be sensitive to study small changes in the tissue barrier resulting from the application of an electric field. It is important to understand such aspects of ocular iontophoresis for the future improvement and utilization of this drug delivery technique.
Previously, it was found that passive transport of Mn2+ and of manganese ethylenediaminetetraacetic acid (MnEDTA2−) into the eye after subconjunctival injection could not be detected by magnetic resonance imaging (MRI) over a 2 h period after injection in vivo.20 However, when the animals were sacrificed before subconjunctival injection in a postmortem study, significant penetration of Mn2+ and MnEDTA2− into the ciliary body, anterior chamber, and posterior chamber near the injection site was observed after the injection, suggesting the importance of a blood vasculature barrier in the subconjunctival route. In these postmortem experiments (i.e., without clearance) ion permeation into the vitreous was still minimal after subconjunctival injection, indicating the existence of a significant tissue barrier such as the retinal epithelial membrane in transscleral transport. Three important questions have been raised. Does electroporation occur during ocular iontophoresis that alters the transscleral tissue barriers such as the retinal epithelium barrier? If iontophoresis alters the tissue barriers, is this effect reversible? What is the role of clearance during iontophoresis and can iontophoresis overcome the clearance barrier to allow better transscleral delivery into the anterior chamber and/or vitreous?
The purpose of the present study was to examine the barriers in transscleral iontophoresis and the effects of iontophoresis upon these barriers. More specifically, the following were to be investigated: (1) the possible role of the electric field in altering tissue barriers during transscleral iontophoresis, and (2) the importance of clearance upon ocular delivery into the eye. To achieve the present objectives, transport experiments were conducted with excised sclera in side-by-side diffusion cells in vitro and with rabbits in vivo and postmortem. The postmortem studies served as a model to investigate the mechanisms of transscleral iontophoresis without the interference of ocular clearance. MRI was employed in the in vivo and postmortem studies because this technique allowed the examination of the distribution of the probe permeants in the eye such as in the vitreous, which would be otherwise difficult to determine in conventional pharmacokinetic studies.
MATERIALS AND METHODS
Strategy
The goal of the present study was to characterize the transscleral barriers during ocular iontophoresis and the effects of iontophoresis upon these barriers. The flux enhancing mechanisms of ocular iontophoresis such as electroporation and their effects on these barriers were to be investigated. Table 1 provides a list of in vitro, in vivo, and postmortem experiments to be conducted and analyzed in the present study. Experiments A to E were the experiments performed in the present study. Experiments I to IV were from previous studies;20-22 these results were to be compared with those obtained in the present study. Experiment A was to study possible electric field-induced alteration of the tissue barrier during iontophoresis in vivo. Comparison of the results in Experiments A and III would allow the examination of barrier alteration as a possible flux enhancing mechanism in transscleral iontophoresis. Experiment B studied this effect without ocular clearance such as blood vasculature clearance using postmortem rabbits. The results from Experiment B were compared with those from Experiment IV. Experiment E was designed to use an in vitro system to quantify the results observed in the in vivo and postmortem experiments under similar iontophoresis conditions (Experiments A and B, respectively). The effects of clearance upon ocular delivery to the posterior segment of the eye were assessed by comparing the results in the studies in vivo (Experiments I and II) and postmortem (Experiments C and D).
Table 1.
Summary of Experiments Conducted and Analyzed in the Present Study
| Experiment | System | Procedure |
|---|---|---|
| A | In vivo | Iontophoresis pretreated subconjunctival injection |
| I | In vivo | Transscleral iontophoresis near limbus, 2 mA, 20 mina |
| II | In vivo | Transscleral iontophoresis near fornix, 2 mA, 20 mina |
| III | In vivo | Subconjunctival injectionb |
| B | Postmortem | Iontophoresis pretreated subconjunctival injection |
| C | Postmortem | Transscleral iontophoresis near limbus, 2 mA, 20 min |
| D | Postmortem | Transscleral iontophoresis near fornix, 2 mA, 20 min |
| IV | Postmortem | Subconjunctival injectionb |
| E | In vitro | Reversibility/iontophoresis pretreated passive |
Materials
MnCl2 tetrahydrate was purchased from Spectrum Chemical (Gardena, CA). Ethylenediamine tetraacetic acid (EDTA) and xylazine were purchased from Sigma Chemical (St. Louis, MO). Sodium chloride 0.9% USP (pH between 5 and 7) was from Baxter Healthcare (Deerfield, IL). Ketamine hydrochloride injectable USP (100 mg/ mL) was from Hospira, Inc. (Lake Forest, IL). Na2MnEDTA solutions (with excess EDTA at 1:1.5 Mn:EDTA ratios) were prepared by the chemicals as received and the pH was adjusted to between 5 and 7 by the addition of concentrated NaOH. 3H-mannitol, 14C-mannitol, and 14C-tetraethyl ammonium (TEA) at >98% purity were purchased from New England Nuclear, PerkinElmer (Boston, MA) and tested for purity using methods specified by the supplier.
For the electrode devices in the in vivo and postmortem studies, Silastic® flexible tubing (Tygon, 3/16 I.D. × 5/16 O.D.) was purchased from VWR Scientific (San Francisco, CA). Silver foil (1.5″ × 0.004″) was obtained from EM Science (Gibbstown, NJ). Liquid silver paint was from LADD Research Industries (Williston, VT). The electrode devices were constructed as previously described.21 Ag was the electrode in the donor chamber in anodal iontophoresis (anode as the donor) and Ag/AgCl was the donor electrode in cathodal iontophoresis (cathode as the donor). Phoresor II Auto constant current iontophoretic device (Model No. 850, Iomed, Inc., Salt Lake City, UT) was the electric current dose controller.
Animals
New Zealand white rabbits of 3–4 kg were purchased from Western Oregon Rabbit Co. (Philomath, OR) for the in vivo and postmortem experiments under the approval of the Institutional Animal Care and Use Committee at the University of Utah. The sclera tissues in the present in vitro experiments were obtained from both the superior and inferior temporal sections of an excised eye away from the limbus. The enucleated rabbit eyes were obtained from other studies at the University of Utah Animal Resource Center. Briefly, the eye was harvested from the rabbit carcasses immediately after euthanasia and freed from adhering debris such as the conjunctiva and extraocular muscles. A cut was made in the cornea along the limbus and the eye was carefully dissected into two hemispheres with surgical scissors. The vitreous humor was then removed and the sclera–choroid–retina layer was used directly without choroid and retina removal. Histology was performed on the tissues with Haematoxylin and Eosin (H&E) stain to verify the dissection method.
In Vivo Rabbit Experiments
The in vivo study was designed to examine the effect of iontophoresis on the barrier properties of the eye for transscleral delivery (Experiment A). After the rabbits were anesthetized with 25–50 mg/kg ketamine IM and 5–10 mg/kg xylazine IM, an electrode device (diffusion surface area of ∼0.2 cm2) containing only saline was placed on the conjunctiva/sclera near the limbus at the pars plicata/pars plana in the superior cul-de-sac (under the upper eyelid). The return electrode (approximately 4 cm2 surface area) was placed on a piece of gauze pad (approximately 40 cm2 surface area) wetted in saline and clamped to the ear on the opposite side of the treated eye. Blank anodal iontophoresis of 2 mA with saline (the pretreatment) was performed for 20 min. Hence, the current density at the surface of the eye was ∼10 mA/cm2 and that across the skin on the ear was ∼0.05 mA/cm2. The applied voltage was generally in the range of 10–20 V the majority of the time in the experiment. Subconjunctival injection of 0.1 mL 40 mM MnCl2 in saline or 0.1 M MnEDTA2− in distilled water was then performed immediately after iontophoresis at the position of the electrode placement. Reversibility of the barrier was examined by subconjunctival injection given at approximately 7 or 30 h after iontophoresis. The delivery and distribution of the probe permeants in the eye were monitored by MRI after subconjunctival injection.
Postmortem Rabbit Experiments
Within 15 min after the rabbits were sacrificed, experiments of subconjunctival injection with iontophoresis pretreatment were conducted (Experiment B). The protocol was similar to that in the in vivo experiments. In these experiments, blank 2-mA anodal iontophoresis (iontophoresis with saline) was performed for 20 min on the postmortem rabbits. Immediately following iontophoresis, a subconjunctival injection of 0.1 mL of 40 mM Mn2+ was given at the position of the electrode placement. The permeation and distribution of the probe in the eye were then monitored with MRI.
In Experiments C and D, transscleral iontophoresis was performed as described previously.22 Two different electrode placements were evaluated. An electrode device with 0.4, 4.0, or 40 mM MnCl2 in saline or 0.1 M Na2MnEDTA in water was placed either on the conjunctiva/sclera at the pars plana next to the limbus (Experiment C) or far away from the limbus near the fornix (Experiment D) in the superior cul-de-sac for transscleral iontophoresis. The position of the electrode was maintained by fixing the wire of the electrode to the MRI coil with adhesive tape and by the pressure from the eyelid. During iontophoresis, the position of the electrode was checked by comparing the relative electrode position to the anatomical structures of the eye in the MR images. The wires to supply the direct current (DC), which ran between the DC current supply device and the electrodes, were twisted and the loop area was minimized to reduce possible noise contribution to MRI. Constant DC of 2 mA, approximately 10 mA/cm2, was applied to the eye for 20 min. For MnCl2, the donor electrode was the anode. For Na2MnEDTA, the donor electrode was the cathode. The delivery and distribution of the permeants in the eye were monitored by MRI during and after iontophoresis.
Animal MRI and Data Interpretation
Due to the availability of MRI scanners, two MRI systems were used. Unless otherwise stated, animal MRI experiments were carried out with a GE SIGNA 1.5-T (NV/CVi gradient) clinical MRI system (GE Medical System, Inc., Milwaukee, WI) with a temporal mandibular joint coil (TMJ coil: a double surface phased array coil set with 3″ diameter) similar to those described previously.21 Dynamic MR imaging was performed, with T1 (spin–lattice relaxation time) weighted spin–echo imaging technique. The imaging parameters were 400 ms TR, 9 ms TE, 256 readout matrix with 160 phase-encoding, and two signal averages to increase signal-to-noise ratio (SNR). Imaging field of view (FOV) was 12 cm in readout direction. Rectangular FOV with 50 or 75% reduction factor was used to reduce the imaging time in phase-encoding direction. The slice thickness was 2 mm with no spacing, resulting in spatial resolution of 0.47 × 0.47 × 2.0 mm3. Each scan provided at least 10 transaxial image slices to cover the whole eye, but only the images across the center of the eye will be presented in this paper. Prescanning was performed only once for the first data set at the beginning of the experiment, and the imaging parameters included the prescan results, such as transmitter and receiver gains. Imaging time for single time data was approximately 1 min. In the reversibility study—all subconjunctival injections 7 and 30 h after iontophoresis pretreatment and a number of subconjunctival injections immediately after iontophoresis pretreatment for direct comparison—the MRI experiments were conducted with a clinical 3-T MRI system (Trio with Sonata gradient, Siemens Medical Solution, Erlangen, Germany) and a human wrist coil (a transmit/receive volume coil). The imaging parameters were 400 ms TR, 11 ms TE, 256 readout matrix with 192 phase-encoding, and two signal averages. Rectangular FOV of 10 cm in readout direction with 75% reduction factor was used. The slice thickness was 1.0 mm with no spacing. The spatial resolution was 0.4 × 0.4 × 1.0 mm3. Each scan provided at least twenty transaxial image slices to cover the whole eye. Imaging time for single time data was approximately 2 min. All MRI experiments were carried out at least in triplicate.
In manganese-enhanced MRI, Mn2+ ion and MnEDTA2− complex (the model permeants) enhance the relaxivity of the water protons, reduce the proton spin–lattice relaxation times, and enhance the signal in the MR images. The calibration curves of manganese-enhanced signal intensity versus Mn2+ and MnEDTA2− concentration at 1.5 T were presented in previous studies.20,21 According to the data in these studies, the relationships between signal intensity and permeant concentration are parabolic. The very bright voxels (high signal intensities) in aqueous medium and vitreous humor in the MR images represent approximately 0.8 mM Mn2+ or 4 mM MnEDTA2−. Above and below these concentration levels, the signal intensities gradually declined (darker voxels). Below approximately 0.02 mM Mn2+ and 0.1 mM MnEDTA2−, there was essentially no signal enhancement. Due to the parabolic behavior, a range of different permeant concentration (e.g., 4.0 and 40 mM MnCl2) was used in the present study to allow MRI data interpretation. In addition, the concentration of the permeants in the tissues and other segments of the eye cannot be evaluated with the present technique because of possible interferences from Mn-tissue binding and the tissue proton relaxation times.23,24 MR images obtained from the GE system were analyzed with Scion Image program (Scion Corp., Frederick, MD), and Siemens DICOM images were first converted to the analyze format and analyzed using NIH ImageJ. Tissues away from the region of interest (fat tissues behind the eye) were selected and their signals were used as references in the MR images. MR images with tissue reference signals outside the one-standard deviation range of the reference average were not used in the study. The distances between two points or two regions of interest were determined by the number of pixels between the two points in the images.
In Vitro Transport Experiments
The objectives of the in vitro transport experiments were to identify possible electric field-induced membrane alteration during iontophoresis and to quantify this effect in an in vitro setting. A three-stage protocol (Stages 1, 2, and 3) was employed to examine the effects of the electric fields upon the barrier of excised rabbit sclera tissue. Fresh sclera was prepared from the same day of eye excision in an attempt to preserve the retina. The sclera tissue (with the retina) was sandwiched between the two half-cells of a two-chamber side-by-side diffusion cell with the retina facing the receiver chamber.10 Each compartment of the diffusion cell has a 2-mL volume and an effective diffusional area of around 0.2 cm2. The diffusion cell was placed in a circulating water bath at 37 ± 1°C. Saline (2 mL) and donor solution (2 mL) were pipetted into the receiver and donor chambers, respectively. Trace amounts of radiolabeled TEA and mannitol in saline or 40 mM Mn2+ in saline or 0.1 M MnEDTA2− in deionized water was the donor solution. Under the three-stage protocol, Stage 1 was passive transport experiment. Blank anodal iontophoresis of 2 mA was applied for 20 min in Stage 2 with saline in the absence of the permeants in the donor. Phoresor II Auto constant current iontophoretic device (Model No PM 850, Iomed, Inc., Salt Lake City, UT) was the electric current dose controller to provide the electric current. Silver foil and Ag/AgCl coated Ag foil were the conductive elements in the donor and receiver chambers, respectively. Stage 2 was followed by the passive permeability experiment in Stage 3. In the passive transport experiments of Stages 1 and 3, samples were withdrawn from the donor and receiver chambers at predetermined time (30–60 min) intervals. Typically, 20-μL aliquots were taken from the donor chamber, and 1 mL aliquots were withdrawn from the receiver chamber. The same volume of fresh saline was added back to the receiver chamber after each aliquot removal to maintain a constant volume. The duration of the passive transport experiments was 2–3 h, which was at least eight times longer than the transport lag time. The total permeability coefficient (P) of the sclera and retina tissue composite was calculated at steady state under sink conditions (receiver concentrations ≤10% of the donor concentrations):
| (1) |
where CD is the concentration of the permeant in the donor chamber, AD is the diffusional surface area, and ΔQ/Δt is the slope of the cumulative amount of the permeant transported across the membrane into the receiver chamber versus time plot. The total permeability coefficient is a function of the permeability coefficient of the sclera–choroid composite and that of the retina:
| (2) |
where Pretina is the permeability coefficient of the retina, and Psclera is the permeability of the combined sclera and choroid membrane. In the experiments with Mn2+ and MnEDTA2−, the donor and receiver samples were analyzed by a colorimetric method.20 In the experiments with radiolabeled permeants, the samples were analyzed using scintillation cocktail (Ultima Gold, Packard Instrument, Meriden, CT) and a liquid scintillation counter (Packard TriCarb Model 1900TR Liquid Scintillation Analyzer).10 The permeability coefficients of the tissue for the permeants in Stages 1 and 3 were then compared. The in vitro study was to mimic the conditions of the in vivo and postmortem studies in Experiments A and B for comparison of the in vitro results with those obtained in vivo and postmortem.
RESULTS
In vivo Rabbit Experiments
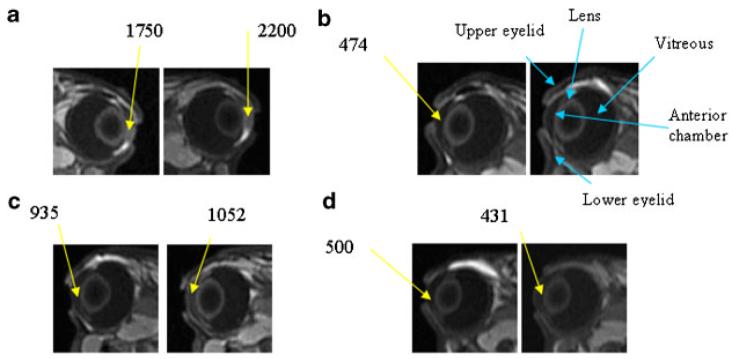
Figure 1 is a diagram of an eye to compare with the labeled MR image in Figure 2b. Figure 2 shows the MR images of Experiment A with subconjunctival injection after iontophoresis pretreatment in vivo. The MR images of Experiment III in a previous study are also presented in the figure for direct comparison.20 In this previous study, the anterior chamber, posterior chamber, and vitreous show no sign of the Mn2+ and MnEDTA2− (i.e., being below the detection limits) for over 2 h after subconjunctival injection in vivo without pretreatment. With iontophoresis pretreatment in the present study, permeants were generally observed to enter the anterior chamber. Rabbit-to-rabbit variability was also observed. Five rabbits in the present Mn2+ experiments showed significant permeant penetration as in Figure 2a, and four rabbits showed less penetration enhancement (n = 9). In the MnEDTA2− experiments, all rabbits showed some penetration enhancement as in Figure 2c (n = 3). Possible reasons of the lower signal intensity observed with MnEDTA2− compared to that with Mn2+ include the lower diffusion coefficient of the larger molecular size MnEDTA2− and the weaker MR signal enhancement for MnEDTA2− at the same concentration.20,21 In general, the effects of penetration enhancement were also observed to decrease over time after iontophoresis in the 7- and 30-h pretreatment experiments, suggesting that these effects were reversible. When subconjunctival injection was given at 7 h after iontophoresis, some penetration enhancement was observed in one rabbit, and little or no enhancement was observed in four rabbits (n = 5, data not shown). At 30 h after iontophoresis, the barriers were almost completely recovered as shown by the lack of a significant difference between the electric field pretreated eye and the control without pretreatment (n = 4, data not shown).
Figure 1.
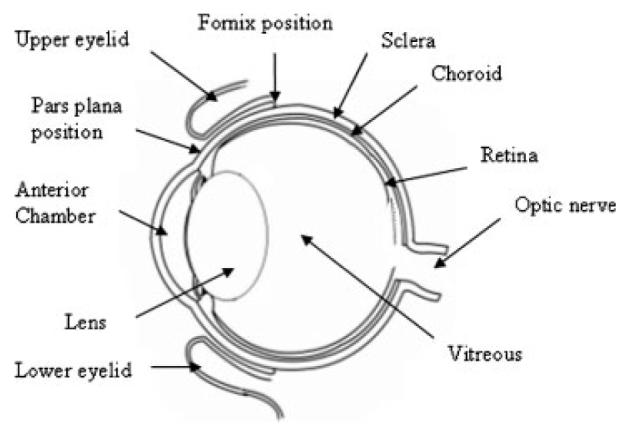
Rabbit eye diagram.
Figure 2.
Representative MR images of in vivo studies in Experiments A (blank iontophoresis of 2 mA followed by 0.1 mL subconjunctival injection) and Experiment III (0.1 mL subconjunctival injection without iontophoresis pretreatment). a: Experiment A, injection of 40 mM Mn2+ showing significant penetration enhancement, (b) Experiment III, injection of 40 mM Mn2+, (c) Experiment A, injection of 0.1 M MnEDTA2− showing some penetration enhancement, and (d) Experiment III, injection of 0.1 M MnEDTA2−. From left to right, images obtained at (a) 40 and 70 min, (b) 30 and 138 min, (c) 21 and 124 min, and (d) 40 and 120 min after injection, respectively. Images in (b) and (d) are obtained from a previous study.20 The numbers indicate the MR signals from the region of interest (average of 9 voxels or 4 mm3). The orientations of the eyes in the images (facing left or right) denote the left or right eye.
As an additional note, the results in Experiments A are similar to (but with a slower delivery rate than) those of iontophoresis in vivo22 and subconjunctival injection postmortem.20 Pretreatment of the eye tissue with an electric field was capable of facilitating permeant diffusion from the subconjunctival space into the anterior chamber. This result suggests that clearance is not the only barrier for transscleral transport in vivo. Other tissue barriers may also exist that can be influenced by treatment with an electric field. The alteration of these barriers enhances permeant absorption from the subconjunctival space into the anterior segment of the eye. It was also noted in the MR images (Fig. 2a) that the MR signals of the iris and ciliary body away from subconjunctival depot were enhanced. This finding was confirmed by MRI using a slice orientation that was orthogonal to the transverse orientation normally used in the present study (data not shown). The enhancement of penetration in the iris and ciliary body around the eye, faster than expected from passive diffusion, suggests that pretreatment with an electric field may enhance permeant delivery via local blood circulation in the ciliary body into the anterior chamber.
Postmortem Rabbit Experiments
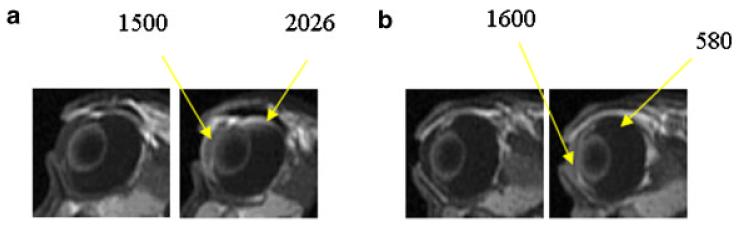
The MR images of the subconjunctival injection postmortem study with iontophoresis pretreatment (Experiment B, n = 3) are presented in Figure 3. The MR images in a previous study (Experiment IV) are also shown in the figure for direct comparison.20 Compared with the MR images of subconjunctival delivery without pretreatment in this previous study, a larger amount of permeants was observed in the eye. The difference between the subconjunctival results with and without iontophoresis pretreatment again demonstrates the effect of electric field-induced barrier alteration. The enhancement due to the pretreatment was magnified in the rabbit postmortem relative to those in vivo. Particularly transport enhancement into the vitreous was observed postmortem. This suggests that the electric field can alter the transscleral barrier to enhance subconjunctival (passive) delivery into the vitreous, in addition to altering the anterior barrier at the ciliary body. This effect could be masked by the effects of clearance in vivo, and was only observed in the animal postmortem. The present postmortem result is consistent with the hypothesis that the electric field alters the transscleral tissue barrier, possibly the retinal epithelial membrane.
Figure 3.
Representative MR images of postmortem studies in (a) Experiment B, blank iontophoresis of 2 mA followed by 0.1 mL subconjunctival injection of 40 mM Mn2+ and (b) Experiment IV, 0.1 mL subconjunctival injection of 40 mM Mn2+. From left to right, for (a) and (b), images obtained at 30 and 60 min after injection. Images in (b) are obtained from a previous study.20 The numbers indicate the MR signals from the region of interest (average of 9 voxels or 4 mm3).
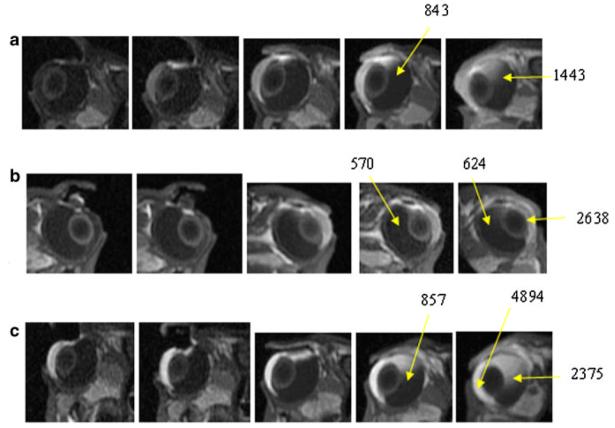
Figure 4 shows the MR images in the 2-mA iontophoresis study postmortem when the electrode was placed on the sclera next to the limbus (Experiment C). These results show the full extent of the effect of iontophoretic enhanced transport without the interference of clearance and other physiological factors. In the figure, significant amounts of Mn2+ and MnEDTA2− were observed to be delivered to the anterior segment of the eye, greater than those observed in the experiment in vivo.22 At the completion of the 20 min iontophoresis, the permeants penetrate as far as 2 mm into the eye from the device/conjunctiva interface. In less than 1 h after iontophoresis, penetration of Mn2+ and MnEDTA2− into the ciliary body, anterior chamber, and posterior chamber near the electrode position was observed, especially when a high permeant concentration such as 40 mM Mn2+ and 0.1 M MnEDTA2− was used. The permeants continued to diffuse into the posterior segment of the eye after iontophoresis and reached as far as 5–6 mm into the vitreous from the position of the electrode placement approximately 2 h after iontophoresis. Such permeant diffusion and distribution after iontophoresis were not observed in the in vivo experiments22 when clearance can be a major factor.
Figure 4.
Representative MR images of postmortem transscleral iontophoresis experiment of 2 mA of (a) 0.1 M MnEDTA2−, (b) 4 mM Mn2+, and (c) 40 mM Mn2+ in Experiment C. From left to right, images obtained at (a) 9 and 19 min during iontophoresis, and 10, 55 and 140 min after iontophoresis, (b) 10 and 19 min during iontophoresis, and 40, 60, and 190 min after iontophoresis, and (c) 9 and 18 min during iontophoresis, and 10, 60, and 140 min after iontophoresis. Note that high Mn2+ and MnEDTA2− concentrations such as 40 mM Mn2+ and 0.1 M MnEDTA2− in the electrode device are represented by dark voxels in the images due to the parabolic signal versus concentration relationship. The numbers indicate the MR signals from the region of interest (average of 9 voxels or 4 mm3).
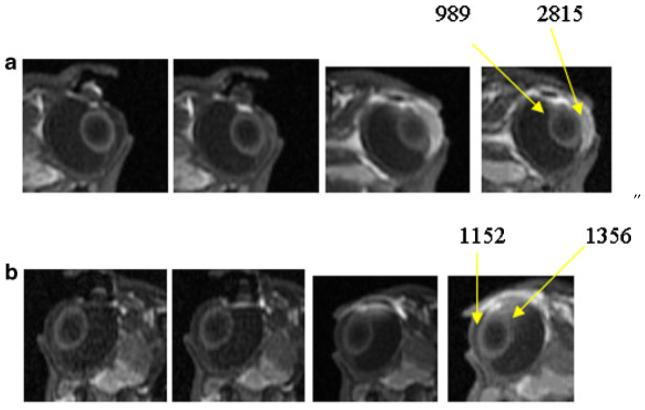
Figure 5 presents the MR images after 20-min 2-mA iontophoresis application postmortem with the two different positions of electrode placement in Experiments C and D. Different permeant distributions were observed in the eye when the electrodes were placed at these two positions. The results of 4 mM Mn2+ condition are shown in the figure because they provide the most contrast demonstrating the effects of electrode placement. From the MR images in the figure, when the electrode was placed near the limbus, permeants were mainly delivered to the anterior chamber and the anterior parts of the eye. When the electrodes were placed on the sclera away from the limbus towards the fornix, little ion penetration into the anterior chamber was initially observed. In this case, iontophoresis primarily delivered the permeants to the posterior segment of the eye with a larger amount of permeant observed in the posterior segment relative to that in the anterior segment.
Figure 5.
Representative MR images of postmortem transscleral iontophoresis of 4 mM Mn2+ and 2 mA postmortem in Experiments C and D. From left to right, images obtained at (a) 10 and 19 min during iontophoresis, and 40 and 60 min after iontophoresis, with electrode at the pars plana and (b) 11 and 20 min during iontophoresis, and 40 and 86 min after iontophoresis, with electrode at the back of the eye near the fornix. The numbers indicate the MR signals from the region of interest (average of 9 voxels or 4 mm3).
In Vitro Results
Table 2 shows the passive permeability results with the sclera (sclera–choroid–retina) in the three stage passive, anodal iontophoresis, and passive experiments in vitro (Experiment E). The iontophoresis condition in this experiment was similar to those in the iontophoresis-pretreated subconjunctival injection studies in vivo and postmortem (Experiments A and B). The data in Table 2 show no significant difference between the transscleral permeability coefficients in the passive transport runs (Stages 1 and 3) before and after iontophoresis (Stage 2) for TEA, mannitol, Mn2+, and MnEDTA2− (p > 0.5 for all permeants). Comparison of the passive permeability coefficients of sclera with retina in the present study to those of sclera without retina in a previous study20 in an analysis using Eq. 2 allowed the estimation of the permeability coefficients of retina for TEA, mannitol, Mn2+, and MnEDTA2−. The permeability coefficients of retina were estimated to be in the order of 5 × 10−5 cm/s for these permeants of small molecular sizes.
Table 2.
Permeability Coefficients of Passive Transscleral Transport Before and After Iontophoresis Treatment (Experiment E)a
| Permeability Coefficient Before Iontophoresis, P1 (10−5 cm/s) |
Permeability Coefficient After Iontophoresis Pretreatment, P3 (10−5 cm/s) |
Ratio P1/P3 | |
|---|---|---|---|
| TEA | 2.3±1.2 | 2.6±1.0 | 0.9±0.2 |
| Mannitol | 1.7±0.9 | 1.9±0.9 | 0.9±0.1 |
| 40 mM Mn2+ | 1.5±0.5 | 1.7±0.5 | 0.9±0.1 |
| 0.1 M MnEDTA2− | 1.4±0.4 | 1.5±0.2 | 0.9±0.2 |
Mean±SD (n≥3).
DISCUSSION
MRI and Postmortem Model
The present study utilizes the advantages of the noninvasive pharmacokinetic MRI technique and a postmortem model to investigate possible electric field-induced barrier alteration and the effects of clearance upon transscleral delivery during iontophoresis. MRI was employed to examine the distribution of the probe permeants in the eye in transscleral transport with and without the pretreatment of an electric field. The postmortem model provided a method to investigate the properties of the tissue barriers in transscleral iontophoresis without the interference of ocular clearance.20,25,26 The probe permeants allow the assessment of the ocular barrier with and without iontophoresis (or pretreatment of iontophoresis) for polar and ionic drugs as drug permeation is mainly related to its physicochemical properties such as molecular size, charge, and diffusion coefficient when there is no specific interaction between the drug and ocular tissues. However, caution must be exercised to extrapolate the results of these probe permeants to lipophilic drugs and macromolecules.
Two recent MRI studies have been performed in this laboratory to investigate ocular iontophoresis.21,22 In a previous study in vivo, the transport and distribution of ions into the eye during and after transscleral and transcorneal iontophoresis were compared using MRI.21 The use of MRI as a technique to evaluate ocular delivery was also discussed. In a subsequent in vivo study, the effects of electrode placement, electric current density, and duration of iontophoresis application upon the transscleral transport during and after transscleral iontophoresis were investigated.22 The least resistance pathway during transscleral iontophoresis for direct iontophoretically enhanced delivery was determined, and the potential of iontophoresis to directly deliver an ion deep into the vitreous and to the back of the eye was assessed. In a separate study to examine the effect of ocular clearance upon passive transscleral transport, the delivery, and distribution of ions after subconjunctival injection was investigated with MRI utilizing the postmortem model.20 The present study was designed to address some of the remaining questions such as the effect of ocular clearance upon transscleral iontophoretic transport and possible barrier alteration during iontophoresis. The findings in the present study, which are built upon the data from the previous subconjunctival injection and iontophoresis studies, are now discussed.
Tissue Barrier Alteration: Postmortem and In vivo Studies
The 20-min 2-mA iontophoresis conditions employed in the present study were observed to be within human tolerable limits7 and this electric current level was suggested not to alter the structures of ocular tissues in animals.8 There was no evidence of gross damage to the general structure of the eye caused by the electrode device with and without the electric current according to the MR images and visual observation of the eye surface in the present in vivo and postmortem studies (data not shown). Comparisons of the postmortem results of blank iontophoresis followed by subconjunctival injection (Experiment B) and those of subconjunctival injection without iontophoresis pretreatment20 (Experiment IV) suggest electric field-induced barrier alteration during ocular iontophoresis. The differences between the in vivo results of subconjunctival injection after iontophoresis pretreatment (Experiment A) and subconjunctival injection without pretreatment20 (Experiment III) are consistent with this assessment. Although the in vivo results are more relevant, the postmortem data are more reliable in demonstrating tissue barrier alteration because the postmortem data are not compromised by possible electric field-induced physiological changes that might affect the in vivo data. The in vivo postmortem data together are evidence of electric field-induced barrier alteration as a flux enhancing mechanism under the conditions of the present transscleral iontophoresis study.
It was suggested in a previous subconjunctival injection study20 that the least resistive transport pathway in transscleral transport was at the ciliary body, particularly, the pars plana region. This is consistent with the morphology of the ciliary body.27 The result also suggests the existence of a transscleral barrier hindering the delivery of permeants into the vitreous postmortem. The present study shows that the transscleral barriers can be altered by the electric field during iontophoresis. It is speculated that the retinal epithelial membrane is the barrier28,29 because the sclera is relatively inert to electric field-induced structure alteration.10,30 The exact mechanisms of this are not fully understood. For example, electroporation can facilitate transport through the blood vasculature to the anterior chamber by affecting the endothelial cell31 lining the blood vessels and/or altering the tissue barrier for direct permeation enhancement in the eye. Electric fields can also enhance absorption across retina cell membrane.32 Further studies are required to determine the actual mechanisms of electroporation in ocular iontophoresis.
It should be noted that the term electroporation is used here to describe the mechanism of enhanced transport due to reversible barrier alterations or disruption induced by an electric field in the present study. This definition is different from the method-related definition which mainly covers the application of short high voltage pulses in transdermal14 and ocular delivery.33,34 Under the definition in the present study, electroporation occurs in 20 min 2-mA ocular iontophoresis as evidenced by the increase in transscleral permeation after iontophoresis. Electroporation generally occurs under an electric field of 0.2–1 V per lipid bilayer depending on the composition of the bilayer membrane.14,17 The exact electrical resistance of the transscleral barrier and the electrical potential across this barrier during ocular iontophoresis are not available in the present study. If the barrier resistance is greater than 0.1 kΩcm2, the current density used in the present study (10 mA/cm2) will provide an electrical potential greater than 1 V across the barrier. This electrical potential is strong enough to induce cell membrane electroporation, and the mechanism of electroporation in ocular iontophoresis should not be ruled out. Further studies are needed to confirm this hypothesis.
In vitro Transscleral Model
The influence of an electric field on the permeation across the sclera and retina was also investigated in vitro (Experiment E). In these experiments, the permeability coefficients of passive transscleral transport before and after the 20 min iontophoresis were determined to be essentially the same, and this contradicts the MR data observed in the postmortem study of blank iontophoresis followed by subconjunctival injection (Experiment B). In the postmortem study, larger amounts of permeants were observed to be delivered into the vitreous when the sclera was pretreated with iontophoresis. The different observations in the in vitro and postmortem studies suggest the existence of a transscleral transport barrier in postmortem rabbits that is absent in the side-by-side diffusion cell system in vitro, and that this barrier can be altered by the application of the electric field. It is possible that this barrier was compromised during tissue preparation in vitro, so the permeability results of the passive transport experiments before and after iontophoresis treatment were not significantly different. This hypothesis is supported by previous data. In previous human retina studies in vitro and in vivo, the permeability coefficients of retina for FITC-dextrans of low molecular weight were determined to be in the order of 10−3 cm/s in vitro,35 but those of retina transport for fluorescein were in the order of 10−6 cm/s in vivo.36 The different tissue permeability results in these two previous studies suggest that the retina may be easily compromised in tissue preparation in vitro. There may also be other methodology differences in the in vitro and in vivo studies, resulting in the different permeability values. Hence, the inability of the present in vitro method to identify electric field-induced barrier alteration does not seem to be related to the particular in vitro methodology employed in the present study but to the general approach of using excised tissue in vitro to evaluate transscleral transport in intact eyes.
Effects of Ocular Clearance
To investigate the tissue barrier without the interference of ocular clearance, a postmortem model was utilized. To examine the effects of clearance upon iontophoretic transport using the postmortem data, a main assumption was that the eye tissues such as the choroid, retina, and endothelial cell tight junctions remained functionally and structurally intact postmortem. A previous study suggests that the eye tissues remain viable for several hours postmortem,25 and a comparison of the MR images in vivo and postmortem would allow studying the effects of the blood vasculature barrier and clearance. In the present postmortem study, the results show significantly larger amounts of permeants delivered into the eye compared with those in vivo.22 Deeper penetration of Mn2+ into the eye from the iontophoresis application site was observed during iontophoresis. The permeant continued to diffuse into the posterior segment of the eye after iontophoresis and filled more than half of the vitreous, which was not observed in vivo. Also noted in the present study was the significant absorption enhancement into the anterior chamber but not into the vitreous in the in vivo iontophoresis pretreatment study. This suggests significant clearance toward the back of the eye preventing transscleral penetration in vivo. Previous studies have demonstrated the importance of clearance such as blood vasculature clearance in transscleral delivery.26,29,37 The present results continue to support this hypothesis. The vasculature blood barrier is likely to be related to the choroid.29 Other factors such as lymphatic clearance 25,26 and elimination via the aqueous humor outflow38 may also contribute to the differences observed in vivo and postmortem. It is also possible that the transscleral barrier is compromised postmortem leading to the larger amount of permeant delivered into the eye or magnifying the electroporation effect of iontophoresis in the postmortem studies.
Iontophoresis Delivery
The postmortem results can also serve as a baseline model for comparison with a previous in vivo iontophoresis study22 showing the full extent of the effect of iontophoretically enhanced transport without the interference of clearance. A comparison of the extent and penetration depth of permeant delivery in the eye in the present postmortem iontophoresis study (Experiment C) with those after subconjunctival injection in the previous postmortem study20 clearly demonstrates the effect of the electric field upon drug delivery into the eye over passive diffusion. In addition, the effects of electrode placements upon transscleral delivery during transscleral iontophoresis can also be studied effectively in the postmortem experiments without blood circulation and clearance. A previous study has suggested that the location of electrode placement during ocular iontophoresis affected ocular iontophoretic delivery due to the anatomy of the eye.22 The anatomical barriers in drug delivery to the anterior segment of the eye are likely to be different from those of the posterior segment.31 A direct comparison of the penetration pathways of transscleral iontophoresis when the electrode was placed on the sclera near the pars plana (Experiment C) versus that near the fornix (Experiment D) was performed postmortem in the present study. This comparison demonstrates the importance of electrode placement in ocular iontophoresis and supports the previous in vivo study conclusion that the lack of posterior iontophoretic delivery with electrode placement near the fornix is related to ocular clearance.22
CONCLUSION
The present study demonstrates the utility of the postmortem animal model and MRI in transscleral transport study. In contrast, the transscleral barriers such as the retinal epithelial barrier in the in vitro model are believed to be compromised. The present in vivo and postmortem results suggest that electroporation (or electropermeabilization) is a flux enhancing mechanism of transscleral iontophoresis. The subconjunctival injection study shows that pretreatment with iontophoresis enhances transscleral delivery to the anterior chamber in vivo, which is reversible, and to the posterior segment of the eye postmortem. This is consistent with the hypothesis that the tissue barrier hindering transscleral transport is altered by the electric field during iontophoresis. The data also suggest that clearance such as blood vasculature clearance significantly reduces ocular delivery to the posterior segment of the eye during and after iontophoresis.
ACKNOWLEDGMENTS
This research was supported in part by NIH Grant EY 015181. The authors thank Dr. Paul S. Bernstein for helpful discussion and Anthony L. Tuitupou and Dr. Rajan P. Kochambilli for their help.
REFERENCES
- 1.Yoo SH, Dursun D, Dubovy S, Miller D, Alfonso E, Forster RK, Behar-Cohen FF, Parel JM. Iontophoresis for the treatment of paecilomyces keratitis. Cornea. 2002;21:131–132. doi: 10.1097/00003226-200201000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Eljarrat-Binstock E, Raiskup F, Frucht-Pery J, Domb AJ. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release. 2005;106:386–390. doi: 10.1016/j.jconrel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Behar-Cohen FF, Parel JM, Pouliquen Y, Thillaye-Goldenberg B, Goureau O, Heydolph S, Courtois Y, De Kozak Y. Iontophoresis of dexamethasone in the treatment of endotoxin-induced-uveitis in rats. Exp Eye Res. 1997;65:533–545. doi: 10.1006/exer.1997.0364. [DOI] [PubMed] [Google Scholar]
- 4.Lam TT, Edward DP, Zhu XA, Tso MO. Transscleral iontophoresis of dexamethasone. Arch Ophthalmol. 1989;107:1368–1371. doi: 10.1001/archopht.1989.01070020438050. [DOI] [PubMed] [Google Scholar]
- 5.Behar-Cohen FF, El Aouni A, Gautier S, David G, Davis J, Chapon P, Parel JM. Transscleral coulomb-controlled iontophoresis of methylprednisolone into the rabbit eye: Influence of duration of treatment, current intensity, and drug concentration on ocular tissue and fluid levels. Exp Eye Res. 2002;74:51–59. doi: 10.1006/exer.2001.1098. [DOI] [PubMed] [Google Scholar]
- 6.Voigt M, Kralinger M, Kieselbach G, Chapon P, Anagnoste S, Hayden B, Parel JM. Ocular aspirin distribution: A comparison of intravenous, topical, and coulomb-controlled iontophoresis administration. Invest Ophthalmol Vis Sci. 2002;43:3299–3306. [PubMed] [Google Scholar]
- 7.Parkinson TM, Ferguson E, Febbraro S, Bakhtyari A, King M, Mundasad M. Tolerance of ocular iontophoresis in healthy volunteers. J Ocul Pharmacol Ther. 2003;19:145–151. doi: 10.1089/108076803321637672. [DOI] [PubMed] [Google Scholar]
- 8.Hughes L, Maurice DM. A fresh look at iontophoresis. Arch Ophthalmol. 1984;102:1825–1829. doi: 10.1001/archopht.1984.01040031483028. [DOI] [PubMed] [Google Scholar]
- 9.Eljarrat-Binstock E, Domb AJ. Iontophoresis: A noninvasive ocular drug delivery. J Control Release. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Li SK, Zhang Y, Zhu H, Higuchi WI, White HS. Influence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transport. J Pharm Sci. 2005;94:847–860. doi: 10.1002/jps.20293. [DOI] [PubMed] [Google Scholar]
- 11.Kasting GB. Theoretical models for iontophoretic delivery. Adv Drug Deliv Rev. 1992;9:177–199. [Google Scholar]
- 12.Peck KD, Srinivasan V, Li SK, Higuchi WI, Ghanem AH. Quantitative description of the effect of molecular size upon electroosmotic flux enhancement during iontophoresis for a synthetic membrane and human epidermal membrane. J Pharm Sci. 1996;85:781–788. doi: 10.1021/js950044j. [DOI] [PubMed] [Google Scholar]
- 13.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Del Rev. 1992;9:201–237. doi: 10.1016/s0169-409x(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 14.Chizmadzhev YA, Indenbom AV, Kuzmin PI, Galichenko SV, Weaver JC, Potts RO. Electrical properties of skin at moderate voltages: Contribution of appendageal macropores. Biophys J. 1998;74:843–856. doi: 10.1016/S0006-3495(98)74008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li SK, Ghanem A-H, Peck KD, Higuchi WI. Pore induction in human epidermal membrane during low to moderate voltage iontophoresis: A study using AC iontophoresis. J Pharm Sci. 1999;88:419–427. doi: 10.1021/js980331y. [DOI] [PubMed] [Google Scholar]
- 16.Glaser RW, Leikin SL, Chernomordik LV, Pastushenko VF, Sokirko AI. Reversible electrical break-down of lipid bilayers: Formation and evolution of pores. Biochim Biophys Acta. 1988;940:275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Smye SW, Robinson MP, Evans JA. Membrane electroporation theories: A review. Med Biol Eng Comput. 2006;44:5–14. doi: 10.1007/s11517-005-0020-2. [DOI] [PubMed] [Google Scholar]
- 18.Maurice DM. Iontophoresis of fluorescein into the posterior segment of the rabbit eye. Ophthalmology. 1986;93:128–132. doi: 10.1016/s0161-6420(86)33781-3. [DOI] [PubMed] [Google Scholar]
- 19.Kralinger MT, Voigt M, Kieselbach GF, Hamasaki D, Hayden BC, Parel JM. Ocular delivery of acetylsalicylic acid by repetitive coulomb-controlled iontophoresis. Ophthalmic Res. 2003;35:102–110. doi: 10.1159/000069129. [DOI] [PubMed] [Google Scholar]
- 20.Li SK, Molokhia SA, Jeong EK. Assessment of subconjunctival delivery with model ionic permeants and magnetic resonance imaging. Pharm Res. 2004;21:2175–2184. doi: 10.1007/s11095-004-7669-3. [DOI] [PubMed] [Google Scholar]
- 21.Li SK, Jeong EK, Hastings MS. Magnetic resonance imaging study of current and ion delivery into the eye during transscleral and transcorneal iontophoresis. Invest Ophthalmol Vis Sci. 2004;45:1224–1231. doi: 10.1167/iovs.03-0821. [DOI] [PubMed] [Google Scholar]
- 22.Molokhia SA, Jeong E-K, Higuchi WI, Li SK. Examination of penetration routes and distribution of ionic permeants during and after transscleral iontophoresis with magnetic resonance imaging. Int J Pharm. 2007;335:46–53. doi: 10.1016/j.ijpharm.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiller M, Brown RD, Koenig SH, Wolf GL. Longitudinal proton relaxation rates in rabbit tissues after intravenous injection of free and chelated Mn2+ Magn Reson Med. 1988;8:293–313. doi: 10.1002/mrm.1910080307. [DOI] [PubMed] [Google Scholar]
- 24.Berkowitz BA, Roberts R, Goebel DJ, Luan H. Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Invest Ophthalmol Vis Sci. 2006;47:2668–2674. doi: 10.1167/iovs.05-1588. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Robinson MR, Lizak MJ, Tansey G, Lutz RJ, Yuan P, Wang NS, Csaky KG. Controlled drug release from an ocular implant: An evaluation using dynamic three-dimensional magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2004;45:2722–2731. doi: 10.1167/iovs.04-0091. [DOI] [PubMed] [Google Scholar]
- 26.Robinson MR, Lee SS, Kim H, Kim S, Lutz RJ, Galban C, Bungay PM, Yuan P, Wang NS, Kim J, Csaky KG. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res. 2006;82:479–487. doi: 10.1016/j.exer.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Noske W, Stamm CC, Hirsch M. Tight junctions of the human ciliary epithelium: Regional morphology and implications on transepithelial resistance. Exp Eye Res. 1994;59:141–149. doi: 10.1006/exer.1994.1092. [DOI] [PubMed] [Google Scholar]
- 28.Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- 29.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PubMed] [Google Scholar]
- 30.Li SK, Zhu H, Higuchi WI. Enhanced transscleral iontophoretic transport with ion-exchange membrane. Pharm Res. 2006;23:1857–1867. doi: 10.1007/s11095-006-9010-9. [DOI] [PubMed] [Google Scholar]
- 31.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 32.Andrieu-Soler C, Doat M, Halhal M, Keller N, Jonet L, BenEzra D, Behar-Cohen F. Enhanced oligonucleotide delivery to mouse retinal cells using iontophoresis. Mol Vis. 2006;12:1098–1107. [PubMed] [Google Scholar]
- 33.Blair-Parks K, Weston BC, Dean DA. High-level gene transfer to the cornea using electroporation. J Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrieu-Soler C, Bejjani RA, de Bizemont T, Normand N, BenEzra D, Behar-Cohen F. Ocular gene therapy: A review of nonviral strategies. Mol Vis. 2006;12:1334–1347. [PubMed] [Google Scholar]
- 35.Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Invest Ophthalmol Vis Sci. 2003;44:2141–2146. doi: 10.1167/iovs.02-1027. [DOI] [PubMed] [Google Scholar]
- 36.Sander B, Larsen M, Moldow B, Lund-Andersen H. Diabetic macular edema: Passive and active transport of fluorescein through the blood-retina barrier. Invest Ophthalmol Vis Sci. 2001;42:433–438. [PubMed] [Google Scholar]
- 37.Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye II: Development and validation of a simple pharmacokinetic model for subconjunctival injection. J Ocul Pharmacol Ther. 2004;20:43–53. doi: 10.1089/108076804772745455. [DOI] [PubMed] [Google Scholar]
- 38.Wu JC, Jesmanowicz A, Hyde JS. Anterior segment high resolution MRI: Aqueous humor dynamics observed using contrast agents. Exp Eye Res. 1992;54:145–148. doi: 10.1016/0014-4835(92)90079-8. [DOI] [PubMed] [Google Scholar]