Abstract
We recently identified a Transposase domain protein called Metnase, which assists in repairing DNA double-strand breaks (DSB) via non-homologous end-joining (NHEJ), and is important for foreign DNA integration into a host cell genome. Since integration is essential for productive lentiviral infection we examined whether Metnase expression levels could have an influence on lentiviral genomic integration. Using cells stably transduced to either over- or under-express Metnase we determined that the expression level of Metnase did indeed correlate with live lentiviral integration. Changes in Metnase levels were accompanied by changes in the number of copies of integrated lentiviral cDNA. While Metnase levels affected lentiviral integration, it had no effect on the amount of either total cellular viral RNA, cDNA or 2-LTR circles. Therefore, Metnase enhances the integration of lentivirus DNA into the host cell genome.
Keywords: DNA repair, Lentivirus, Genomic Integration, Transposase, Histone Methylation
1. Introduction
In a previous study we identified a novel protein termed Metnase, which promotes double strand break (DSB) repair by non-homologous end-joining (NHEJ) and is important for the integration of foreign DNA into the host cell genome [1]. Metnase contains two functional domains: a SET domain and a Transposase/Integrase domain, which contains a DDE motif that is common to the transposase and retroviral integrase family. We found that Metnase under-expression resulted both in decreased DNA DSB repair by NHEJ and decreased integration of foreign DNA [1]. Genomic integration of lentiviruses such as HIV has three distinct steps. The first is the processing of viral and genomic DNA ends to create overhangs, followed by the NHEJ of the viral DNA into the host DNA, resulting in an integration intermediate with gaps in the flanking host DNA sequence. The final step is filling in these gaps in the host DNA [2]. The completion of the integration process by repairing the gapped DNA intermediates may involve a number of DNA repair proteins, including the NHEJ repair pathway [2-4], Poly (ADP-ribose)-polymerase (PARP) [5, 6], ATM [7], ATR [8] and RAD18 proteins [9]. Our previous study demonstrated that Metnase was important for integration of free fragments of foreign DNA [1]. Given that Metnase contained a domain similar to transposase and integrase family members, we therefore examined whether Metnase played a role in lentiviral genomic integration.
2. Materials and methods
2.1 Metnase over- and under-expressing cell lines
HEK293 cells were transfected with pcDNA3.1-Metnase or pcDNA3.1 (vector control, Invitrogen, Carlsbad, CA) and selected with G418 for stable over-expression of Metnase as described previously (1). For Metnase under-expression HEK293 cells were transfected with pRNA-U6/hygro-siMET or pRNA-U6/hygro (vector control expressing a scrambled siRNA; Genscript, Piscataway, NJ), selected with hygromycin and individual colonies were screened for Metnase under-expression by reverse transcription-PCR (RT-PCR) [1]. HEK293 cells were also stably transfected with pCMV-FLAG (vector control, Sigma-Aldrich, Milwaukee, WI) and pCMV-FLAG constructs of wild type Metnase or the Metnase mutant D483A, which changes the first D in the conserved DDE motif required for transposase and HIV integrase function [1]. Expression for all lines was monitored before each experiment by Western immunoblotting [1]. Equal loading of the protein extracts was demonstrated using an antibody specific for β-actin.
2.2 Lentiviral infection
Cells were infected with supernatant containing equal titers of VSV-G pseudotyped HIV1-backbone lentivirus (pLenti6/GW-LacZ; Invitrogen, Carlsbad, CA) for 24 hours in the presence of 8 μg/ml polybrene. The viral supernatant was removed and cells washed. Infected cells were either harvested for RNA and genomic DNA at 24, 48 and 72 hours post infection (without selection) or were selected with blasticidin (5 μg/ml) for 10 days. Colonies indicated single cells with integrated lentivirus. Each experiment was performed three distinct times in triplicate with differing viral titers with the same results.
2.3 Real time quantitative PCR
Real time quantitative PCR was carried out for viral copy number and 2-LTR circles [10]. The primers used were as follows: for copy number FPLV-2 5’-acctgaaagcgaaagggaaac and RPLV-2 5’-cacccatctctctccttctagcc; for 2-LTR circles MH535 5’-aactagggaacccactgcttaag and MH536ELZ 5’-tacaagcaaaaagcagatcttgtc; for the loading control GAPDH FOR: 5’-ccccacacacatgcacttacct and GAPDH REV: 5’-ccctagtcccagggctttgatt. Copy number was assessed using a standard curve made with 10-fold dilutions of the pLenti6/GW-LacZ plasmid [10]. 2-LTR circles were quantified using the ratio of the C(T) of the sample divided by the C(T) of the GAPDH loading control [10]. Each experiment was performed three distinct times.
2.4 RT-PCR and PCR
RNA was isolated from cells and subjected to RT-PCR using the primers for the blasticidin gene, Forward: 5’-atcaacagcatccccatctc and Reverse: 5’-caagatgcccctgttctcat. Equal loading was determined using primers for 18S as previously described [1]. Genomic DNA was used in PCR to investigate the total amount of virus in the cell lines as well as the amount of integrated virus in each of the infected cell lines. Total viral species were measured using the primers MH531 5’-tgtgtgcccgtctgttgtgt and MH532 5’-gagtcctgcgtcgagagagc [10]. To test for integration two rounds of PCR were carried out with the first round using the primers ALU3 5’-tcccagctactcgggaggctgagg and LTR5 5’-aggcaagctttattgaggcttaag, and the second round using the primers NEST1 5’-cacacacaaggctacttccct and NEST2 5’-gccactccccagtcccgccc [4]. Each experiment was performed three distinct times.
3. Results and Discussion
Metnase over- (MET) and under-expressing (siMET) cell lines and the appropriate controls (pCD, U6) used here have been described previously (1). Metnase was over-expressed 4-fold compared to the control and Metnase expression was reduced 3-fold compared to the control. These lines were used to assess the effect of varying Metnase levels on lentiviral integration.
Metnase could affect the initial infectivity of the live lentivirus. Therefore, the presence of intracellular Blasticidin RNA and lentiviral cDNA were assessed. Following lentiviral infection we harvested RNA from all the cell lines at 48 hours post infection and performed RT-PCR using primers to the blasticidin gene (Fig. 1). Metnase expression levels have no effect on the presence of intracellular lentivirally-produced Blasticidin RNA. This analysis was also done at 24 and 72 hours post infection and the results were the same (data not shown).
FIGURE 1.
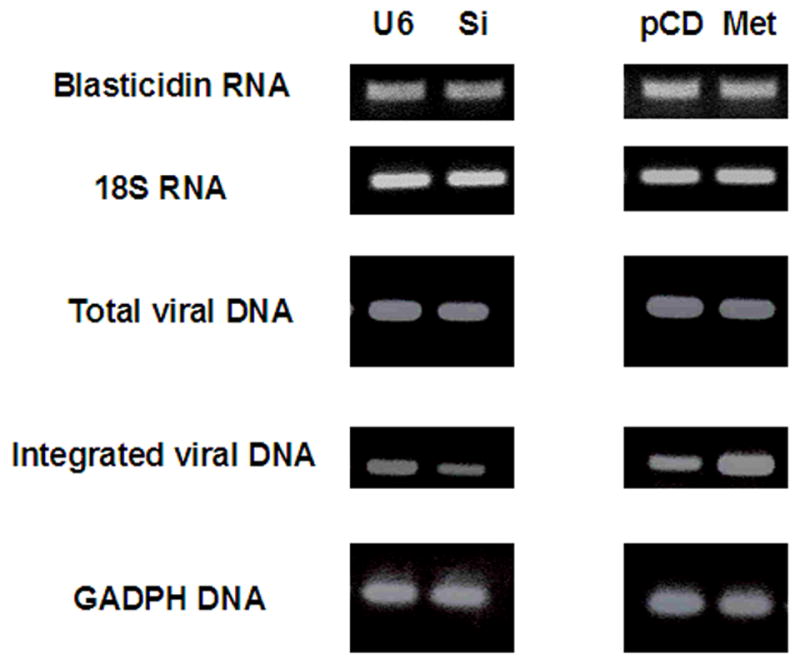
Blasticidin RNA from the lentivirus after infection is not changed by Metnase levels. Genomically integrated viral DNA but not total cellular viral DNA is affected by Metnase expression 48 hours post infection. 18S is the RNA loading control and GAPDH is DNA loading control. U6- siRNA vector control, Si- Metnase under-expressors, pCD- vector control, Met- Metnase over-expressors.
Viral infection results in a number of possible forms of intracellular viral DNA representing different stages of integration. Using PCR on genomic DNA we analyzed both total viral DNA and integrated viral DNA at 48 hours post infection in the Metnase over- and under-expressors. The expression levels of Metnase did not have an affect on the amount of total lentiviral intracellular cDNA following infection (Fig. 1). The lack of any difference between Blasticidin RNA and total intracellular lentiviral cDNA implies that Metnase levels did not appreciably affect the initial infectivity of the lentivirus.
However, using primers that were specific for integrated lentiviral DNA we did observe differences in the amount of lentiviral DNA integration that correlated with the expression of Metnase. Over-expression of Metnase resulted in an almost 4-fold increase in integrated lentiviral DNA while under-expression of Metnase decreased about 2-fold the integrated lentiviral DNA (Fig. 1). Real time-qPCR was used to analyze the formation of 2-LTR circles, and no differences were observed for this viral DNA form between any of the cell lines studied (data not shown).
Total and integrated lentiviral DNA was also analyzed at 24 and 72 hours post infection and the results were identical to that shown for 48 hours (data not shown). Mock infected cells were negative for all PCRs (data not shown).
Since Metnase expression did correlate with integration of viral DNA, cell lines that had been infected with the lentivirus were then selected with blasticidin. Once selection was complete the plates were stained and colonies counted (Fig. 2). Blasticidin-resistant colonies, indicating genomic integration of the lentivirus, were increased 1.8-fold in the Metnase over-expressors (p=0.0074) and decreased 3.2-fold in the Metnase under-expressors (p=0.0066) compared to the vector controls (Fig. 2).
FIGURE 2.
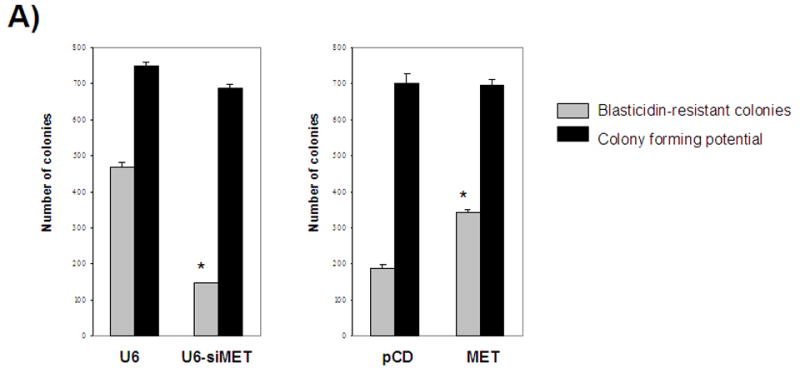
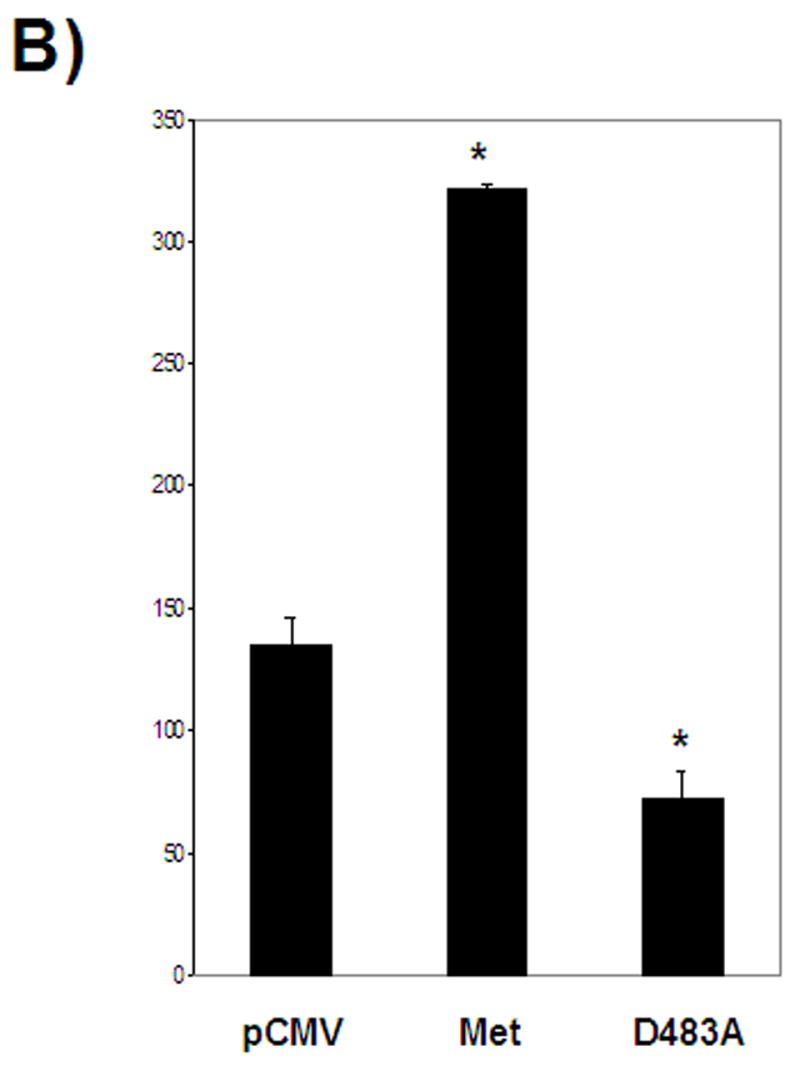
A- Selection for lentiviral genomic integration with blasticidin indicates a correlation between Metnase expression and the number of blasticidin-resistant colonies. B- Mutation of an essential residue in the transposase domain leads to decreased viral integration compared to wild-type Metnase U6- vector control, siMET- Metnase under-expressors, pCD- vector control, MET- Metnase over-expressors, D438A- over-expression of Metnase mutated in the transpose domain. *- statistically significant at p<0.05. All cell lines have the same colony forming potential without blasticidin selection.
Metnase contains the essential DDE motif common to transposases and lentiviral integrases (1). We generated cell lines that over-expressed either wild type Metnase or a mutant Metnase, D483A, which changes the first D residue of the motif in the transposase domain. Wild type Metnase resulted in a 2.4-fold increase in the number of blasticidin-resistant colonies (p=0.0013) compared to the pFLAG vector control (Fig. 2). Stable expression of the Metnase D483A mutant resulted in a 1.9-fold decrease in the number of blasticidin-resistant colonies (p=0.0199) compared to the pFLAG vector control (Fig. 2). Therefore, the DDE motif is important in mediating the effect of Metnase on lentiviral integration.
We next determined whether there were differences in the number of copies of lentiviral DNA integrated into the host genome between the Metnase over-expressing and under-expressing clones. We analyzed integration via real time-quantitative PCR using primers specific for the LTR sequences in the lentiviral DNA compared to a standard curve to obtain a measure of the copy number of viral integration per ng of host cell DNA [4, 10]. From this analysis of viral copy number we determined that Metnase over-expression increased integrated viral copies by 1.9-fold compared to the pCD vector control (1.77×105 compared to 9.4×104 copies per ng DNA, p=0.0274), whereas Metnase under-expression decreased integrated viral copies by 3.1-fold compared to the U6 vector control (9.04×104 compared to 2.84×105 copies per ng DNA, p<0.0001). Therefore, alterations in Metnase expression appear to correlate with both the number of colony-forming single cells with integrated lentivirus and the number of copies of lentiviral DNA in the host cell DNA.
While NHEJ repair proteins have been reported to have important roles in lentiviral integration, this idea remains controversial. Some studies show an effect on viral integration upon loss or down regulation of known DNA repair proteins (2-9). However, other studies suggest that NHEJ proteins are not important for viral integration [11-13]. It appears that the lentivirus titer is a major difference seen in these studies. High titers (>1 MOI) in cells deficient in NHEJ proteins such as DNA-PKcs did result in reduced viral integration. Conversely, low viral titers (<1 MOI) resulted in no difference in viral integration between wild type and DNA-PKcs-deficient cells [11-13].
Metnase has a transposase/integrase domain that is required for its DNA repair activity [1]. Others have shown that this domain has some but not all of the characteristics of transposases [14-16]. Here we found that this domain was also important for assisting in lentiviral genomic integration, a function not unlike transposition.
In our study there is a reproducible effect on viral integration with differing Metnase expression levels and differing lentiviral titers. Mutating an amino acid in Metnase known to be required in transposase domain and lentiviral integrase proteins reduced the ability of Metnase to enhance lentiviral integration. Thus, a functional transposase/integrase domain is required for this activity of Metnase. In addition, Metnase has histone methylase activity [1], and could also assist lentiviral integration by modifying chromatin at the insertion site. Therefore, Metnase may be having two effects on integration, via chromatin modification to assist in integration of the virus DNA, and then in repairing the overhangs resulting from the insertion of the virus DNA into the chromosomal DNA.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee SH, Oshige M, Durant ST, Rasila KK, Williamson EA, Ramsey H, Kwan L, Nickoloff JA, Hromas R. The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc Natl Acad Sci USA. 2005;102:18075–18080. doi: 10.1073/pnas.0503676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Olvera JM, Yoder KE, Mitchell RS, Butler SL, Lieber M, Martin SL, Bushman FD. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 2001;20:3272–3281. doi: 10.1093/emboj/20.12.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel R, Katz RA, Skalka AM. A role for DNA-PK in retroviral DNA integration. Science. 1999;284:644–647. doi: 10.1126/science.284.5414.644. [DOI] [PubMed] [Google Scholar]
- 4.Jeanson L, Subra F, Vaganay S, Hervy M, Marangoni E, Bourhis J, Mouscadet JF. Effect of Ku80 depletion on the preintegrative steps of HIV-1 replication in human cells. Virology. 2002;300:100–108. doi: 10.1006/viro.2002.1515. [DOI] [PubMed] [Google Scholar]
- 5.Gaken JA, Tavassoil M, Gan SU, Vallian S, Giddings I, Darling DC, Galea-Lauri J, Thomas MG, Abedi H, Schreiber V, Menissier-de Murcia J, Collins MK, Shall S, Farzaneh F. Efficient retroviral infection of mammalian cells is blocked by inhibition of poly(ADP-ribose) polymerase activity. J Virol. 1996;70:3992–4000. doi: 10.1128/jvi.70.6.3992-4000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha HC, Juruli K, Zhou Y, Leung S, Hermankova M, Snyder SH. Poly(ADP-ribose) polymerase-1 is required for efficient HIV-1 integration. Proc Natl Acad Sci USA. 2001;98:3364–3368. doi: 10.1073/pnas.051633498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel R, Katz RA, Merkel G, Little JC, Yen TJ, Skalka AM. Evidence that the retroviral DNA integration process triggers an ATR-dependent DNA damage response. Mol Cell Biol. 2001;21:1164–1172. [Google Scholar]
- 8.Daniel R, Kao G, Taganov K, Greger JG, Favorova O, Merkel G, Yen TJ, Katz RA, Skalka AM. Evidence that the retroviral DNA integration process triggers an ATR-dependent DNA damage response. Proc Natl Acad Sci USA. 2003;100:4778–4783. doi: 10.1073/pnas.0730887100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulder LC, Chakrabarti LA, Muesing MA. Interaction of HIV-1 integrase with DNA repair protein hRad18. J Biol Chem. 2002;277:27489–27493. doi: 10.1074/jbc.M203061200. [DOI] [PubMed] [Google Scholar]
- 10.Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- 11.Baekelandt V, Claeys A, Cherepanov P, De Clercq E, De Strooper B, Nuttin B, Debyser Z. DNA-Dependent protein kinase is not required for efficient lentivirus integration. J Virol. 2000;74:11278–11285. doi: 10.1128/jvi.74.23.11278-11285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariumi Y, Turelli P, Masutani M, Trono D. DNA damage sensors ATM, ATR, DNA-PKcs, and PARP-1 are dispensable for human immunodeficiency virus type 1 integration. J Virol. 2005;79:2973–2978. doi: 10.1128/JVI.79.5.2973-2978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilzer JM, Stracker T, Beitzel B, Meek K, Weitzman M, Bushman FD. Roles of host cell factors in circularization of retroviral DNA. Virology. 2003;314:460–467. doi: 10.1016/s0042-6822(03)00455-0. [DOI] [PubMed] [Google Scholar]
- 14.Miskey C, Papp B, Mates L, Sinzelle L, Keller H, Izsvak Z, Ivics Z. The ancient mariner sails again: transposition of the human Hsmar1 element by a reconstructed transposase and activities of the SETMAR protein on transposon ends. Mol Cell Biol. 2007:4589–45600. doi: 10.1128/MCB.02027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Bischerour J, Siddique A, Buisine N, Bigot Y, Chalmers R. The human SETMAR protein preserves most of the activities of the ancestral Hsmar1 transposase. Mol Cell Biol. 2007;27:1125–1132. doi: 10.1128/MCB.01899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordaux R, Udit S, Batzer MA, Feschotte C. Birth of a chimeric primate gene by capture of the transposase gene from a mobile element. Proc Natl Acad Sci USA. 2006;103:8101–8106. doi: 10.1073/pnas.0601161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


