Abstract
In 2001, the National Cancer Institute funded three centers to test the feasibility of establishing a cohort of American Indian and Alaska Native people. Participating tribal organizations named the study EARTH (Education and Research Towards Health). This paper describes the study methods. A computerized data collection and tracking system was developed using audio computer-assisted survey methodology with touch screens. Data were collected on diet, physical activity, lifestyle and cultural practices, medical and reproductive history, and family history of heart disease, diabetes, and cancer. In addition, a small panel of medical measurements was obtained, including height, weight, waist and hip circumferences, blood pressure, and a lipid panel plus glucose. At the completion of the enrollment visit, data were used to provide immediate health feedback to study participants. During the initial funding period, the authors anticipate enrolling 16,000 American Indian and Alaska Native participants. The age distribution of the study population was similar to that reported in the 2000 US Census for the relevant populations. A component critical to the success of the EARTH Study has been the partnerships with tribal members. The study has focused on involvement of American Indian and Alaska Native communities in development and implementation and on provision of feedback to participants and communities.
Keywords: Alaska; cohort studies; diet; Indians, North American; methods
Rates of chronic diseases vary substantially among American Indian and Alaska Native (AIAN) communities and tribes for reasons that are largely unknown. Trends in chronic disease incidence and mortality are not well documented for most tribes, but it does seem clear that diabetes and heart disease incidence and mortality have been increasing over the past two decades and that cancer incidence rates vary among different groups of AIAN people (1–3). Variations in disease rates could be the result of differences in diet and lifestyle patterns, many of which have undergone recent changes among AIAN people.
In the past 25 years, cohort studies have been used to discover several important, modifiable risk factors for chronic diseases in the United States. The Framingham study, a small community-based US cohort, was one of the first established cohorts that provided substantial information on cardiovascular diseases (4). Current large cohorts, mostly consisting of non-Hispanic Whites, have been funded by the National Institutes of Health to study women (5,6), men (7), an undefined group of volunteers (8), California teachers (9), agricultural workers in the Midwest (10), Seventh-Day Adventists (11), 150,000 Chinese women living in Shanghai (12), women as part of the Women’s Health Initiative (13), and a cohort of 40,000 women using dietary supplements (14). The First and Second National Health and Nutrition Examination Surveys, originally designed as cross-sectional studies, have been converted into longitudinal cohort studies (15).
Ethnic minorities have been underrepresented in federally funded cohort studies until recently. A large multiethnic cohort study including non-Hispanic Whites, Hispanics, African Americans, Asian Americans, and about 6,500 Native Hawaiians (16), and another large cohort of African-American women, have been funded by the National Cancer Institute (17). The Southern Community Cohort also was initiated with 70,000 Black and 35,000 non-Black participants (18). While numerous cohorts have been established in the last 25 years, the AIAN population remains the single racial population in the United States that has not been included in any existing cohort studies, with the exception of the Strong Heart Study, a cohort of approximately 4,500 American Indians established to study cardiovascular disease risk factors (19). Understanding chronic disease development in AIAN populations may help alleviate the existing health disparities between this group of Americans and other populations.
There are many challenges to conducting research with AIAN populations. To obtain adequate numbers for a cohort of AIAN populations and to benefit from the diversity within the AIAN population, enrollment from communities and tribal members from around the country is needed. Data have to be immediately useful to the participant and to tribal organizations. AIAN people often live in remote areas, making access difficult. In addition, there are many tribal languages, some of which are spoken rather than written. Most cohort studies collect data through mailed, self-administered questionnaires, a method that is not feasible in AIAN populations because personal involvement with participants and with tribal groups is important for research success in these communities.
The original funding by the National Cancer Institute supported development of study instruments and collection of data at an initial baseline visit for a large group of AIAN people. Expansion and long-term follow-up are contingent upon continued support and funding. The goal of the study, subsequently named EARTH (Education and Research Towards Health), was to obtain a better understanding of how diet, physical activity, body size, lifestyle, and cultural factors relate to the development and progression of diseases. In this paper, we describe the methods used to establish the foundation for the EARTH Study.
MATERIALS AND METHODS
The EARTH Study was funded by the National Cancer Institute in the fall of 2001. Three field centers and one coordinating center were funded to develop standardized study methods and implement recruitment and data collection. The three grants funded in phase I of the EARTH Study were for 1) The University of Utah, including the Navajo Nation field center and the study coordinating center; 2) the Alaska Native Tribal Health Consortium; and 3) the Black Hills Center for American Indian Health. Tribal partnerships were established, and the study was approved by the Navajo Nation Institutional Review Board, the Alaska Area Institutional Review Board, the Aberdeen area Institutional Review Board, the Indian Health Service National Institutional Review Board, and The University of Utah Institutional Review Board. Additionally, regional, local, and village health boards and chapters within local health boards reviewed and approved the study.
Both a Tribal Advisory Board and a Scientific Advisory Board were established (figure 1). Participating tribes appointed individuals to be members of the Tribal Advisory Board; the Tribal Advisory Board chair was elected by the Tribal Advisory Board members. The primary responsibility of the Tribal Advisory Board was to review the study protocol and questionnaires, provide insight into field center operations, and serve as a liaison with the tribe they represented. The Scientific Advisory Board’s role was to provide advice on scientific issues relevant to the study; therefore, the Scientific Advisory Board included people with relevant scientific expertise. Annual meetings included both Scientific Advisory Board and Tribal Advisory Board members along with study investigators and staff.
FIGURE 1.
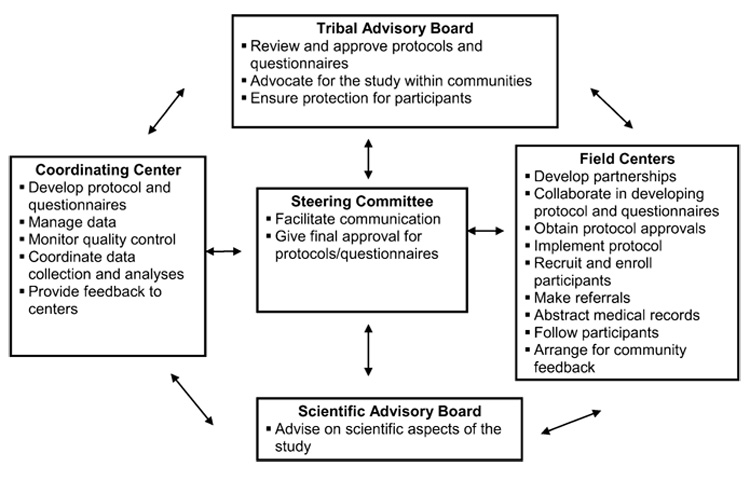
Organization of the Education and Research Towards Health (EARTH) Study of American Indian and Alaska Native people.
The initial study goals were to
Develop methods to collect valid data on diet, physical activity, and lifestyle factors among AIAN populations living in the United States
Develop methods to provide rapid feedback to individuals and communities
Develop cost-efficient methods to recruit and enroll AIAN people in a cohort
Establish methods to track participants and their health over time
The projected enrollment for phase I of the study was targeted at 4,000 participants in Alaska, 5,000 participants in the Plains and Gila River, and 7,500 participants from the Navajo Nation. Eligibility criteria included
At least 18 years of age
AIAN person and eligible for health care through the Indian Health Service
Resident of the study area
Not pregnant at the time of the baseline visit (deferred enrollment)
Not actively undergoing cancer treatment (deferred enrollment)
Physically and mentally able to read and understand a consent form and complete survey instruments and medical tests
Able to complete the interview in English or a tribal language
Convenience sampling within communities was used, except for the people enrolled from the Gila River location. A convenience sample allowed the study to be open to all tribal members, not just those randomly selected, which was the provision of many tribal approvals. In Gila River, a random systematic household sample was used.
Recruitment
Recruitment methods varied by study site to meet local needs. Methods included presentations to tribal groups and health-care providers; hiring of local recruiters who were tribal members; informational tables staffed by study personnel at community events; house-to-house recruiting; brochures and flyers in public locations; and public service announcements on local radio and in newspapers. At some field centers, tribal employees were given release time from work to participate in the study. Baseline study visits were conducted in a variety of settings including stationary locations in the larger population areas, temporary study centers in remote villages, and mobile vans that traveled from community to community. Clinics were set up to assure participant confidentiality for all study components. Participants were given small incentives such as caps, pens, tote bags, T-shirts, water bottles, and gas, phone, cash, or grocery gift cards. The value of these items varied by site but was approximately $20–30.
Baseline study visit components
The baseline study visit consisted of informed consent, an intake questionnaire, medical measurements, an audio computer-assisted self-interview diet history questionnaire, an audio computer-assisted self-interview health and lifestyle questionnaire, an exit interview, and individual feedback (health report to each participant at the conclusion of the study visit). Study participants were asked to fast for 9 hours prior to the study visit. Participants who did not fast were given the option of completing the visit except for the blood test and returning at a later date for the fasting blood test or of completing the study visit with a nonfasting lipid panel.
Computer-assisted data collection and tracking
A study goal was to provide participants with immediate feedback about the results of the study visit. Therefore, a computer-assisted data collection system, SCAPES (Study Computer Assisted Participant Evaluation System), was designed so that study visit data would be available immediately upon completion of the study visit. SCAPES was developed to guide study participants through the various components of the study (figure 2) as well as to collect questionnaire data using computer-assisted interviewing, both interviewer administered and self-administered, and to allow input of medical data. The SCAPES system printed forms for recording medical data as well as participant feedback at visit end. Smart cards (i.e., cards with computer chips that allow them to store and process data) were used to identify employees and participants. The SCAPES shell also contained various administrative functions for management of the smart card infrastructure and database backup and export.
FIGURE 2.
Tracking system bar used in the Study Computer Assisted Participant Evaluation System (SCAPES) of the Education and Research Towards Health (EARTH) Study of American Indian and Alaska Native people. Hr, hours; Min, minutes; DHQ, diet history questionnaire; HLPA, Health, Lifestyle, and Physical Activity Questionnaire.
Medical measurements
Medical tests included seated blood pressure, height, weight, waist and hip circumference measurements, and serum lipid and glucose levels. Participants were asked to fast for at least 9 hours before the tests.
Blood pressure measurements were taken by using the Omron IntelliSense Blood Pressure Monitor (Hem-907/907XL; Omron Healthcare Inc., Vernon Hills, Illinois) (20). Cuff size was determined by measuring the bare arm circumference. After participants were seated for 5 minutes in a quiet environment, three blood pressure measurements were taken automatically at 1-minute intervals and the average blood pressure calculated from the last two measurements.
Waist and hip circumferences and height and weight were measured with the participants wearing loose clothing without shoes. Waist and hip circumference measurements were recorded to the nearest 0.5 inch (1.27 cm) by using either the Novel Products Figure Finder tape (Novel Products Inc., Rockton, Illinois) or the Gulick II Plus tape (Country Technology Inc., Gays Mills, Wisconsin). Participants were standing for both waist and hip measurements. Waist was measured at the smallest point between the 10th rib and the iliac crest; hip circumference was measured at the level of maximum protrusion of the gluteal muscles. Measurements were taken in duplicate. If the two measurements differed by more than 1 inch (2.54 cm), a third set of measurements was taken. The average of the two measurements was then used.
Weight was recorded by using a Tanita digital scale (BWP800/BWP627A; Tanita Corporation of America Inc., Arlington Hills, Illinois). Standing height was measured with the Road Rod Stadiometer (Seca, Hamburg, Germany). Two height and weight measurements were taken and the average of the two measurements used. If the two height measurements differed by more than 1 inch or the weight measurements differed by more than 2 pounds (0.91 kg), measurements were repeated.
The Cholestech LDX (Cholestech, Hayward, California) (21) machine tested fasting glucose, triglycerides, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, and very low density lipoprotein cholesterol. A cassette that measured lipid profile and glucose was used.
Study questionnaires
Four separate questionnaires were developed: intake; diet history; Health, Lifestyle, and Physical Activity; and exit. Development of these questionnaires involved several stages, including
Reviewing other study questionnaires for appropriateness given study objectives, mode of data collection, and cultural diversity of the study population
Meeting with small groups and individual tribal people to obtain input on cultural practices and health concerns of AIAN people
Meeting with scientific investigators to determine diet and lifestyle exposures that could potentially have biologic implications for chronic diseases
Testing questionnaires by tribal members
Translating questionnaires into Navajo and Yupik and audio-recording of questionnaires
All questionnaires were programmed for computer-assisted administration. The intake and exit questionnaires were programmed for interviewer-assisted administration because they contained questions that required entering information from the computer keyboard, such as address, type of cancer, and details about specific medical conditions. The diet history questionnaire and the Health, Lifestyle, and Physical Activity Questionnaire were programmed to be administered primarily as an audio computer-assisted self-interview. The audio component was developed to enforce reading questions prior to responding. Audio files were developed for each question and were merged with text files. Audio files were recorded by a Native speaker in English, Navajo, and Yupik. The underlying program of all of the questionnaires was part of SCAPES.
Intake questionnaire
The EARTH intake questionnaire was used to collect contact information to track participants in the future and to determine a participant’s fasting status. Additionally, questions about the participant’s current job, educational level, household information, Social Security number (to facilitate linking with medical and death data), and race/ethnicity were asked.
Diet history questionnaire and Health, Lifestyle, and Physical Activity Questionnaire
Table 1 shows the components of the questionnaires and the source of questions for adaptation. The referent period for diet and activity was the past year to address seasonality issues, especially in Alaska. Questions had to be designed for the computerized questionnaire. Another important component of questionnaire development was working with tribal members to obtain health information that they thought would be important and was sensitive to tribal issues.
TABLE 1.
Components of the diet history questionnaire and the Health, Lifestyle, and Physical Activity Questionnaire used in the Education and Research Towards Health (EARTH) Study
| Component | Sources of questions for adaptation |
|---|---|
| Dietary intake | The diet history questionnaire was adapted from the CARDIA diet history to include foods commonly eaten by Alaska Natives and American Indians (29). |
| Physical activity | The physical activity questions were adapted from the MESA questionnaire (30) and also incorporated components of the Taylor physical activity questionnaire (31, 32). There were two sections to the questionnaire. In the first, participants reported on seven activities performed during the past week; these activities change less frequently over a year’s time. The second list of 21 activities may vary more throughout the year; therefore, the time frame was the past year. Participants reported on any of the activities performed for more than 10 minutes at a time, how many months the activity was performed, as well as how much time was spent in the activity. The selected list of activities was intended to encompass the major sources of physical activity performed by American Indian and Alaska Native populations. |
| Medical history* | Participants were asked whether they had ever been diagnosed with the following conditions by a health-care provider: high blood pressure, high cholesterol, gallbladder disease including gallstones, kidney failure, liver disease including cirrhosis and hepatitis, thyroid disease, bone fracture or break as an adult, arthritis, asthma, chronic bronchitis, emphysema or chronic obstructive pulmonary disease, glaucoma, cataracts, depression, diabetes, and cancer. If yes, the age at diagnosis was asked. |
| Reproductive history* | Questions included information on menstrual status, age at menarche and menopause, pregnancy history, use of hormonal contraception, use of hormone replacement therapy, and history of hysterectomy and oophorectomy. |
| Cancer screening* | This section asked participants whether they had certain age- and sex-specific cancer-screening tests, including Papanicolaou tests, prostate-specific antigen tests, mammogram, and sigmoidoscopy or colonoscopy. |
| Over-the-counter medicine† | This section asked about use of a limited number of medications, including aspirin, nonsteroidal antiinflammatory drugs, and traditional medicines. |
| Health status | The standardized SF12 Health Survey (Quality Metric, Lincoln, Rhode Island) was included. |
| Tobacco* | The tobacco section included questions about use of various types of tobacco products, including cigarettes, pipes, cigars, chewing tobacco, or snuff and homemade chew made from tobacco leaves mixed with ash such as iq’mik. The amount smoked and number of years of smoking were obtained. |
| Alcohol | The alcohol section included binge drinking questions from the Behavioral Risk Factor Surveillance System as well as the CAGE questions (33, 34). |
| Other health risk behaviors | Questions regarding safety were adapted from the Behavioral Risk Factor Surveillance System (35). |
| Environment† | Twelve questions about environmental exposures were included at the request of the Tribal Advisory Board. Participants were asked whether they ever worked at least once a month for 6 months or more with or around asbestos; cadmium or mercury; lead; mineral or mining dust; pesticides including insecticides, weed killers, or fungicides; gasoline or petroleum products; and radioactive materials. Additional questions included ever having done silversmithing or welding at least once a month for 6 months or more; ever served in the US military; and, if served in the military, whether exposed to biologic or chemical agents. |
| Family history† | Participants were asked about their family history of heart attack; stroke; diabetes; and colorectal, breast, ovarian, prostate, and other cancers. Questions were designed to ascertain first-degree relatives with these conditions, although information on non-first-degree relatives was ascertained. |
| Socioeconomic status† | Participants were asked to report category of household income and number of people supported on that income. |
| Culture and traditional lifestyle† | Participants were asked whether they spoke their Native language at home, how well they identified with Native and non-Native culture, and whether they attended or participated in Native dances and events. |
Questions were adapted from other questionnaires to meet requirements of mode of questionnaire administration.
Questions were developed by study staff.
Exit interview
The EARTH exit questionnaire was used to obtain additional detailed information about medical conditions and screening tests the participant reported on the Health, Lifestyle, and Physical Activity Questionnaire. The questions asked for information about when and where the participant was last seen for a condition or for a screening test. Additional clinical information was gathered for some conditions such as heart disease, cancer, and arthritis.
Participant feedback
After the medical follow-up questions were asked, a four-page feedback report was generated by SCAPES and was reviewed by study personnel with the participant (refer to the Web Appendix, which is posted on the Journal’s website (http://aje.oupjournals.org/)). If medical referrals were needed (table 2), participants were referred to clinics or physicians. Feedback was developed on the basis of current standard health guidelines and was reviewed by local health-care providers (22–28). The goal was to give participants information on modifiable risks and to encourage healthy behaviors.
TABLE 2.
Critical values for medical screening tests for participants in the Education and Research Towards Health (EARTH) Study
| Test | Emergency/immediate referral (“Consult a physician immediately/ today.”) | Urgent referral (“Consult a physician within 1 week to 1 month.” Research staff make the referral.) | Routine referral (“Consult a physician within 1 month or at the first convenient appointment.”) | No referral (Give participants their values and health education information.) |
|---|---|---|---|---|
| Systolic blood pressure(mmHg) | ≥200 | 140–199 | <140 | |
| Diastolic blood pressure (mmHg) | ≥105 | 90–104 | <90 | |
| Height | All values | |||
| Weight | All values | |||
| Total cholesterol(mg/dl) | ≥300 | 240–299 | <240 | |
| High density lipoprotein cholesterol (mg/dl) | <40 | ≥40 | ||
| Triglycerides (mg/dl) | >650 | 200–650 | <200 | |
| Low density lipoprotein cholesterol (mg/dl) | No diabetes/heart disease: ≥190; diabetes or heart disease:≥ 160 | No diabetes/heart disease: 160–189; diabetes or heart disease: 130–159 | No diabetes/heart disease: <160; diabetes or heart disease:<130 | |
| Fasting glucose (mg/dl) | No diabetes: <50 and ≥400; diabetes: <60 and ≥400 | No diabetes: 126–399; diabetes: 60–89 | No diabetes: 110–125; diabetes: 126–399 | No diabetes: 50–109; diabetes: 90–130 |
Quality control
The goal of quality control was to monitor adherence to study protocol through training, certification, monitoring, and equipment maintenance. To reach this goal, the following procedures were implemented: 1) range checks programmed into the SCAPES system whenever possible to require confirmation of unlikely values; 2) double entry of height, weight, waist, hip, and Cholestech values to eliminate data entry error; 3) computerized self-administered questionnaires to enforce questionnaire logic and thereby eliminate most item nonresponse; 4) automatic reports to identify potential problems; 5) and data backup and data transmittal techniques to eliminate potential data loss.
EARTH staff were certified to complete the study visit, including obtaining consent, managing data, providing instructions for questionnaires, completing interviewer-administered components of the questionnaire (intake and exit interviews), explaining the audio computer-assisted self-interview questionnaires, completing anthropometric measurements, performing blood pressure measurements, collecting the capillary blood sample, handling biohazard materials, and providing participant feedback. Certified study staff were recertified on a quarterly basis to perform components of the examination according to protocol. Equipment was calibrated monthly, except that Cholestech QC optic checks were performed daily.
Once the study was under way, field center site visits were performed by a site visit team that consisted of one survey methodologist and one programmer from the coordinating center, one study coordinator, and one study investigator. The purposes of the site visits were to improve adherence to the study protocol; to assess the degree to which there was cross-center inconsistency in protocol implementation; to review and assess the administration and management of the field center, including issues of communication and decision making; to provide feedback on performance, both positive and correctional; and to recognize areas in which the protocol or data collection system needed change.
Data management
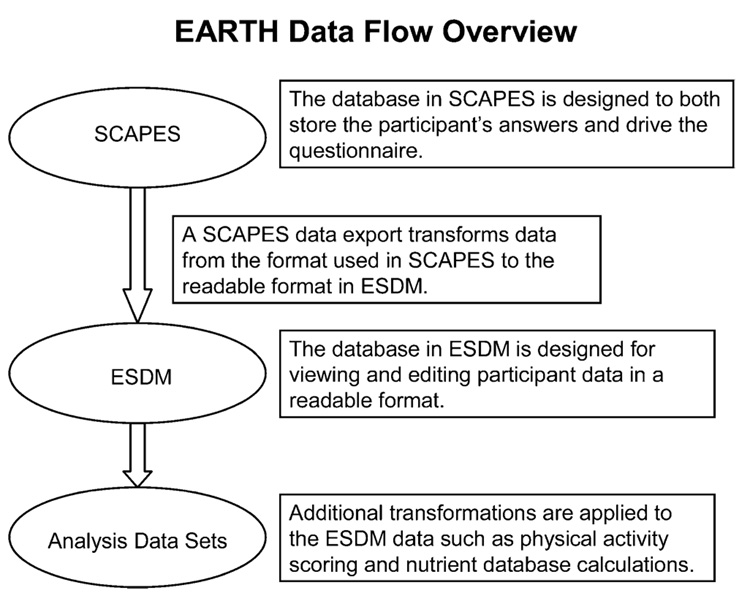
An overview of the data flow is shown in figure 3. The EARTH Data Management System was designed by coordinating center staff to control data flow through the EARTH Study, allow limited data editing and cleaning in that only certain fields could be changed, and offer basic data reporting capability. The EARTH Data Management System was loaded onto study computers at the EARTH data collection sites, EARTH regional centers, and the EARTH coordinating center. Various data edits and reporting can be done at any stage, although the EARTH Data Management System controls editing to protect data integrity. All SCAPES participant data exports were imported into the EARTH Data Management System at field center sites. Data were then submitted to the coordinating center from participating sites, where additional transformations were applied.
FIGURE 3.
Data flow overview. Data are initially entered into the Study Computer Assisted Participant Evaluation System (SCAPES) and are managed through the Education and Research Towards Health (EARTH) Data Management System (ESDM) for the EARTH Study of American Indian and Alaska Native people.
Participant confidentiality
Several steps were taken to assure that all participant and tribal data were treated confidentially. All staff received training regarding human subjects research protections at several levels, including the US Department of Health and Human Services, The University of Utah, the Indian Health Service, and local clinics. Data were de-identified by having a separate identification generated by SCAPES that was merged to the tracking identification in a data table separate from the study data. Prior to data being exported to the coordinating center, all identifiers were removed.
Participant monitoring
The EARTH Study is unique in that follow-up can be done in part through the Indian Health Service and regional tribal health organizations, which maintain a centralized computerized medical record system, the Resource Patient Management System. Staff abstract key data elements at both the Alaska and Black Hills centers. At the Navajo site, a computerized program pulls off key items approved by the Navajo Nation Institutional Review Board. Additionally, follow-up has been ongoing through center-specific mailings or telephone calls that include brief follow-up questionnaires, newsletters, and local disease registries. The content of subsequent visits and the extent of follow-up will depend on future funding levels.
RESULTS
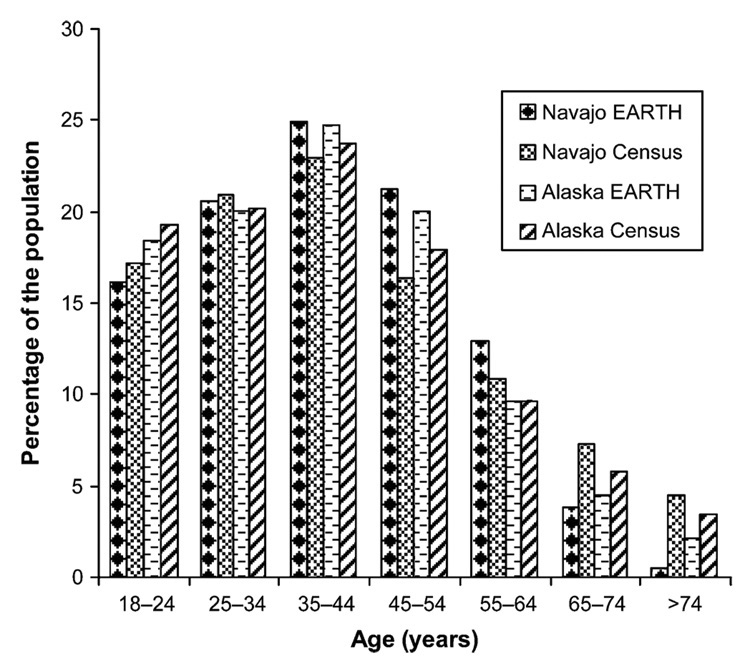
The age distribution of Alaska and Navajo study populations was similar to that reported for the 2000 US Census (figure 4). Our study population consisted of 40 percent men and 60 percent women, whereas the US Census data showed more equal proportions of men and women.
FIGURE 4.
Comparison of age distribution between Alaska and Navajo study participants in the Education and Research Towards Health (EARTH) Study to 2000 US Census data.
Baseline characteristics of the first 11,142 study participants seen through January 2006 are shown in table 3. The mean age of the population was approximately 40.2 years, and mean body mass index (weight (kg)/height (m)2) was over 30.0 kg/m2. The average number of livebirths reported by women who had been pregnant was 3.4. Of participants, 23.9 percent reported currently smoking cigarettes, 31.4 percent reported using any tobacco products, 37.1 percent had acquired a post–high school education, 39.1 percent reported no medical conditions (table 1 lists the medical conditions), and 31.6 percent perceived their overall health to be excellent or very good. Forty-one percent of participants reported not knowing their family history of cancer. Of participants who knew their family history, 38.4 percent reported having a family history of cancer, and 47.9 percent reported a family history of heart disease. The most common reason given for hearing about the study was word of mouth (48.2 percent).
TABLE 3.
Characteristics of participants enrolled in the Education and Research Towards Health (EARTH) Study through January 2006
| Everyone |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Center | ||||||
| Alaska | 2,459 | 22.1 | 931 | 21.1 | 1,528 | 22.7 |
| Navajo | 4,331 | 38.9 | 1,535 | 34.8 | 2,796 | 41.5 |
| Plains/Arizona | 4,352 | 39.0 | 1,945 | 44.1 | 2,407 | 35.8 |
| Age (years)* (mean (SD†)) | 40.2 (14.2) | 39.5 (14.1) | 40.6 (14.2) | |||
| Household size (mean (SD)) | 4.2 (2.3) | 4.0 (2.4) | 4.3 (2.3) | |||
| Educational level‡ | ||||||
| <High school | 3,134 | 28.6 | 1,342 | 30.9 | 1,792 | 27.0 |
| High school | 3,761 | 34.3 | 1,710 | 39.4 | 2,051 | 30.9 |
| Vocational/technical/associate’s degree/college | 3,591 | 32.7 | 1,178 | 27.1 | 2,413 | 36.4 |
| Bachelor’s/master’s/doctoral degree | 482 | 4.4 | 110 | 2.5 | 372 | 5.6 |
| Currently smoking cigarettes§ | 2,621 | 23.9 | 1,248 | 28.7 | 1,373 | 21.7 |
| Using any tobacco products | 3,445 | 31.4 | 1,661 | 38.2 | 1,766 | 26.6 |
| Body mass index (kg/m2) (mean (SD)) | 30.3 (6.5) | 29.0 (5.9) | 31.1 (6.7) | |||
| Parity¶ (mean (SD)) | 3.4 (2.7) | NA† | 3.4 (2.7) | |||
| Any medical condition (no.)# | ||||||
| 0 | 4,326 | 39.1 | 2,023 | 46.2 | 2,303 | 34.5 |
| 1 | 2,751 | 24.9 | 1,038 | 23.7 | 1,713 | 25.7 |
| ≥2 | 3,973 | 36.0 | 1,315 | 30.1 | 2,658 | 39.8 |
| Overall health** | ||||||
| Excellent/very good | 3,479 | 31.6 | 1,503 | 34.5 | 1,976 | 29.7 |
| Good | 4,422 | 40.2 | 1,716 | 39.3 | 2,706 | 40.7 |
| Fair/poor | 3,110 | 28.2 | 1,142 | 26.2 | 1,968 | 29.6 |
| Family history of cancer†† | 2,498 | 38.4 | 661 | 32.1 | 1,837 | 41.3 |
| Family history of heart disease‡‡ | 2,783 | 47.9 | 842 | 45.9 | 1,941 | 48.8 |
| Family history of diabetes§§ | 4,524 | 74.8 | 1,359 | 71.3 | 3,165 | 76.4 |
Values for means are limited to Alaska Native and Navajo people.
SD, standard deviation; NA, not applicable.
Missing values for education: 28 Alaska, 36 Navajo, 110 Plains/Arizona.
Missing values for refusing to answer or did not know about smoking: 1 Alaska, 5 Navajo, 81 Plains/Arizona.
Restricted to women who had ever been pregnant.
Missing values for medical conditions: 4 Alaska, 81 Navajo, 18 Plains/Arizona.
Missing values for overall health: 3 Alaska, 74 Navajo, 54 Plains/Arizona.
Missing values (includes preferred not to answer and unknown family history) for family history of cancer: 865 Alaska, 1,877 Navajo, 1,827 Plains/Arizona. Includes individuals with any reported family history of cancer, heart disease, or diabetes.
Missing values for family history of heart disease: 1,137 Alaska, 2,060 Navajo, 2,060 Plains/Arizona. Includes individuals with any reported family history of cancer, heart disease, or diabetes.
Missing values for family history of diabetes: 1,132 Alaska, 1,995 Navajo, 1,895 Plains/Arizona. Includes individuals with any reported family history of cancer, heart disease, or diabetes.
Feedback information is summarized in table 4, which shows that 9.1 percent were given a routine referral for cholesterol follow-up and 1.2 percent a referral for urgent cholesterol follow-up. Of participants, 50.1 percent were given a statement that they should reduce their weight (body mass index ≥30 kg/m2). Among women, 73 percent met the recommendations for Papanicolaou screening, and 51.6 percent met the recommendations for mammography. Of people older than age 50 years, 79.5 percent were told that they should have colonoscopy or sigmoidoscopy screening. In terms of safety behaviors, 54.9 percent used safety belts when driving, 84.1 percent did not drink alcohol and drive, and 20.2 percent wore helmets when bicycling or riding an all-terrain vehicle.
TABLE 4.
Summary of feedback provided to the first 11,142 participants in the Education and Research Towards Health (EARTH) Study
| No. | % | |
|---|---|---|
| Cholesterol level (mg/dl) | ||
| ≤200 | 7,103 | 65.0 |
| 201–239 | 2,701 | 24.7 |
| 240–299 | 998 | 9.1 |
| ≥300 | 132 | 1.2 |
| Body mass index (kg/m2) | ||
| <25 | 2,099 | 19.3 |
| 25–<30 | 3,326 | 30.6 |
| ≥30 | 5,438 | 50.1 |
| Papanicolaou test in the past 3 years (women) | ||
| Yes | 4,037 | 73.0 |
| No | 1,490 | 27.0 |
| Mammogram in the past 2 years (women aged ≥40 years) | ||
| Yes | 1,504 | 51.6 |
| No | 1,409 | 48.4 |
| Colonoscopy/sigmoidoscopy in the past 5 years (age ≥50 years) | ||
| Yes | 489 | 20.5 |
| No | 1,898 | 79.5 |
| Cigarette smoking | ||
| Never | 6,586 | 60.0 |
| Former | 1,775 | 16.2 |
| Current | 2,621 | 23.9 |
| Alcohol use | ||
| No or infrequent | 4,515 | 50.6 |
| Binge drinking in the past year | 4,415 | 49.4 |
| Always use safety belt | ||
| Yes | 5,980 | 54.9 |
| No | 4,911 | 45.1 |
| Always wear a helmet | ||
| Yes | 851 | 20.2 |
| No | 3,358 | 79.8 |
| Drink alcohol and drive | ||
| Yes | 1,740 | 15.9 |
| No | 9,178 | 84.1 |
DISCUSSION
Close communication and trust between tribal members and study investigators were key to the development and implementation of this study. Partnerships with tribes were established before the study was funded, and a national Tribal Advisory Board was convened after the project was funded. In addition to reviews by appropriate institutional review boards, all study communities reviewed and approved the project through the appropriate village, tribal, or chapter authority.
The methods developed for this study allowed collection of data in rural, remote settings as well as more urbanized areas. Use of Scientific Advisory Board questionnaires required fewer staff than would have been necessary with interviewer-administered questionnaires. Staff were recruited from participating communities. The fact that the most effective method of recruitment was word of mouth is further evidence of study acceptance by the population.
The EARTH cohort is unique in that, in addition to collecting information from study participants, health information was provided to study participants immediately at the conclusion of their study visit. Researchers working with AIAN populations have been criticized for enrolling participants, asking questions and taking biologic samples, but not providing feedback to communities and participants. Providing immediate feedback to participants helps to address this issue. Our approach to participant feedback focused on positive health behaviors reported by participants and on additional ways they could improve their health. This approach makes the study visit a positive experience, again confirmed by the fact that the majority of people participating in the study did so after hearing about the study by word of mouth. Research findings also are provided to communities and tribal organizations.
In conclusion, this study was developed in collaboration with tribal organizations and has been well accepted. AIAN people were included not only as participants but also as part of the study team. Standardized instruments were developed that can be used by other tribes in the United States. Audio computer-assisted interviews provided confidentiality in small communities and a way to use standardized questionnaires with multiple languages. The use of computers and the length of the baseline visit were acceptable to the participants. Participants appreciated the immediate report of their health status. Reports of aggregated data are being given to individual communities and tribal health organizations. It is hoped that these data will provide information to focus health promotion and disease prevention efforts and thus improve the health status in the AIAN populations.
ACKNOWLEDGMENTS
This study was funded by grants CA88958, CA89139, and CA96095 from the National Cancer Institute. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute.
The authors acknowledge the contributions and support of the Navajo Nation, Indian Health Service, Alaska Native Tribal Health Consortium Board of Directors, South Central Foundation, Southeast Alaska Regional Health Consortium, Yukon-Kuskokwim Health Corporation, Cheyenne River Sioux Tribe, Oglala Sioux Tribe, Gila River Indian Community, and Fort Defiance and Shiprock Health Boards. They also acknowledge Dr. Franklin Freeland; Dr. Ruth Etzel; Dr. Joseph Klejka; Kari Lundgren; Dr. Cindy Schraer; Tribal Advisory Board members, including Beverley Pigman, George Ridley, Ileen Sylvester, Tim Gilbert, Fritz George, Terry Pourier, and Jayme Longbrake; the staff of the Navajo Nation, including Clarina Clark, Amy Rogers, Carmen George, and Wesley George; the many health data analysts; the staff in Alaska, including Jennifer Johnson, Diana Redwood, Katie Rose Hulett, Sharon Lindley, Cheri Hample, Maybelle Filler, Antoinelle Thompson, and Jayleen Wheeler; and the staff associated with the Black Hills Center, including Joyce Colombe, Marcia O’Leary, Betty Jarvis, Dr. Patricia Nez Henderson, Dr. Marie Russell, Kurt Schweigman, Bert Lewis, Wendy Lawrence, Daniel Kougl, Marie Gross, Jay Kunf, Lauri Bickle, Francine Red Willow, Arie Shiroma, Lois Bettlyoun, Mary Merrivale, and Lillian Brown. They also thank James Bryner and Kelly Cunningham for computer programming and KheNi Ma for statistical analyses; Omron Health Care, Inc., who provided the Omron Hem 907 to the study at a reduced cost; and Sharlane Donaldson of Alaska Scientific, Inc., who assisted with development of the Cholestech protocol and staff training.
Abbreviations
- AIAN
American Indian and Alaska Native
- EARTH
Education and Research Towards Health
- SCAPES
Study Computer Assisted Participant Evaluation System.
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Rhoades ER. American Indians and Alaska Natives—overview of the population. Public Health Rep. 1996;111 suppl 2:49–50. [PMC free article] [PubMed]
- 2.US Indian Health Service, Office of Planning, Evaluation and Legislation, Division of Program Statistics. Rockville, MD: US Indian Health Service; 1996 trends in Indian health. 1997
- 3.US Indian Health Service, Office of Planning, Evaluation and Legislation, Division of Program Statistics. Rockville, MD: US Indian Health Service; Regional differences in Indian health. 1997
- 4.Fox CS, Evans JC, Larson MG, et al. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 8.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 9.Horn-Ross PL, Hoggatt KJ, West DW, et al. Recent diet and breast cancer risk: the California Teachers Study (USA) Cancer Causes Control. 2002;13:407–415. doi: 10.1023/a:1015786030864. [DOI] [PubMed] [Google Scholar]
- 10.Alavanja MC, Sandler DP, Lynch CF, et al. Cancer incidence in the agricultural health study. Scand J Work Environ Health. 2005;31 suppl 1:39–45. discussion 5–7. [PubMed] [Google Scholar]
- 11.Mills PK, Beeson WL, Phillips RL, et al. Prospective study of exogenous hormone use and breast cancer in Seventh-day Adventists. Cancer. 1989;64:591–597. doi: 10.1002/1097-0142(19890801)64:3<591::aid-cncr2820640305>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 13.Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 14.Satia-Abouta J, Patterson RE, King IB, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the Vitamins and Lifestyle Study. Am J Epidemiol. 2003;157:944–954. doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HW, Hailpern SM, Fang J, et al. Sodium intake and mortality in the NHANES II follow-up study. Am J Med. 2006;119(275):e7–e14. doi: 10.1016/j.amjmed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Galanis DJ, Kolonel LN, Lee J, et al. Anthropometric predictors of breast cancer incidence and survival in a multi-ethnic cohort of female residents of Hawaii, United States. Cancer Causes Control. 1998;9:217–224. doi: 10.1023/a:1008842613331. [DOI] [PubMed] [Google Scholar]
- 17.Russell C, Palmer JR, Adams-Campbell LL, et al. Follow-up of a large cohort of Black women. Am J Epidemiol. 2001;154:845–853. doi: 10.1093/aje/154.9.845. [DOI] [PubMed] [Google Scholar]
- 18.Signorello LB, Hargreaves MK, Steinwandel MD, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–979. [PMC free article] [PubMed] [Google Scholar]
- 19.Stoddart ML, Jarvis B, Blake B, et al. Recruitment of American Indians in epidemiologic research: the Strong Heart Study. Am Indian Alsk Native Ment Health Res. 2000;9:20–37. doi: 10.5820/aian.0903.2000.20. [DOI] [PubMed] [Google Scholar]
- 20.White WB, Anwar YA. Evaluation of the overall efficiency of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit. 2001;6:107–110. doi: 10.1097/00126097-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Cobbaert C, Boerma GJ, Lindemans J. Evaluation of the Cholestech L.D.X. desktop analyser for cholesterol, HDL-cholesterol, and triacylglycerols in heparinized venous blood. Eur J Clin Chem Clin Biochem. 1994;32:391–394. [PubMed] [Google Scholar]
- 22.National Institutes of Health, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program. Bethesda, MD: National Institutes of Health; The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. 1997 (NIH publication no. 98-4080) [PubMed]
- 23.National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung and Blood Institute; Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2001
- 24.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26:33S–50S. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 26.US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; Guide to clinical preventive services 2001–2004. (3rd ed.) ( http://www.ahrq.gov/clinic/gcpspu.htm)
- 27.National Institutes of Health, National Heart, Lung, and Blood Institute with the National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. 1998 (NIH publication no. 98-4083)
- 28.US Department of Health and Human Services. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Physical activity and health: a report of the Surgeon General. 1996
- 29.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–1112. [PubMed] [Google Scholar]
- 30.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 32.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 33.Remington PL, Smith MY, Williamson DF, et al. Design, characteristics, and usefulness of state-based behavioral risk factor surveillance: 1981–7. Public Health Rep. 1988;103:366–375. [PMC free article] [PubMed] [Google Scholar]
- 34.Saremi A, Hanson RL, Williams DE, et al. Validity of the CAGE questionnaire in an American Indian population. J Stud Alcohol. 2001;62:294–300. doi: 10.15288/jsa.2001.62.294. [DOI] [PubMed] [Google Scholar]
- 35.Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; Behavioral Risk Factor Surveillance System Survey Questionnaire.