Abstract
Objectives
We describe the histopathology of ossicular grafts and implants so as to provide insight into factors that may influence functional results after surgery for chronic otitis media.
Methods
Histopathologic observations were made on 56 cases: 50 surgical specimens and 6 temporal bone cases in which the graft was sectioned in situ.
Results and Conclusions
Autogenous malleus, incus, and cortical bone grafts behaved in a similar manner and maintained their morphological size, shape, and contour for extended periods of time, at least up to 30 years. These histopathologic observations support the continued use of autograft ossicular and cortical bone grafts for middle ear reconstruction. Cartilage grafts developed chondromalacia with resulting loss of stiffness and showed a tendency to undergo resorption. Synthetic prostheses made of porous plastic (Plastipore, Polycel) elicited foreign body giant cell reactions with various degrees of biodegradation of the implants. Prostheses made of hydroxyapatite and Bioglass were enveloped by a lining of connective tissue and mucosal epithelium. The Bioglass material was broken down into small fragments and partially resorbed by a host response within the middle ear. These results warrant caution in the use of prostheses made of porous plastic or Bioglass.
Keywords: histopathology, implant, middle ear, ossicular graft
INTRODUCTION
The main goals of tympanomastoid surgery for chronic otitis media (COM) are eradication of disease, prevention of recurrence, and improvement of hearing. Tympanomastoid surgery for COM has a high success rate of 80% to 90% in controlling infection.1 However, functional hearing results continue to be modest, especially when the ossicular chain has to be reconstructed. For example, long-term closure of the air-bone gap to 20 dB or less occurs in 40% to 70% of cases when the incus is missing and in only 35% to 60% when both the incus and stapes superstructure are missing.2
A large number of grafts and prostheses have been described for use in the middle ear (ME) to reconstruct the ossicular chain. These include autograft and homograft struts, as well as a wide variety of synthetic ossicular replacement prostheses (ORPs). Ossicular grafts and prostheses are unique in many ways compared with implants placed elsewhere in the body. Ossicular implants must couple well at their ends to bone (stapes or manubrium) or to soft tissue (tympanic membrane [TM] or fascial graft), but must remain suspended in air elsewhere to avoid unwanted ankylosis (eg, to the promontory or facial nerve canal). They must maintain their shape, size, and acoustic transmission properties over long periods of time — ideally, several decades. The recipient ME milieu in COM is hostile as a result of active or arrested inflammatory disease or negative static pressure in the ME, both of which can predispose an implant to undergo resorption or extrusion. Finally, homograft and synthetic ossicular implants are potentially subject to immune-mediated rejection.
Histopathologic study of ossicular implants can provide insight into some of the factors that determine success after ossicular reconstruction. Two previous reports from our laboratory have described the histopathology of ossicular grafts and implants. The first report, in 1985, described the pathologic findings in 25 surgical specimens removed at the time of revision surgery.3 The second report, in 1994, described an additional 3 cases, including 1 postmortem temporal bone specimen in which a cartilage graft was sectioned in situ.4 Since the 1994 report, we have studied an additional 28 cases, including 5 ears in which the temporal bone specimen was removed after death and the ossicular graft was sectioned in situ. The availability of in situ cases is of value as compared to surgical specimens, because the in situ cases are not biased toward ears with recurrent disease or failed implants. One can also assess the coupling of an implant to the TM and to the other ossicles, as well as study histopathologic reactions at the tissue-implant interface. The present report describes our observations in all 56 cases contained in our temporal bone collection.
MATERIALS AND METHODS
The material available for the present study consisted of 50 surgical specimens and 6 cases in which the graft was sectioned in situ. The 56 cases included 24 malleus or incus grafts, 7 cortical bone grafts, 8 cartilage grafts, and 17 synthetic prostheses. All specimens were prepared for histopathologic study for light microscopy in the standard manner, including fixation in 10% formalin, decalcification with ethylenediaminetetraacetic acid or trichloracetic acid, embedment in celloidin, serial sectioning at a thickness of 20 μm, and staining of every 5th or 10th section with hematoxylin and eosin.5
Implants made of calcium salts and phosphate (hydroxyapatite [HA] and Bioglass) are difficult to study by the standard technique, because the process of decalcification dissolves the implant. To overcome this problem, we placed implants made of HA and Bioglass within a small amount of brain tissue and then subjected them to decalcification, embedding, and staining. The surrounding brain tissue “held” the implants in place, permitting us to study the host response in relation to the implant.
Clinical data gathered in each case included the age of the patient, the duration of implantation, and the indications for revision surgery in the case of surgical specimens. Histologic sections were examined under bright field and polarized light. In the case of incus and malleus grafts, all stained serial sections of each graft were assessed to arrive at approximate quantitative estimates of the amount of viable bone, the degree of revascularization, and the amount of fibrous tissue replacement of the graft. Viable bone was identified on the basis of the presence of osteocytes (cell bodies and nuclei) within the lacunae.3 In each section, the amount of viable bone in comparison to the total bone was estimated by visual inspection and stratified into bins (less than 25%, 25% to 50%, 50% to 75%, and more than 75%). The estimates were averaged across all sections examined. Similarly, the vascularity of the graft was determined by examining each section and estimating the percentage of haversian canals that contained blood vessels, followed by averaging across all sections through the graft. A similar analysis was done for fibrous tissue replacement of the graft, which was judged as the percentage of the graft that consisted of connective tissue rather than bone. The presence of osteitis was also noted, defined as resorption of bone and infiltration of bone by inflammatory cells.3
RESULTS
INCUS AND MALLEUS GRAFTS
Surgical Specimens
We examined 21 ossicular grafts that had been sculpted from the incus or malleus (Table 1). One of these was a homograft (case 1), and all the others were autografts. The duration of implantation ranged from 5 months to 25 years. The age of the patients at the time of surgery varied from 7 to 62 years. The indications for revision procedures included recurrent or persistent conductive hearing loss, as well as recurrent COM.
TABLE 1.
INCUS AND MALLEUS GRAFTS
| Case | Patient Age (y) | Implant Time | Graft Type | Indications for Revision Surgery | Viable Bone (% Replacement of Graft)* | Vascularity of Graft (%)† | Fibrous Replacement of Graft (%) | Osteitis of Graft |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 2 y 4 mo | I/H | Slipped strut, M ankylosis | <5 | 5–25 | 25–50 | Mild |
| 2 | 38 | 5 mo | M/A | Slipped strut | 5–25 | <5 | 25–50 | None |
| 3 | 20 | 1 y 6 mo | I/A | Slipped strut, perforation | <5 | <5 | 25–50 | Mild |
| 4 | 52 | 11 y | M/A | Stapes fixation | <5 | <5 | 5–25 | None |
| 5 | 16 | 6 y | I/A | Stapes fixation | 50–75 | 50–75 | <5 | None |
| 6 | 13 | 2 y | I/A | Recurrent cholesteatoma | <5 | 5–25 | 50–75 | Severe |
| 7 | 34 | 12 mo | I/A | Graft lateralization, active COM | 25–50 | 50–75 | 50–75 | Mild |
| 8 | 7 | 1 y 10 mo | I/A | Slipped strut, perforation | 5–25 | <5 | 5–25 | None |
| 9 | 13 | 12 mo | M+I/A | Middle ear atelectasis, fibrosis | 5–25 | 25–50 | 5–25 | None |
| 10 | 32 | 9 y 4 mo | I/A | Slipped strut, fibrosis | 25–50 | 25–50 | 5–25 | None |
| 11 | 32 | 9 y 4 mo | I/A | Slipped strut | 25–50 | >75 | 5–25 | None |
| 12 | 22 | 5 y 10 mo | I/A | Slipped strut | 5–25 | 5–25 | 5–25 | None |
| 13 | 62 | 9 mo | I/A | Active COM | 5–25 | 50–75 | 25–50 | Mild |
| 14 | 44 | 20 y | I/A | Active COM | 25–50 | 50–75 | 25–50 | None |
| 15 | 48 | 8 y | I/A | Recurrent cholesteatoma | 5–25 | 50–75 | 50–75 | Moderate |
| 16 | 60 | 5 y | I/A | Active COM | 25–50 | 50–75 | 5–25 | None |
| 17 | 46 | 2 y | I/A | EAC stenosis, graft atelectasis | 25–50 | 25–50 | <5 | None |
| 18 | 45 | 11 y | I/A | Active COM | <5 | 5–25 | 5–25 | None |
| 19 | 52 | 25 y | I/A | Slipped strut, recurrent cholesteatoma | <5 | 5–25 | 25–50 | Mild |
| 20 | 29 | 9 y | I/A | Recurrent cholesteatoma | <5 | 5–25 | 25–50 | Moderate |
| 21 | 52 | 24 y | I/A | Slipped strut | 25–50 | 25–50 | <5 | None |
| 22 | 62 | 12 y | I/A | Temporal bone specimen (in situ) | 25–50 | 25–50 | 25–50 | None |
| 23 | 87 | 26 y | M/A | Temporal bone specimen (in situ) | 5–25 | 5–25 | 25–50 | Mild |
| 24 | 87 | 28 y | I/A | Temporal bone specimen (in situ) | 5–25 | 25–50 | 5–25 | None |
I — incus; H — homograft; M — malleus; A — autograft; COM — chronic otitis media; EAC — external auditory canal.
Percentage of lacunae filled with osteocytes.
Percentage of haversian canals that showed blood vessels.
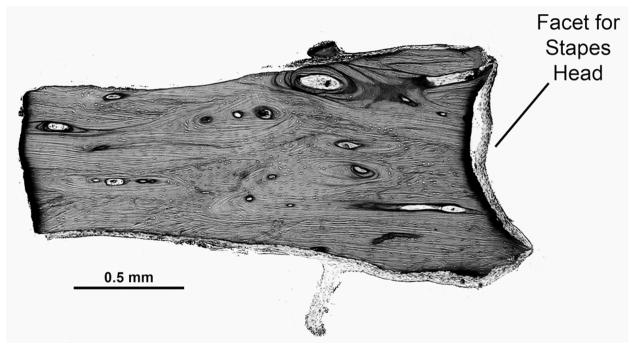
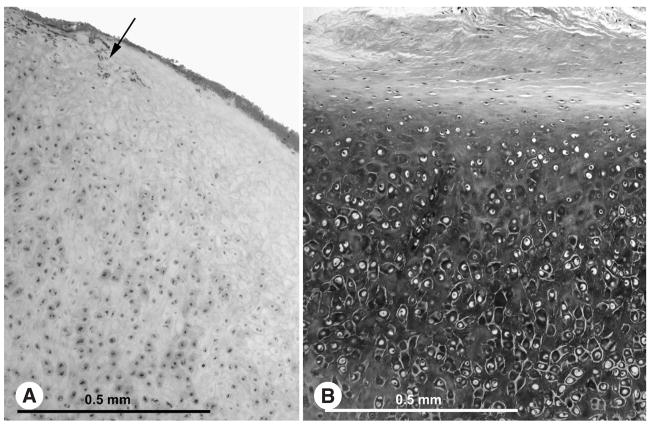
These ossicular grafts were found to be covered by a mucosal lining of flat or cuboidal epithelial cells. The grafts consisted of various amounts of viable bone characterized by the presence of osteocytes within lacunae (Figs 1 and 2). Such viable bone was typically observed surrounding revascularized haversian canals within the core of the ossicle and at its periphery. Some grafts contained virtually no viable bone (less than 5%), whereas other specimens showed viable bone in 50% or more of the graft. Examination under polarized light showed that areas of viable and nonviable bone maintained the lamellar arrangement of collagen fibers. There was an approximate correlation between the degree of revascularization of the graft and the amount of viable bone. There was no correlation between the duration of implantation and the amount of viable bone. It is noteworthy that the original shape and size of the ossicle strut was maintained, even in those cases in which more than 50% of the strut had viable bone (Fig 1).
Fig 1.

(Case 17) Incus autograft implanted for 2 years. Nearly complete remodeling of graft is seen in this particular section, as evidenced by presence of osteocytes throughout graft. Note that overall shape and size of strut has remained unchanged despite extensive remodeling.
Fig 2.
(Case 18) Incus autograft implanted for 11 years. There are very few osteocytes within bone strut.
Many grafts showed small areas of fibrous replacement by expansion of narrow spaces that did not alter the shape, size, or integrity of the graft. Some grafts showed more extensive areas of resorption and replacement by connective tissue associated with osteitis resulting from suppuration within the ME or cholesteatoma. Some grafts also showed an intense basophilic staining area in which a surgical drill had been used to sculpt the graft (Fig 3). We believe that this represents thermal trauma that can lead to necrosis and resorption of the graft in some cases. The single homograft ossicle in our series showed preservation of its architecture, with minimal new bone formation and no evidence of immune-mediated rejection.
Fig 3.
(Case 3) Incus autograft implanted for 1 year 6 months. There is intense staining of area in which drill had been used to create facet for stapes head. This presumably resulted from thermal injury. In another section (not shown), this area of devitalized bone had undergone full-thickness resorption with replacement by fibrous tissue.
Comment
We found that autograft incus and malleus struts maintained their contour, size, shape, and physical integrity for long periods of time, spanning at least 25 years. After implantation, it is assumed that such grafts become nonviable because of loss of blood supply, which is characterized histologically by empty haversian canals devoid of blood vessels.3,6–8 The fate of osteocytes is not entirely known, but the evidence indicates that loss of blood supply leads to apoptosis and dropout of the majority of osteocytes contained in the graft.7 The autograft undergoes remodeling of bone surrounding revascularized haversian canals at a very slow pace, termed “creeping substitution.”3,7 The factors that determine the rate of creeping substitution in ossicular grafts are unknown at the present time.
The histologic changes observed in our specimens are similar to those reported by other investigators studying ossicular grafts,9–15 and are also similar to those seen in bone autografts used in orthopedic and maxillofacial surgery.6–8 Neo-osteogenesis and remodeling of an ossicular autograft are not necessary from a functional standpoint. Grafts that predominantly consist of nonviable bone appear to maintain their morphological structure and integrity and transmit sound just as well as those consisting of viable bone. In this respect, they differ from bone grafts used in orthopedic surgery, in which weight-bearing stresses make nonviable bone grafts prone to fatigue fractures. However, both nonviable and viable ME grafts are subject to resorption by osteitis when there is recurrent ME suppuration, similar to that observed in ossicles in COM. They are also subject to resorption when thermal trauma is imparted during the sculpturing process. The generous use of irrigation is recommended to avoid such trauma.
In Situ Specimens
There were 3 ears that underwent ossiculoplasty during life for COM (Table 1; cases 22 to 24). These 3 ears included 2 incus grafts and 1 malleus graft. The ages of the patients at death ranged from 62 to 87 years. The duration of implantation ranged from 12 to 28 years. In all 3 ears the ossicular grafts were invested by a mucosal epithelium, and they had maintained their shape, size, and contour (Fig 4). They showed various amounts of viable bone, characterized by the presence of osteocytes and blood vessels within haversian canals. The histologic appearance of these grafts was similar to that observed in the surgical specimens.
Fig 4.
(Case 24) In situ temporal bone specimen. Incus autograft placed 28 years before death. A) Low-power view shows graft to be in good position between stapes and reconstructed tympanic membrane. B) Higher-power view of graft, same section as in A. Graft has maintained its shape and is covered by healthy middle ear mucosa. There are no areas of active bone resorption or new bone formation. New blood vessels are evident in its core. Notch drilled for stapes head is at some distance from stapes in this section. There is better alignment between stapes head and notch in other sections (not shown).
The availability of these in situ specimens allowed histologic examination at the interface between the grafts and surrounding ossicles and other structures. There was often bony fusion between the ossicle strut and an adjoining ossicle, such as the manubrium or lenticular process (Fig 5). Bony ankylosis was also observed at points of contact between the ossicle strut and bone of the fallopian canal or promontory. In the 3 in situ cases there were 8 points of contact between the ossicle strut and either another ossicle or the bony wall of the tympanic space. Bony fusion occurred at 5 of the 8 points of contact.
Fig 5.
(Case 23) In situ temporal bone specimen. Autograft was shaped from head of malleus and placed onto stapes head 26 years before death. There is bony fusion between autograft and manubrium. Tympanic membrane (TM) is retracted onto autograft.
Comment
In general, the histologic changes seen in ossicular struts in the in situ cases were similar to those in the surgical specimens. Because the in situ cases were not biased toward ears with recurrent disease or failed implants, we can conclude that our observations made on surgical specimens, as well as those of other investigators, are valid. The in situ cases showed a propensity for ossicular struts to undergo ankylosis if the strut came into contact with a bony surface. Such bony fusion is probably desirable when the strut is in contact with another ossicle such as the malleus or stapes. On the other hand, the strut can also undergo ankylosis to the fallopian canal, promontory, etc, with resulting compromise in sound transmission through the ME. Thus, it is important for the otologic surgeon to reduce the size of autograft struts by sculpturing before implantation.
CORTICAL BONE GRAFTS
Seven cortical bone autografts were removed after implantation times of 7 months to 10 years from patients 8 to 62 years of age (Table 2). Revision surgery was performed in 4 cases for conductive hearing loss and in 3 for recurrent cholesteatoma. Six of the 7 grafts maintained their shape, size, and integrity, with a healthy covering of mucosa and various amounts of viable bone containing osteocytes and blood vessels (Fig 6). The overall amount of viable bone was less than that seen in incus and malleus autografts. One case (case 31) showed extensive osteitis and resorption as a result of recurrent cholesteatoma.
TABLE 2.
CORTICAL BONE GRAFTS
| Case | Patient Age (y) | Implant Time | Indications for Revision Surgery | Viable Bone(% Replacement of Graft)* | Vascularity of Graft (%)† | Fibrous Replacement of Graft (%) | Osteitis of Graft |
|---|---|---|---|---|---|---|---|
| 25 | 17 | 9 mo | Slipped strut, fixed malleus | <5 | 5–25 | <5 | None |
| 26 | 51 | 10 y 7 mo | Slipped strut, recurrent cholesteatoma | <5 | 5–25 | <5 | None |
| 27 | 22 | 8 mo | Slipped strut | <5 | 25–50 | 5–25 | None |
| 28 | 8 | 7 mo | Slipped strut, recurrent cholesteatoma | 5–25 | 50–75 | 5–25 | None |
| 29 | 20 | 7 mo | Fixation to oval window | 5–25 | 50–75 | 5–25 | None |
| 30 | 22 | 5 y 10 mo | Slipped strut, lateralized graft | 25–50 | 50–75 | 25–50 | Mild |
| 31 | 62 | 9 mo | Recurrent cholesteatoma | >75 | >75 | 50–75 | Severe |
Percentage of lacunae filled with osteocytes.
Percentage of haversian canals that showed blood vessels.
Fig 6.
(Case 25) Autograft cortical bone strut implanted for 9 months. Graft has maintained its shape, size, and contour and is invested by thin covering of mucosa. Very few osteocytes are evident within graft.
Comment
Autogenous cortical bone struts had a histologic fate similar to that of autograft ossicular bone grafts. They maintained their shape, size, and contour and did not show exuberant new bone formation or excessive bone resorption. Thus, autogenous cortical bone struts are useful and viable alternatives for ossiculoplasty. Similar findings and conclusions have been reported by other investigators.15–17
CARTILAGE GRAFTS
We examined 8 cartilage grafts: 7 surgical specimens and 1 in situ graft (Table 3). The 7 surgical specimens included 6 conchal cartilage autografts (2 placed as ossicular struts and 4 introduced as buffers between a total ORP [TORP] and the TM) and 1 septal cartilage homograft strut placed as an ossicular strut. The cartilage grafts had been in place for 9 months to 14 years, and the patient ages ranged from 12 to 62 years. The grafts were removed at revision surgery because of recurrent cholesteatoma in 4 cases, conductive hearing loss in 2 cases, and active COM in 1 case. On gross examination prior to fixation in formalin, all of the cartilage grafts from surgical specimens felt rubbery and appeared to have lost some rigidity. On light microscopy, all specimens showed characteristic features of chondromalacia, including pyknosis of cell nuclei, dropout of chondrocytes, and myxoid changes of the ground substance (Fig 7). Blood vessels and inflammatory cells were often seen to invade the periphery of these grafts, signifying vascularization and chondritis. The degree of chondromalacia varied from mild to severe, with the most severe change observed in the specimens that had been in place the longest (12 and 14 years; cases 39 and 38, respectively). The histologic changes were similar in both autograft and homograft specimens.
TABLE 3.
CARTILAGE GRAFTS
| Case | Patient Age (y) | Implant Time | Graft Type | Indications for Revision of Surgery | Use of Graft | Chondromalacia of Graft |
|---|---|---|---|---|---|---|
| 32 | 56 | 1 y | Conchal cartilage (A) | Fascial graft lateralization, fibrosis | Buffer | Moderate |
| 33 | 12 | 4 y | Conchal cartilage (A) | Recurrent cholesteatoma | Strut | Moderate |
| 34 | 18 | 6 y | Conchal cartilage (A) | Recurrent cholesteatoma, perforation | Strut | Mild |
| 35 | 20 | 3 y 5 mo | Conchal cartilage (A) | Extruding TORP, atelectasis | Buffer | Mild |
| 36 | 22 | 9 mo | Septal cartilage (H) | Recurrent cholesteatoma, perforation, stapes fixation | Strut | Mild |
| 37 | 44 | 4 y 7 mo | Conchal cartilage (A) | Recurrent cholesteatoma | Buffer | Mild |
| 38 | 21 | 14 y | Conchal cartilage (A) | Active COM | Buffer | Severe |
| 39 | 62 | 12 y | Conchal cartilage (A) | Temporal bone specimen | Strut | Severe |
A — autograft; TORP — total ossicular replacement prosthesis; H — homograft; COM — chronic otitis media.
Fig 7.
A) (Case 35) Cartilage autograft implanted for 3 years 5 months as buffer between prosthesis and tympanic membrane shows chondromalacia evidenced by loss of chondrocytes, faint staining of matrix, and vascularization of graft (arrow). B) Normal cartilage shown for comparison. Matrix stains darkly in normal case.
The in situ case was that of a cartilage “boomerang” strut placed between the stapes footplate and the fascial graft 12 years before death at the time of a canal wall–up mastoidectomy with tympanoplasty for COM (Fig 8). A remnant of cartilage was seen embedded on the medial aspect of the fascial graft, and it showed widespread and severe chondromalacia. A small remnant of cartilage was seen at the stapes footplate, which was devoid of cells and embedded in fibrous tissue. The remainder of the strut, between the stapes footplate and the fascial graft, had undergone complete resorption. The ME and mastoid were well aerated, without effusion or cholesteatoma. The submucosa of the ME and mastoid showed scattered chronic inflammatory cells.
Fig 8.
(Case 39) In situ temporal bone specimen. L-shaped cartilage strut had been placed between tympanic membrane (TM) and stapes footplate 12 years before death. That part of strut between TM and footplate is missing because of resorption. Middle ear is healthy, without active inflammation or infection.
Comment
The evidence from our small series of cartilage grafts indicated that they underwent chondromalacia, characterized by ingrowth of blood vessels, dropout of chondrocytes, and degeneration of ground substance. This resulted in loss of stiffness and rigidity, and in some cases, it resulted in resorption of the cartilage. Similar findings have been published by other investigators.12,18–20 Vascularization of cartilage can also lead to calcification of the matrix, rather than resorption, and has been reported previously.12,13,19,21
Our observations from this small number of specimens suggest that cartilage struts are likely to be suboptimal for reconstruction of the sound transmission system, although they are probably satisfactory as buffers between a synthetic prosthesis and TM grafts or for reconstruction of surgical defects. Clinical experience has demonstrated that cartilage grafts used to reconstruct a TM or to serve as buffers over TORPs and partial ORPs (PORPs) can remain in contact with bony surfaces without inducing new bone formation and without adversely affecting sound transmission through the ME.1
SYNTHETIC PROSTHESES
We examined 17 synthetic PORPs and TORPs: 7 made of Plastipore (high-density polyethylene sponge), 2 made of HA (a calcium phosphate compound that has the same chemical composition as living bone), 2 made of Bioglass (similar to HA in its composition of calcium salts and phosphate, except that the phosphate is combined with silicon and sodium salts to create a glass), 2 made of polyethylene, and 1 made of Polycel (thermal-fused high-density polyethylene sponge). An additional 3 prostheses were composites, consisting of Plastipore and HA (2 cases) and Plastipore and Teflon (1 case; Table 4). The duration of implantation ranged from 2 months to 41 years. The ages of the patients ranged from 7 years to 91 years. Revision surgery was performed for conductive hearing loss in 10 cases, for cholesteatoma in 4 cases, and for active COM in 1 case.
TABLE 4.
SYNTHETIC PROSTHESES
| Case | Patient Age (y) | Implant Time | Type of ORP | Composition | Indications for Revision Surgery | Multinucleated Giant Cells* | Resorption of ORP & Replacement by Fibrous Tissue† |
|---|---|---|---|---|---|---|---|
| 40 | 53 | 21 y | TORP | Plastipore | Cholesteatoma in mastoid, displaced ORP | ++++ | Moderate to severe |
| 41 | 53 | 18 y | TORP | Plastipore | Displaced ORP | ++++ | Moderate |
| 42 | 37 | 17 y | PORP | Plastipore | Beginning extrusion | ++++ | Moderate |
| 43 | 43 | 14 y | TORP | Plastipore | Recurrent cholesteatoma | ++++ | Mild |
| 44 | 60 | 4 y 10 mo | TORP | Plastipore shaft, hydroxyapatite top | Displaced ORP, beginning extrusion | ++++ Plastipore, − hydroxyapatite | Minimal Plastipore, none hydroxyapatite |
| 45 | 10 | 4 y 4 mo | TORP | Plastipore | Beginning extrusion | ++++ | Mild to moderate |
| 46 | 20 | 3 y 5 mo | TORP | Plastipore shaft, hydroxyapatite top | Beginning extrusion, recurrent cholesteatoma | ++++ Plastipore, − hydroxyapatite | Minimal |
| 47 | 56 | 1 y | TORP | Plastipore top, Teflon shaft | Fascial graft lateralization, fibrosis | +++ Plastipore, + Teflon | Minimal Plastipore, none Teflon |
| 48 | 44 | 2 mo | PORP | Plastipore | Displaced ORP | ++++ | Minimal |
| 49 | 52 | 10 y 7 mo | TORP | Plastipore | Persistent AB gap | ++++ | Mild |
| 50 | 30 | 6 y 2 mo | TORP | Hydroxyapatite | Displaced ORP | − | None |
| 51 | 44 | 4 y 7 mo | TORP | Hydroxyapatite | Residual cholesteatoma | − | Minimal |
| 52 | 45 | 15 y | TORP | Bioglass | Persistent AB gap | − | Fragmented, partly resorbed |
| 53 | 21 | 14 y | TORP | Bioglass | AB gap, active COM | − | Fragmented, partly resorbed |
| 54 | 7 | 11 mo | TORP | Polycel | Persistent AB gap | +++ | Minimal |
| 55 | 91 | 41 y 8 mo | TORP | Polyethylene | Temporal bone specimen | − | Cannot assess |
| 56 | 85 | 32 y | PORP | Polyethylene | Temporal bone specimen | + | None |
ORP — ossicular replacement prosthesis; TORP — total ossicular replacement prosthesis; PORP — partial ossicular replacement prosthesis; AB — air-bone; COM — chronic otitis media.
– — None; + — occasional; ++ — cells in peripheral areas only; +++ — cells in majority of ORP; ++++ — cells throughout ORP or extreme cellular infiltration.
Minimal — occasional; mild — up to one third of total volume; moderate — one third to two thirds of total volume; severe — more than two thirds of total volume.
All prostheses showed an investing covering of mucosa, and some showed a capsule of fibrous tissue. Plastipore and Polycel are porous when examined by light microscopy (Figs 9 and 10). In both types of implants, the porous spaces were infiltrated by fibroblasts, round cells, and foreign body giant cells. The foreign body giant cell response was clearly visible in the specimen retrieved 2 months after implantation (case 48) and appeared to be most intense in the prosthesis that had been implanted the longest (case 40). Under polarized light, particulate matter was visible in the cytoplasm of foreign body giant cells in all cases, suggesting active resorption. In 4 cases, biodegradation of the prosthesis had progressed to a significant degree, with replacement of at least one third of the volume of the implant.
Fig 9.
(Case 43) Plastipore prosthesis implanted for 14 years. A) Dense capsule of fibrous tissue surrounds prosthesis. Outline of prosthesis is no longer circular because of partial resorption at its periphery. B) Higher-power view of implant with numerous foreign body giant cells that are causing microdegradation of Plastipore material.
Fig 10.
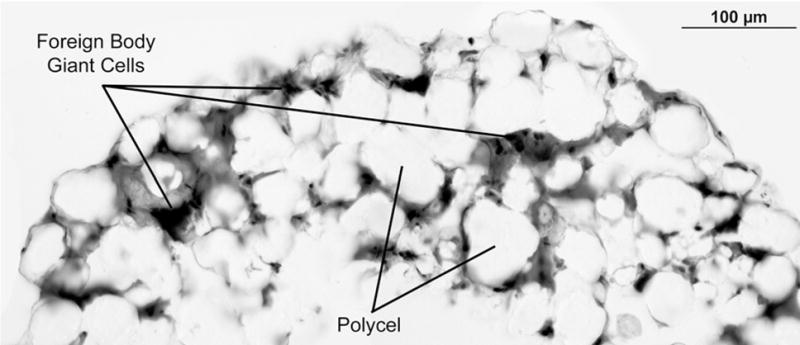
(Case 54) Polycel prosthesis implanted for 11 months. Material is porous, similar to Plastipore. There is foreign body giant cell response with microscopic biodegradation of Polycel.
In the case of HA and Bioglass, the prosthesis itself was dissolved by the process of decalcification. Inferences can be made on the basis of the histologic appearance of the surrounding tissue. In both cases of HA implants (cases 50 and 51), the prosthesis was surrounded by a thin capsule of fibrous tissue that consisted of several layers of fibroblasts and collagen fibers (Fig 11). Foreign body giant cells or inflammatory cells were not evident in this capsule. Between the capsule and the prosthesis, a few small linear areas of osteoid-like tissue with scattered calcification were observed in both cases. There was no apparent ingrowth of tissue into the HA per se.
Fig 11.
(Case 51) Hydroxyapatite prosthesis implanted for 4 years 7 months. Prosthesis is surrounded by capsule of healthy connective tissue without any significant inflammation or foreign body giant cells. Also seen is piece of cartilage used as buffer between prosthesis and tympanic membrane.
Both prostheses made of Bioglass had became fragmented in the ME, with fibroblastic connective tissue growing around and between the pieces of the implant (Fig 12). There were no obvious foreign body giant cells in this connective tissue. There were a few scattered histiocytic cells and round cells, suggesting a low level of chronic inflammation. Both prostheses had become partially resorbed.
Fig 12.
(Case 53) Bioglass prosthesis implanted for 14 years. Prosthesis has become fragmented. Host response consists predominantly of connective tissue with few scattered inflammatory cells.
The implants made of polyethylene (2 cases) and of Teflon (1 case) remained relatively intact. There were a few foreign body giant cells within the fibrous capsule, but no significant degradation of the implants.
Comment
Synthetic implants made of Plastipore and Polycel had a similar histopathologic appearance, consisting of a host foreign body giant cell and inflammatory response that was well established as early as 2 months and that continued for the duration of implantation. Microdegradation of the plastic material occurred and appeared to increase with time. Such a host response has also been reported by other investigators.13,22–27 The presence of this foreign body response is in contradistinction to some reports in the literature indicating that the porosity of the material would permit ingrowth of host tissue and enhance its biocompatibility.28,29 Some investigators have abandoned the use of Plastipore and similar plastic prostheses, predicting eventual implant dissolution and failure,24,25,30,31 whereas others have argued that the reaction remains at a microscopic level and that the ME can tolerate the material for long periods of time.13,22,26,29,32 Although the small number of cases in our series does not allow for a definite conclusion to be drawn, the intense foreign body reaction seen in all cases, with significant biodegradation in some cases, does warrant caution in the continued otologic use of implants made of porous plastic.
Although HA is a commonly used material for ossiculoplasty and thousands of such implants are used worldwide annually, information about the histopathologic response of the ear to HA is extremely sparse. Clinical experience suggests that HA is well tolerated in the ME and does not readily undergo biodegradation or dissolution.33,34 The 2 cases in our collection did not reveal a foreign body giant cell response, unlike Plastipore and Polycel. Van Blitterswijk and Grote35 reported on 4 HA prostheses removed 6 to 32 months after implantation. These prostheses were covered by epithelial and fibrous tissues. Light microscopy revealed small numbers of macrophages and multinucleated giant cells at the implant-tissue interface, with implant material in the cytoplasm of cells, indicating microscopic biodegradation. They judged that such degradation was enhanced by ME infection. They also stated that all 4 prostheses were intact on gross examination and that they believed the implants performed well even in the presence of ME infection.
Bioglass is similar to HA in its composition of calcium salts and phosphate, but it also contains silica. Both Bioglass prostheses in our study showed a host response that had resulted in partial resorption of the implant and its fragmentation into multiple small pieces.
DISCUSSION
The findings of the present study are similar in many respects to those described in the previous 2 reports from our laboratory.3,4 The present study differs from the earlier ones in 3 ways. First, we now have a greater number of specimens. Second, the present study included 6 cases acquired postmortem in which the implants were sectioned in situ; this practice removes bias toward ears with recurrent disease or failed implants, and also permits histopathologic assessment at the tissue-implant interface. Finally, the present study included synthetic implants made of Polycel, Bioglass, HA, and polyethylene, which were not described in the previous 2 reports.
The findings indicated that autogenous malleus, incus, and cortical bone grafts behave in a similar manner and retain their morphological size, shape, and contour for extended periods, at least up to 30 years and probably much longer. They do not incite formation of exuberant new bone, nor do they show excessive resorption of bone in the absence of infection. They demonstrate various amounts of replacement of nonviable bone by new bone through a slow process of creeping substitution. Sculpturing of such grafts should be done with adequate irrigation to avoid thermal injury and subsequent resorption. These grafts have a tendency to undergo ankylosis when they come into contact with another bony surface such as a neighboring ossicle or the wall of the tympanic cavity. Therefore, autografts should be reduced in size by sculpturing to avoid unwanted bony fusion to surrounding structures such as the facial canal or the promontory. These histologic observations support the continued use of autograft, ossicular, and cortical bone grafts for ME reconstruction. They would be especially valuable in children and young adults, in whom the grafts are expected to last for several decades.
Cartilage autograft and homograft implants show a tendency to develop chondromalacia with a resulting loss of stiffness, and some grafts tend to undergo resorption. These changes appear to intensify with time. Therefore, cartilage grafts are probably not ideal as struts for ME sound transmission, but are probably satisfactory as buffers between synthetic prostheses and the TM, and are also probably satisfactory for reconstructing the TM.
Synthetic prostheses made of porous high-density polyethylene (Plastipore and Polycel) elicit foreign body giant cell reactions with biodegradation of the implants. The biodegradation can be significant, resulting in partial dissolution of the implant in some cases. Implants made of Bioglass can fail because of a host response that can lead to resorption and fragmentation of the prostheses. These findings suggest that implants made of porous plastic and Bioglass may not be suitable for ossiculoplasty. The histologic fate of HA, which is a commonly used material in contemporary otology, needs further investigation. Our study contained only 2 specimens made of HA, both of which showed encapsulation of the implant by a layer of connective tissue without any significant foreign body reaction or inflammation.
Our histopathologic observations of human ME implants are based on a small number of specimens, as are all other similar reports in the literature, especially in comparison with the thousands of tympanoplasty operations performed annually by otolaryngologists worldwide. Much research still needs to be done on the histologic fate of materials such as HA and titanium that are in common use in contemporary otology. Animal studies provide useful information, but animal results cannot always be extrapolated to humans because of species differences as well as unique human factors such as eustachian tube dysfunction and the many variants of COM. Hence, the ultimate test of implant performance still remains the human ME. Research is also needed to better define the biological basis for extrusion of prostheses. Clinical experience has shown that extrusion is rare with autograft implants in comparison with synthetic prostheses.
The choice of what material to use as an ossicular graft or implant is determined by a number of factors, including the status of the diseased ossicular chain, the severity and type of infection, knowledge of the histopathology of ossicular grafts, the cost and availability of implants, and the personal preferences of the otologic surgeon. When performing ossicular reconstruction from the stapes head to the manubrium or TM (minor columella), our preference is to use autografts made of the incus, malleus, or cortical bone. On the other hand, if an ossicular reconstruction is from the footplate to the manubrium or TM (major columella), we will use a synthetic TORP, because we find it difficult to fashion an autograft bone strut with precision for such a reconstruction and because such struts have a risk of delayed ankylosis to the walls of the oval window niche.
The histopathologic observations presented in this study, as well as our clinical experience, lead us to conclude that there is probably a place for both biological and synthetic materials in ME surgery. The choice of implant material is an important contributor to the functional success of tympanoplasty. Other factors that also contribute to the success or failure of tympanoplasty include the skill of the otologic surgeon, the biomechanics of the reconstructed ossicular chain, and the pathological state of the ME.
Acknowledgments
The authors thank Diane Jones for the histologic preparations and Richard Cortese for photomicrography.
Supported by National Institutes of Health grant R01 DC04798 and by Axel Eliasen and Lakshmi Mittal.
References
- 1.Nadol JB Jr, McKenna MJ, editors. Surgery of the ear and temporal bone. 2. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 2.Merchant SN, Ravicz ME, Voss SE, Peake WT, Rosowski JJ. Middle ear mechanics in normal, diseased and reconstructed ear. J Laryngol Otol. 1998;112:715–31. doi: 10.1017/s0022215100141568. [DOI] [PubMed] [Google Scholar]
- 3.Schuknecht HF, Shi SR. Surgical pathology of middle ear implants. Laryngoscope. 1985;95:249–58. [PubMed] [Google Scholar]
- 4.Merchant SN, Nadol JB., Jr Histopathology of ossicular implants. Otolaryngol Clin North Am. 1994;27:813–33. [PubMed] [Google Scholar]
- 5.Schuknecht HF. Pathology of the ear. 2. Philadelphia, Pa: Lea & Febiger; 1993. [Google Scholar]
- 6.Albrektsson T. Repair of bone grafts. A vital microscopic and histological investigation in the rabbit. Scand J Plast Reconstr Surg. 1980;14:1–12. doi: 10.3109/02844318009105731. [DOI] [PubMed] [Google Scholar]
- 7.Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983 Apr;:28–42. [PubMed] [Google Scholar]
- 8.Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987 Dec;:7–16. [PubMed] [Google Scholar]
- 9.Hall A, Rytzner C. Vitality of autotransplanted ossicles. Acta Otolaryngol Suppl (Stockh) 1960;(suppl 158):335–40. doi: 10.3109/00016486009122442. [DOI] [PubMed] [Google Scholar]
- 10.Linthicum FH., Jr Postoperative temporal bone histopathology. Laryngoscope. 1966;76:1232–41. [Google Scholar]
- 11.Kerr AG, Smyth GDL. The fate of transplanted ossicles. J Laryngol Otol. 1971;85:337–47. doi: 10.1017/s0022215100073515. [DOI] [PubMed] [Google Scholar]
- 12.Smyth GDL, Kerr AG, Hassard TH. Homograft materials in tympanoplasty. Otolaryngol Clin North Am. 1977;10:563–80. [PubMed] [Google Scholar]
- 13.Belal A, Jr, Sanna M, Gamelotti R. Pathology as it relates to ear surgery. V Ossiculoplasty. J Laryngol Otol. 1984;98:229–40. doi: 10.1017/s0022215100146481. [DOI] [PubMed] [Google Scholar]
- 14.Lang J, Kerr AG, Smyth GDL. Long-term viability of transplanted ossicles. J Laryngol Otol. 1986;100:741–7. doi: 10.1017/s0022215100100027. [DOI] [PubMed] [Google Scholar]
- 15.Mills RP, Cree IA. Histological fate of cortical bone autografts in the middle ear. Clin Otolaryngol Allied Sci. 1995;20:365–7. doi: 10.1111/j.1365-2273.1995.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 16.Kylén P, Albrektsson T, Ekvall L, Hellkvist H, Tjellstrom A. Survival of the cortical bone columella in ear surgery. Acta Otolaryngol (Stockh) 1987;104:158–65. doi: 10.3109/00016488709109062. [DOI] [PubMed] [Google Scholar]
- 17.Robin PE, Bennett RJ, Gregory M. Study of autogenous transposed ossicles, bone and cartilage in man. Clin Otolaryngol Allied Sci. 1976;1:295–308. doi: 10.1111/j.1365-2273.1976.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 18.Kerr AG, Byrne JET, Smyth GDL. Cartilage homografts in the middle ear: a long-term histological study. J Laryngol Otol. 1973;87:1193–9. doi: 10.1017/s0022215100078166. [DOI] [PubMed] [Google Scholar]
- 19.Steinbach E, Pusalkar A. Long-term histological fate of cartilage in ossicular reconstruction. J Laryngol Otol. 1981;95:1031–9. doi: 10.1017/s0022215100091787. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto E, Iwanaga M, Fukumoto M. Histologic study of homograft cartilages implanted in the middle ear. Otolaryngol Head Neck Surg. 1988;98:546–51. doi: 10.1177/019459988809800602. [DOI] [PubMed] [Google Scholar]
- 21.Belal A, Jr, Linthicum FH, Jr, Odnert S. Fate of cartilage autografts for ossiculoplasty: an electron microscopic study. Clin Otolaryngol Allied Sci. 1981;6:231–6. doi: 10.1111/j.1365-2273.1981.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 22.Belal A, Jr, Odnert S. TORPS and PORPS: a transmission and scanning electron microscopic study. J Laryngol Otol. 1982;96:49–55. doi: 10.1017/s0022215100092227. [DOI] [PubMed] [Google Scholar]
- 23.Brennan G, Kerr AG, Smyth GDL. Porous prostheses: an electron microscopic study. Clin Otolaryngol Allied Sci. 1984;9:229–33. doi: 10.1111/j.1365-2273.1984.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 24.Kerr AG. Proplast and Plastipore. Clin Otolaryngol Allied Sci. 1981;6:187–91. doi: 10.1111/j.1365-2273.1981.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 25.Palva T, Mäkinen J. Histopathological observations on polyethylene-type materials in chronic ear surgery. Acta Otolaryngol (Stockh) 1983;95:139–46. doi: 10.3109/00016488309130927. [DOI] [PubMed] [Google Scholar]
- 26.Makek M, Mattox DE, Schmid S, Fisch U. Histology of synthetic ossicular prostheses. Arch Otolaryngol Head Neck Surg. 1988;114:1127–30. doi: 10.1001/archotol.1988.01860220061024. [DOI] [PubMed] [Google Scholar]
- 27.Jang CH, Wang P-C. Scanning electron microscope and energy dispersive X-ray spectrometry studies on the material surface of extruded Polycel ossicular prostheses. J Laryngol Otol. 2004;118:840–4. doi: 10.1258/0022215042703697. [DOI] [PubMed] [Google Scholar]
- 28.Postma D, Shea JJ. Polyethylene sponge total ossicular replacement prosthesis. A histopathologic study Arch Otolaryngol. 1982;108:801–2. doi: 10.1001/archotol.1982.00790600045010. [DOI] [PubMed] [Google Scholar]
- 29.Emmett JR. Biocompatible implants in tympanoplasty. Am J Otol. 1989;10:215–9. [PubMed] [Google Scholar]
- 30.Smyth GDL. Five-year report on partial ossicular replacement prostheses and total ossicular replacement prostheses. Otolaryngol Head Neck Surg. 1982;90:343–6. [PubMed] [Google Scholar]
- 31.Palva T, Mäkinen J. Polyethylene sponge total ossicular replacement prosthesis: a histopathologic study [Letter] Arch Otolaryngol. 1984;110:765. doi: 10.1001/archotol.1984.00800370067019. [DOI] [PubMed] [Google Scholar]
- 32.Shea JJ, Emmett JR. Biocompatible ossicular implants. Arch Otolaryngol. 1978;104:191–6. doi: 10.1001/archotol.1978.00790040013003. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg RA, Emmett JR. Current use of implants in middle ear surgery. Otol Neurotol. 2001;22:145–52. doi: 10.1097/00129492-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Yung MW. Literature review of alloplastic materials in ossiculoplasty. J Laryngol Otol. 2003;117:431–6. doi: 10.1258/002221503321892244. [DOI] [PubMed] [Google Scholar]
- 35.van Blitterswijk CA, Grote JJ. Biocompatibility of clinically applied hydroxylapatite ceramic. Ann Otol Rhinol Laryngol Suppl. 1990;99(suppl 144):3–11. [PubMed] [Google Scholar]