Abstract
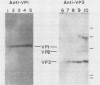
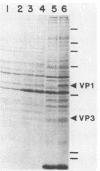
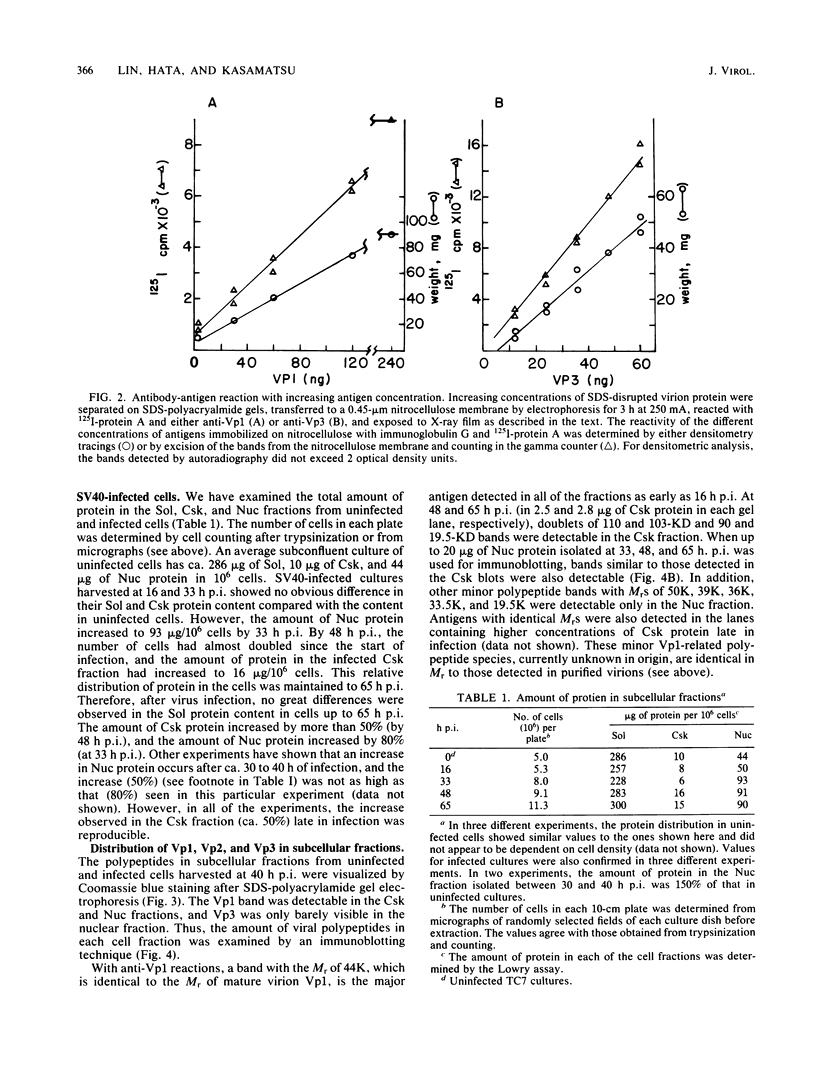
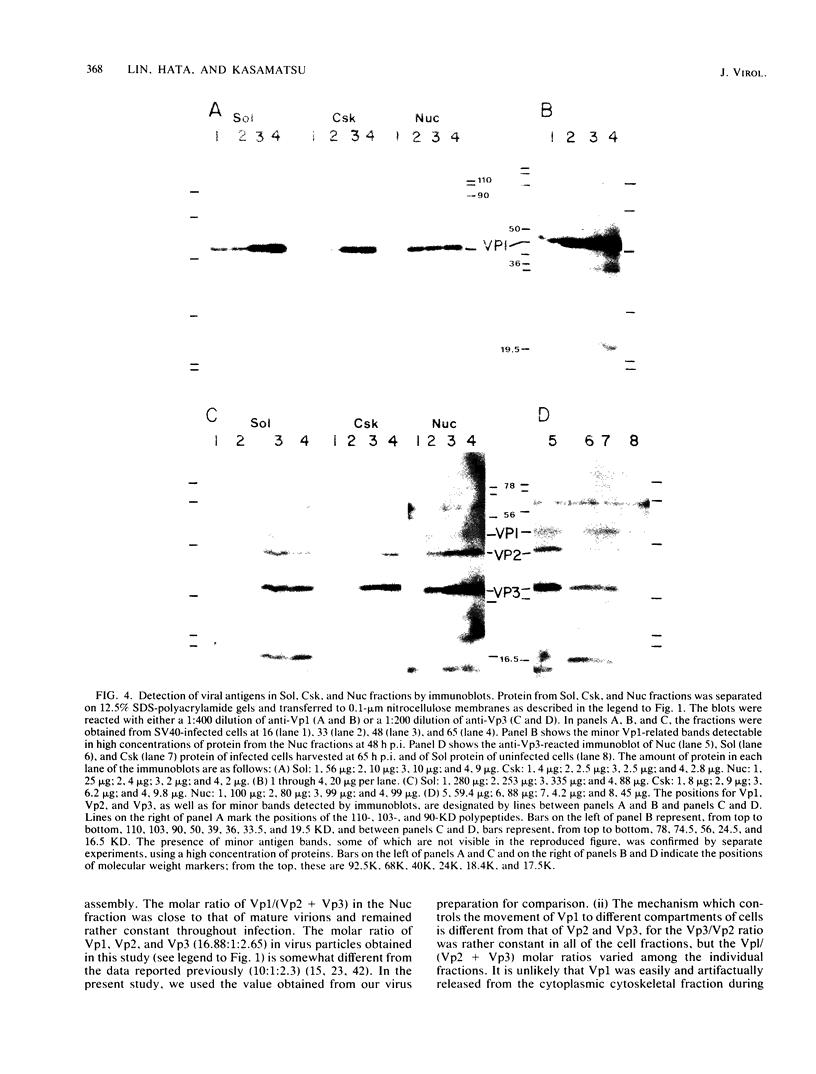
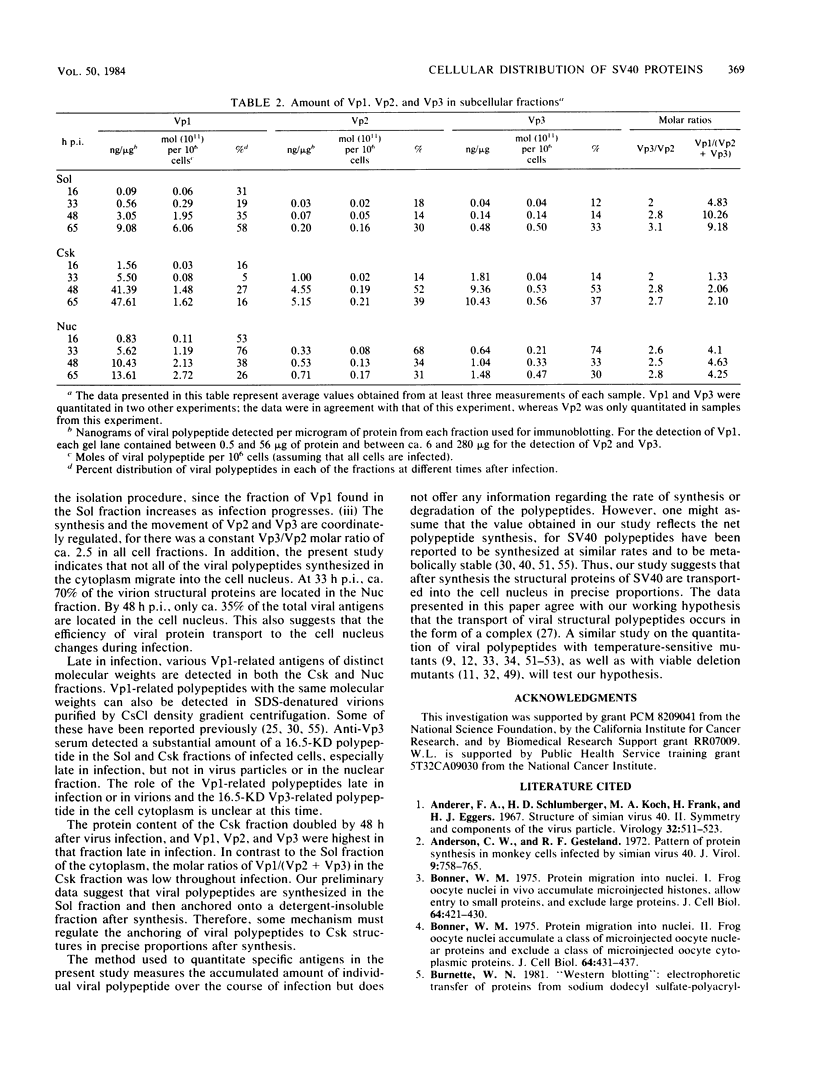
The amounts of simian virus 40 structural polypeptides Vp1, Vp2, and Vp3 in different subcellular fractions at various times after lytic infection were determined by a quantitative immunoblotting procedure. Simian virus 40-infected cells were lysed with a buffer containing Nonidet P-40 to yield a soluble fraction. The Nonidet P-40-insoluble fraction was further fractionated in the presence of deoxycholate and Tween 40 to yield a soluble fraction (cytoskeletal) and an insoluble fraction (Nuc), which is primarily cell nuclei. At 33 h postinfection, the majority of viral structural proteins was found in the cell nucleus, whereas, at 48 to 65 h postinfection, Vp1 was distributed evenly among all cell fractions and Vp2 and Vp3 were found predominantly in the cytoskeletal and Nuc fractions. Thus, not all of the viral polypeptides synthesized in the cytoplasm migrated into the cell nucleus. Throughout infection, the molar ratio (Vp3/Vp2) was rather constant in all subcellular fractions, indicating that the synthesis or processing or both of Vp2 and Vp3 are coordinately regulated. The molar ratio of Vp1/(Vp2 + Vp3) varied among the fractions. The Vp1/(Vp2 + Vp3) molar ratio in the soluble fraction varied during the course of infection; however, constant ratios were maintained in the cytoskeletal and Nuc fractions. Thus, the mechanism which controls the movement of Vp1 to different compartments of the cell appears to be different from that of Vp2 and Vp3. The Vp1/(Vp2 + Vp3) value in the Nuc fraction was similar to the ratio found in virus particles. The constant molar distribution of Vp1, Vp2, and Vp3 in the Nuc fraction throughout infection suggests that there is a specific mechanism which regulates the transport of viral structural proteins. These results support the hypothesis that the structural proteins of simian virus 40 are transported into the cell nucleus in precise proportions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Gesteland R. F. Pattern of protein synthesis in monkey cells infected by simian virus 40. J Virol. 1972 May;9(5):758–765. doi: 10.1128/jvi.9.5.758-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975 Feb;64(2):431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D. J., Tevethia M. J. Characterization of a temperature-sensitive, DNA-positive, nontransforming mutant of simian virus 40. Virology. 1981 Jul 30;112(2):605–624. doi: 10.1016/0042-6822(81)90306-8. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Fischer H., Sauer G. Identification of virus-induced proteins in cells productively infected with simian virus 40. J Virol. 1972 Jan;9(1):1–9. doi: 10.1128/jvi.9.1.1-9.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. A peptide comparison of proteins in the simian virus 40 virion. Virology. 1975 Dec;68(2):539–543. doi: 10.1016/0042-6822(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., LeVine D. Amino acid compositions of simian virus 40 structural proteins. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1221–1227. doi: 10.1016/0006-291x(73)90630-x. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Flory P. J., Jr Synthesis of the SV40 viral polypeptide Vp1 during infection. Virology. 1978 May 15;86(2):344–353. doi: 10.1016/0042-6822(78)90075-2. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Nehorayan A. Intracellular localization of viral polypeptides during simian virus 40 infection. J Virol. 1979 Nov;32(2):648–660. doi: 10.1128/jvi.32.2.648-660.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Nehorayan A. Vp1 affects intracellular localization of Vp3 polypeptide during simian virus 40 infection. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2808–2812. doi: 10.1073/pnas.76.6.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Shyamala M., Lin W. Host antigens in the centriolar region are induced in SV40-infected TC7 cells: SV40 small-T-function requirement. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):243–252. doi: 10.1101/sqb.1980.044.01.028. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Protein-SV40 DNA complex stable in high salt and sodium dodecyl sulfate. Biochem Biophys Res Commun. 1976 Feb 9;68(3):927–936. doi: 10.1016/0006-291x(76)91234-1. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D. Protein metabolism in SV40-infected cells. Virology. 1973 Nov;56(1):313–333. doi: 10.1016/0042-6822(73)90309-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. The B/C gene of simian virus 40. Virology. 1976 Dec;75(2):335–345. doi: 10.1016/0042-6822(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- Lin W., Kasamatsu H. On the electrotransfer of polypeptides from gels to nitrocellulose membranes. Anal Biochem. 1983 Feb 1;128(2):302–311. doi: 10.1016/0003-2697(83)90379-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Tegtmeyer P. Synthesis and assembly of simian virus 40. II. Synthesis of the major capsid protein and its incorporation into viral particles. J Virol. 1972 Jan;9(1):52–60. doi: 10.1128/jvi.9.1.52-60.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C. L., Shure H. Cell-free translation of simian virus 40 16S and 19S L-strand-specific mRNA classes to simian virus 40 major VP-1 and minor VP-2 and VP-3 capsid proteins. J Virol. 1979 Mar;29(3):1204–1212. doi: 10.1128/jvi.29.3.1204-1212.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. N., Pearson M. L. Evidence for post-transcriptional control of the morphogenetic genes of bacteriophage lambda. J Mol Biol. 1974 May 5;85(1):163–175. doi: 10.1016/0022-2836(74)90135-1. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Pearson M. L. Functional inactivation of bacteriophage lambda morphogenetic gene in RNA. Nature. 1975 Feb 20;253(5493):647–650. doi: 10.1038/253647a0. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M., Kuehl L. Microinjection of the nonhistone chromosomal protein HMG1 into bovine fibroblasts and HeLa cells. Cell. 1979 Apr;16(4):901–908. doi: 10.1016/0092-8674(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Mulligan R. C., Gorecki M., Roberts B. E., Rich A. Direct biochemical mapping of eukaryotic viral DNA by means of a linked transcription-translation cell-free system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2747–2751. doi: 10.1073/pnas.73.8.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Intermediate filament systems are collapsed onto the nuclear surface after isolation of nuclei from tissue culture cells. Exp Cell Res. 1982 Mar;138(1):207–214. doi: 10.1016/0014-4827(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Robb J. A., Widmer C., Ozer H. L. Altered protein metabolism in infection by the late tsB11 mutant of simian virus 40. J Virol. 1974 Oct;14(4):997–1007. doi: 10.1128/jvi.14.4.997-1007.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Ripper L. W. Biology of simian virus 40 (SV40) transplantation antigen (TrAg) II. Isolation and characterization of additional temperature-sensitive mutants of SV40. Virology. 1977 Sep;81(2):192–211. doi: 10.1016/0042-6822(77)90137-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Roblin R., Dulbecco R. Protein synthesis in Simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1972 Apr;69(4):921–924. doi: 10.1073/pnas.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]