Abstract
Rho family proteins have been implicated in regulating various cellular processes, including actin cytoskeleton organization, endocytosis, cell cycle, and gene expression. In this study, we analyzed the function of a novel Dictyostelium discoideum Rho family protein (RacC). A cell line was generated that conditionally overexpressed wild-type RacC three- to fourfold relative to endogenous RacC. Light and scanning electron microscopy indicated that the morphology of the RacC-overexpressing cells [RacC WT(+) cells] was significantly altered compared with control cells. In contrast to the cortical F-actin distribution normally observed, RacC WT(+) cells displayed unusual dorsal and peripheral F-actin–rich surface blebs (petalopodia, for flower-like). Furthermore, phagocytosis in the RacC WT(+) cells was induced threefold relative to control Ax2 cells, whereas fluid-phase pinocytosis was reduced threefold, primarily as the result of an inhibition of macropinocytosis. Efflux of fluid-phase markers was also reduced in the RacC WT(+) cells, suggesting that RacC may regulate postinternalization steps along the endolysosomal pathway. Treatment of cells with Wortmannin and LY294002 (phosphatidylinositol 3-kinase inhibitors) prevented the RacC-induced morphological changes but did not affect phagocytosis, suggesting that petalopodia are probably not required for RacC-induced phagocytosis. In contrast, inactivating diacylglycerol-binding motif–containing proteins by treating cells with the drug calphostin C completely inhibited phagocytosis in control and RacC WT(+) cells. These results suggest that RacC plays a role in actin cytoskeleton organization and phagocytosis in Dictyostelium.
INTRODUCTION
Small-Mr GTPases are molecular switches that function to regulate a diverse array of biological processes, including vesicular trafficking, cell cycle progression, oxidative burst, transcriptional activation, and actin cytoskeleton organization (reviewed in Van Aelst and D’Souza-Schorey, 1997). The Rho proteins (one of five subfamilies of Ras-like GTPases) are divided into three classes; Rho, Rac, and Cdc42. Members of the Rho family are primarily involved in the regulation of the actin cytoskeleton of the cell; Rho was shown to control the formation of actin stress fibers (Ridley and Hall, 1992); Cdc42 was shown to regulate filopodia formation (Kozma et al., 1995; Nobes and Hall, 1995); and Rac was shown to coordinate lamellipodia formation (Ridley et al., 1992). More recent data suggest that these GTPases regulate other cellular functions, including gene expression (Coso et al., 1995), tumorigenic activation (Qiu et al., 1995), the oxidative burst of phagocytes (Bokoch, 1995), cell cycle progression (Ridley, 1995; Westwick et al., 1997), and cellular transformation (Qiu et al., 1995, 1997; Roux et al., 1997).
Several lines of evidence suggest that the actin cytoskeleton of many different types of cells plays an important role in regulating processes such as phagocytosis and fluid-phase pinocytosis. For instance, it has been demonstrated that disrupting the actin cytoskeleton inhibited the internalization of the α-factor pheromone and prevented receptor-mediated endocytosis in mammalian cells (Lamaze et al., 1997). Furthermore, studies using cytochalasin A and latrunculin B in Dictyostelium have demonstrated that disrupting the actin cytoskeleton inhibits the processes of phagocytosis and fluid-phase pinocytosis (Temesvari et al., 1996; Hacker et al., 1997).
The fact that the actin cytoskeleton appears to play such an important role in endocytosis implies that proteins that regulate actin distribution, such as Rho family GTPases, may also be important in the regulation of this process. In fact, a recent study has shown that expression of a constitutively active form of Rac1 or Rho inhibited receptor-mediated endocytosis of the transferrin receptor in HeLa cells (Lamaze et al., 1996). In addition, two independent groups have demonstrated that Rho family GTPases play a role in regulating phagocytosis in macrophages and leukocytes (Cox et al., 1997; Hackam et al., 1997).
The simple unicellular eukaryote Dictyostelium discoideum is an ideal system in which to study the function of GTPases because, like the Saccharomyces cerevisiae, Drosophila, and Caenorhabditis elegans systems, it is amenable to genetic and biochemical manipulations. Furthermore, it functions in motility and phagocytosis in a manner similar to that observed for neutrophils. Our laboratory has identified seven Rho family genes in D. discoideum (rac1A, rac1B, rac1C, racA, racB, racC, and racD) (Bush et al., 1993). On the basis of protein homology searches, we have provisionally named these Rho family members “Rac” proteins; however, it remains to be demonstrated whether these small-Mr GTPases function like mammalian Rac proteins and if the proteins in this family share overlapping roles in Dictyostelium. By comparison, three rac-like genes have been identified in mammalian cells (rac1, rac2, and rac3) (Didsbury et al., 1989; Haataja et al., 1997), as well as five Rho-like GTPases (rhoA, rhoB, rhoC, rhoD, and rhoE), and a cdc42-like gene (reviewed in Van Aelst and D’Souza-Schorey, 1997). The three D. discoideum Rho family proteins, Rac1A, Rac1B, and Rac1C, share at least 81% homology to human Rac1, whereas the other Rac-like proteins from D. discoideum share between 58 and 74% homology to human Rac1; therefore, these proteins have been classified as “novel” Rac-like GTPases. Another laboratory has also recently identified an additional D. discoideum rho family gene (racE) that appears to play a role in cytokinesis (Larochelle et al., 1996).
As one approach to investigate the function of novel Rho family proteins, we have generated cell lines that modestly and conditionally overexpress the novel D. discoideum GTPase RacC, which is 61% identical to human Rac1 and Cdc42 in amino acid sequence. We report that RacC WT(+) cells displayed unusual F-actin–based structures on their surface that we have termed “petalopodia,” because they resemble the petals of a flower. Furthermore, the rate of phagocytosis in RacC WT(+) cells was stimulated threefold, whereas the rate of fluid-phase pinocytosis was reduced threefold (probably as the result of an abrogation of macropinocytosis). Finally, the exocytosis of fluid-phase and lysosomal hydrolases was inhibited in RacC WT(+) cells. These results indicate that RacC may function at discrete steps along the endolysosomal pathway, perhaps to regulate actin-based processes, including phagocytosis, pinocytosis, and endolysosomal vesicle trafficking.
MATERIALS AND METHODS
Organism
Dictyostelium strains were grown axenically in HL5 medium (1% oxoid proteose peptone, 1% glucose, 0.5% yeast extract [Difco, Detroit, MI], 1.4 mM Na2HPO4, 3 mM KH2PO4, pH 6.5) in 175-cm2 tissue culture flasks (Sarstedt, Newton, NC) at 19°C. To generate RacC WT(+) cell lines, the parental Ax2 cells were transformed with the D. discoideum RacC expression vector HA-RacC-pVEIIΔATG. To create this vector, full-length racC cDNA was cloned into the SacI site of the D. discoideum expression vector HA-pVEIIΔATG to generate the new vector pDS7, thus placing RacC in-frame with a 10-amino acid–encoding epitope tag from the hemagglutinin (HA) protein of influenza virus. This vector contained the discoidin I promoter, which can be induced with prestarvation factor (Rathi et al., 1991) and repressed by growing cells in the presence of 1 mM folate. Stably transformed cells (Early and Williams, 1987) were maintained under G418 selection (100 μg/ml) and folic acid (1 mM) to ensure that the discoidin I promoter was turned off. Individual G418-resistant clones (a total of four) were then grown in HL5 media in the absence of both folate and G418; after 2 d the cells were harvested and prepared for Western blot or prepared for functional analysis (see below). All four clones showed identical phenotypic properties, and we selected one for more detailed studies.
Antibody Generation
To N-terminally tag racC with GST, racC cDNA was cloned into the XhoI site of the bacterial protein expression vector pGEX (Pharmacia, Piscataway, NJ) 4T-1, thus generating the new vector pDS20. This vector was then transformed into the Stratagene (La Jolla, CA) Escherichia coli strain XL-1 blue, and clonal isolates were grown in the presence of 1 mM isopropyl-1-thio-β-d-galactopyranoside to induce expression of GST–RacC protein. The recombinantly expressed protein was purified using affinity chromatography with glutathione-Sepharose beads, and 100 μg of protein were used to immunize two female white New Zealand rabbits (Cocalico Biologicals, Reamstown, PA), followed by two boosts of 50 μg each at 2-wk intervals. After the second boost, polyclonal antisera was obtained and affinity purified using Sepharose beads that were covalently coupled to recombinantly expressed GST–RacC using a cyanogen bromide coupling system (Pharmacia).
Subcellular Fractionation and Western Blot Analysis
Ax2 cells were collected by centrifugation (1000 × g for 5 min) and resuspended at a density of 2 × 108 cells/ml in MESES buffer (20 mM 2-[N-morpholino]ethane sulfonic acid, 1 mM EDTA, 250 mM sucrose, pH 6.5) supplemented with a protease inhibitor mixture (leupeptin [50 μg/ml], pepstatin A [10 μg/ml], benzamidine [2 mM], PMSF [1 mM]). Cells were homogenized on ice using a Dounce homogenizer (30 strokes), and light microscopy was performed to ensure that at least 95% of the cells were broken. Cells were then subjected to the following conditions: no treatment, 15 min incubation with 1% Triton X-100, or 15 min incubation with 100 mM sodium carbonate (peripherally associated membrane protein stripping agent). Membranes and supernatants were separated by differential centrifugation (100,000 × g for 30 min), and the samples were resuspended in 2× (final) sample buffer (Laemmli, 1970). Proteins in the pellets and supernatants were resolved using discontinuous SDS-PAGE and then blotted to a nitrocellulose membrane (Towbin et al., 1979). Western blots were developed as described (Buczynski et al., 1997a,b).
Scanning Electron Microscopy
Sterile, glass coverslips were inserted into 25-cm2 tissue culture flasks and submerged in 10 ml of HL5 growth medium. Cells at an initial titer of 105 cells/ml were grown for 24 h to allow cell attachment to coverslips. The coverslips were removed and fixed in 1.25% gluteraldehyde buffered in 50 mM sodium cacodylate (pH 6.8). The attached cells were dehydrated in a graded acetone series, and critical point dried with liquid carbon dioxide. The coverslips with adhering dried cells were mounted on specimen stubs, sputter coated with 10 nm gold, and viewed with an ISI DS 130 scanning electron microscope.
Confocal Microscopy Staining
For phalloidin staining, cells were attached to a glass coverslip and grown overnight in HL5 medium, and the coverslip was removed and clamped between the aluminum blocks of a circular Rose chamber (Rose et al., 1958). The medium was replaced by fixing solution (1% formaldehyde, 0.1% glutaraldehyde, 0.01% Triton X-100 in HL5) containing 1 μM rhodamine–phalloidin. The cells were imaged by a Bio-Rad (Richmond, CA) MRC-600 laser scanning confocal microscope equipped with a 25-mW krypton–argon laser (Ion Laser Technology, Salt Lake City, UT) attenuated with a 1% neutral density filter. A 100× (1.30 numerical aperture) Neofluar objective (Carl Zeiss, Thornwood, NY) was used. Fluorescence and differential interference contrast images were collected simultaneously, and confocal z-sections were acquired at 0.72-μm intervals. Images were further analyzed and processed using NIH Image (written by Wayne Rasband, National Institutes of Health, Bethesda, MD, and available from the internet by anonymous FTP from zippy.nimh.nih.gov) and Adobe (Mountain View, CA) Photoshop.
Phagocytosis, Fluid-Phase Pinocytosis, and Exocytosis Assays
Phagocytosis assays were performed as described using fluorescent latex beads (Buczynski et al., 1997a). Fluid-phase endocytosis assays were performed according to the methods of Aubry et al. (1993a). Fluid-phase flux assays were performed as described using FITC–dextran (Buczynski et al., 1997a). Intravesicular pH was measured as described (Cardelli et al., 1989).
Lysosomal Hydrolase Secretion Assays and Radiolabel Pulse–Chase Experiments
Cells in growth media were harvested from T-175 flasks, washed, resuspended in fresh media or 10 mM phosphate buffer at a titer of 5 × 106 cells/ml, and placed in shaking suspension. At various times, cells were centrifuged, and the subsequent pellets and supernatants were assayed for α-mannosidase activity. Radiolabel pulse–chase analysis of α-mannosidase processing was performed as described (Mierendorf et al., 1983).
RESULTS
Endogenous RacC Is Primarily Localized to the Membrane Fraction of D. discoideum
To examine the intracellular distribution of RacC in wild-type cells, an affinity-purified polyclonal antibody against recombinant RacC was generated (see MATERIALS AND METHODS). As shown in Figure 1A, anti-RacC polyclonal antibodies did not recognize bacterially expressed GST–RacB (lanes 4–6), the most closely related Dictyostelium Rho family protein to RacC. In contrast, these antibodies recognized a species of ∼51 kDa in the lanes loaded with GST–RacC (lanes 1–3), which is the predicted size of the GST–RacC fusion protein. RacE, which is 49.5% identical to RacC in amino acid sequence (Larochelle et al., 1996), was also not recognized by these antibodies when expressed in Dictyostelium cells as a GFP–RacE fusion protein (unpublished results).
Figure 1.
RacC is localized in the membrane fraction of lysed D. discoideum cells and can be conditionally overexpressed. (A) Western blot analysis of recombinantly expressed RacB and RacC–GST fusion proteins using LSU37 anti-RacC polyclonal antibodies. The lanes were loaded with increasing amounts of the GST–RacC and GST–RacB fusion proteins, separated by SDS-PAGE, and blotted to nitrocellulose membranes (lanes 1 and 4 contain 3.25 ng fusion protein; lanes 2 and 5 contain 6.5 ng fusion protein; lanes 3 and 6 contain 13 ng fusion protein). The LSU37 anti-RacC antisera reacted specifically with GST–RacC but not GST–RacB. (B) Western blot analysis of membranes and supernatants of D. discoideum Ax2 cells using rabbit polyclonal anti-RacC antisera. Homogenates were prepared as described in MATERIALS AND METHODS. Cells were then incubated with 1% Triton X-100, 100 mM sodium carbonate, or left untreated for 15 min. The cell lysates were then pelleted by differential centrifugation to separate the membranes from the supernatants and then prepared for SDS-PAGE followed by electrophoretic transfer to nitrocellulose membranes. As indicated in the text, 70% of endogenous RacC is located in the membrane fraction (lane 1), whereas 30% remains soluble (lane 2). None of the endogenous RacC was associated with the Triton X-100–insoluble F-actin cytoskeleton (lanes 3 and 4). Finally, RacC is intimately associated with the membranes, because sodium carbonate treatment did not release it from membranes. (C) Western blot analysis of an HA–RacC conditionally overexpressing cell line using D. discoideum RacC-specific LSU37 rabbit polyclonal antisera. Cells were transformed with the pVEIIΔATG plasmid harboring racC cDNA, and G418-resistant clones were obtained. To induce expression of HA–RacC (under control of the discoidin I promoter), folate was removed from the growth medium, and cells were harvested for Western blot analysis at the indicated times.
Published data indicate that GFP–RacC localizes to the plasma membrane of vegetative D. discoideum amoebae (Larochelle et al., 1997), but these studies did not determine what percentage of cellular RacC was actually membrane associated. To answer this question, differential centrifugation of Dictyostelium lysates that were treated with various agents, followed by Western blot analysis of the resultant pellet and supernatant fractions using anti-RacC antibodies, was performed. As shown in Figure 1B, 70% of endogenous RacC protein was localized to the membrane fraction of cellular extracts, whereas 30% of endogenous RacC was cytosolic. To test whether RacC was associated with the F-actin cytoskeleton, cell lysates were incubated with Triton X-100 to solubilize membranes, and the Triton-insoluble F-actin cytoskeleton was pelleted by centrifugation. As shown in Figure 1B, Triton treatment released 100% of endogenous RacC into the supernatant, indicating that RacC was not associated with the F-actin cytoskeleton of D. discoideum. Furthermore, RacC behaved more like an integral membrane protein and not like a peripherally associated membrane protein, because treatment of lysed cells with sodium carbonate did not release additional RacC from the membrane fraction.
RacC Overexpression Induces Changes in Cell Morphology and F-Actin Organization in a Phosphatidylinositol (PI) 3-Kinase–dependent Manner
To begin to define the function of RacC in D. discoideum, cell lines were created that conditionally overexpressed the RacC protein (see MATERIALS AND METHODS). To allow us to differentiate between endogenously expressed and conditionally overexpressed forms of the protein, an HA epitope tag was added to the N terminus of RacC. As shown in Figure 1C, 24 h after folate was removed from the growth medium to induce expression from the discoidin I promoter, HA-RacC was expressed at a level two- to threefold higher than that of endogenous RacC. The same pattern and level of overexpression were consistently seen in four independent strains. After 48 h of induction, RacC was maximally expressed, at a level three- to fourfold higher than endogenous RacC. Interestingly, concurrent with RacC overexpression was the appearance of cells that contained phase-dark areas (membrane blebs) around their periphery (unpublished results). When RacC was maximally expressed (48 h after induction), up to 85% of the cells in a field displayed the “membrane blebs.”
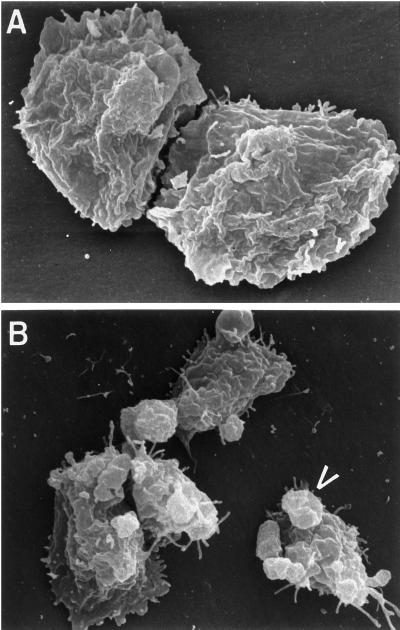
To examine these bleb-like structures in greater detail, scanning electron microscopy was performed. Control Ax2 cells displayed a fairly uniform, ruffled appearance (Figure 2A), with an occasional “crown-like” structure observed. In contrast, RacC WT(+) cells displayed many protrusions that reached out from the dorsal and lateral surfaces of the cell (Figure 2B; see arrowhead). None of the bleb-like structures contained cup-like distal structures frequently found on “crowns.” Because Rho family GTPases have previously been shown to regulate the cellular F-actin cytoskeleton (Ridley and Hall, 1992; Ridley et al., 1992), we hypothesized that RacC overexpression was altering cell morphology by mediating changes in the F-actin cytoskeleton of Dictyostelium.
Figure 2.
Scanning electron microscopy of Ax2 and RacC WT(+) cells. Control Ax2 cells (A) and HA-RacC WT(+) cells (B) were seeded on coverslips without folate, fixed with gluteraldehyde, critical point dried, sputter coated with gold, and prepared for scanning electron microscopy as described in MATERIALS AND METHODS. RacC WT(+) cells are characterized by protrusions that reach out from the surface of the cell (B, arrowhead), whereas the plasma membrane of control cells is characterized by a uniform, ruffled appearance.
To examine the organization of the F-actin cytoskeleton, control Ax2 and RacC WT(+) cells were fixed and incubated with FITC–phalloidin, and serial sections were visualized using confocal microscopy. We observed that the F-actin cytoskeleton of RacC WT(+) cells was strikingly different from that of control Ax2 cells (Figure 3). The F-actin of RacC WT(+) was organized into irregular structures that resembled the petals of a flower; therefore we termed these novel structures “petalopodia” (Figure 3, F–H). Interestingly, these petalopodia structures were distributed over the entire dorsal surface of the RacC WT(+) cells. In contrast, the F-actin of Ax2 cells was organized in a more cortical manner (Figure 3, B–D), with enriched staining at the pseudopod region and reduced amounts of F-actin at the dorsal surface of the cells.
Figure 3.
The F-actin cytoskeleton is altered in RacC WT(+) cells. Control Ax2 (A–D) and RacC WT(+) (E–H) cells were fixed and stained with rhodamine–phalloidin, and confocal z-sections were acquired at 0.72-μm intervals. The fluorescence montage shows every third image in the series (sections from left to right are progressively more dorsal). DIC (A) was collected simultaneously with fluorescence image B, and DIC (E) was collected with image G. RacC WT(+) cells contain rounded F-actin protrusions (for example, petalopodia, arrowhead) over much of the dorsal surface, whereas control Ax2 cells display a normal F-actin cortex and enriched pseudopod staining. Bar, 10 μm.
Previous studies using a double-null mutant cell line, Δddpik1/ddpik2, which contained only 25% of the wild-type level of PI 3,4,5-trisphosphate, indicated that PI 3-lipid kinases were important in regulating the organization of the F-actin cytoskeleton in D. discoideum (Buczynski et al., 1997b; Zhou et al., 1998). Furthermore, treatment of wild-type cells with the PI 3-kinase–specific inhibitors Wortmannin and LY294002 induced the same phenotypic effects observed in the Δddpik1/ddpik2 cell line (our unpublished result). This and other data suggest that DDPIK1 and DDPIK2 may be the major targets of LY294002 and wortmannin. To determine whether Wortmannin- and LY294002-sensitive PI 3-kinases were important for RacC-induced morphological and F-actin cytoskeleton changes, wild-type and RacC WT(+) cells were seeded on coverslips, treated with either LY294002 or wortmannin for 15 min, fixed, permeabilized, and stained with FITC–phalloidin. The cells were then examined using phase-contrast and fluorescence microscopy. As shown in Figure 4D, RacC WT(+) cells displayed a petalopodia-rich F-actin cytoskeleton; however, the addition of LY294002 (final concentration of 20 μM) for 5 min caused the petalopodia to disappear from 95% of the cells, and the F-actin cytoskeleton of the cells assumed a cortical distribution, similar to control Ax2 cells (Figure 4, compare F with B).
Figure 4.
RacC-induced morphology is inhibited by PI 3-kinase inactivation. (A and B) Control Ax2 cells; (C and D) RacC WT(+) cells. RacC WT(+) cells were also treated with the PI 3-kinase inhibitor wortmannin (2 μM) for 20 min (E and F). Cells were seeded on coverslips, pretreated with LY294002 or DMSO, fixed, permeabilized, stained with FITC–phalloidin, and viewed using phase-contrast (A, C, and E) or fluorescence (B, D, and F) microscopy. RacC WT(+) cells display the novel petalopodia actin-based structures on their surface (C and D; see arrowheads), whereas control Ax2 cells display actin in a cortical distribution (A and B). LY294002 treatment of RacC WT(+) cells reversed the formation of petalopodia (E and F) and caused the F-actin to redistribute to a more cortical pattern.
Overexpression of RacC Stimulates Phagocytosis
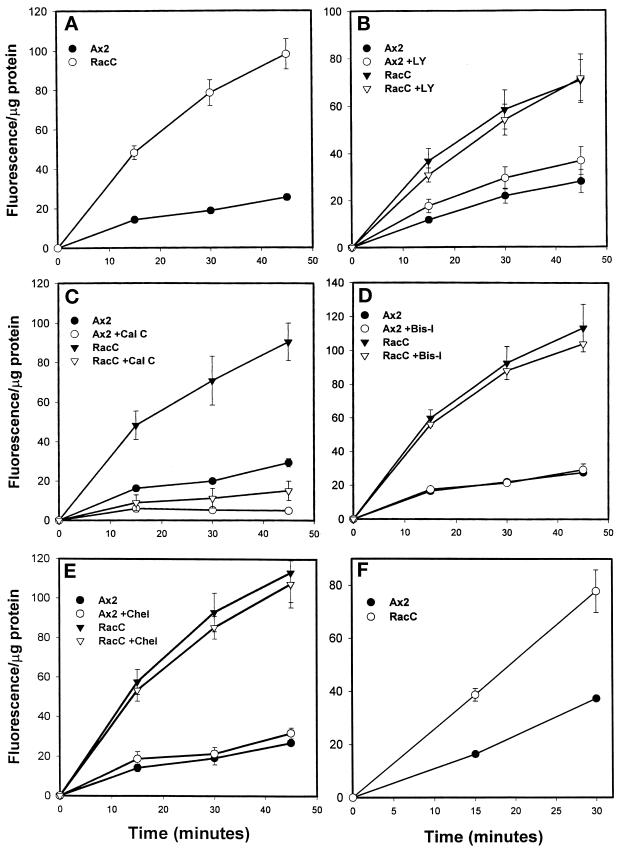
To determine whether RacC overexpression affected phagocytosis, a process dependent on the regulation of F-actin (Maniak et al. 1995; Temesvari et al., 1996; Niewohner et al., 1997), we examined the ability of control Ax2 and RacC WT(+) cells to internalize 1-μm fluorescent latex beads. As shown in Figure 5A, RacC WT(+) cells internalized latex beads at a rate that was two- to threefold faster than control Ax2 cells. All four independent clones showed a comparable increase in phagocytosis, and we selected one clone for further analysis.
Figure 5.
RacC overexpression stimulates phagocytosis. For A–E, cells were incubated with 1-μm fluorescent latex beads for the indicated time after a 20-min pretreatment with the appropriate drug or DMSO (the diluent). Cells were collected by centrifugation, washed, and prepared for fluorimetry. Shown are the rates obtained by averaging the indicated time points from independent experiments that were performed. The data are reported as fluorescence normalized to total protein/sample to account for potential differences in cell size between the two strains. (A) Untreated cells. RacC overexpression stimulates the rate of 1-μm latex bead uptake three- to fourfold over control Ax2. (B) Cells treated with the PI 3-kinase inhibitor LY294002 (20 μM). Inhibition of PI 3-kinase only partially represses the RacC-induced stimulation of phagocytosis. Control cells treated with PI 3-kinase inhibitors actually display a slight stimulation of bead uptake. (C) Cells treated with calphostin C (0.75 μM), an inhibitor of enzymes containing DAG-binding motifs. Calphostin C treatment completely prevented the uptake of latex beads in control and RacC WT(+) cells. (D and E) Cells treated with bis-indolylmaleimide I (10 μM; D) or chelerythrine (10 μM; E), specific inhibitors of PKC. Phagocytosis was unaffected by treating cells with drugs that specifically inhibit PKC. (F) Cells were incubated with FITC-labeled E. coli, and internalization was measured in the same manner as for latex beads. All values are reported as fluorescence per micrograms of protein ± SEM. Statistical analysis was performed on the data obtained for the 15-min (shown below) and 30-min time points to test the significance of the reported increases and decreases using the Student’s two-tailed t test (Instat, IBM, Armonk, NY). (A) No treatment. Ax2, 14.2 ± 1.1 (n = 12); RacC WT(+), 48.2 ± 3.5 (n = 12). (B) LY294002. Untreated: Ax2, 11.6 ± 1.3 (n = 4); RacC WT(+), 36.7 ± 5.4 (n = 4). LY294002-treated: Ax2, 17.5 ± 2.9 (n = 4); RacC WT(+), 30.7 ± 3.0 (n = 4). (C) Calphostin C. Untreated: Ax2, 16.2 ± 0.8 (n = 3); RacC WT(+), 48.4 ± 7.2 (n = 3). Calphostin C-treated: Ax2, 6.0 ± 1.8 (n = 3); RacC WT(+), 8.9 ± 4.0 (n = 3). (D) Bis-indolylmaleimide. Untreated: Ax2, 16.5 ± 1.0 (n = 3); RacC WT(+), 59.9 ± 5.0 (n = 3). Bis-indolylmaleimide I-treated: Ax2, 17.5 ± 1.4; RacC WT(+), 56.1 ± 2.0 (n = 3). (E) Chelerythrine. Untreated: Ax2, 14.2 ± 2.0 (n = 3); RacC WT(+), 57.6 ± 6.4 (n = 3). Chererythine-treated: Ax2, 18.7 ± 3.7; RacC WT(+), 53.4 ± 5.5 (n = 3). (F) FITC–E. coli: Ax2, 16.4 ± 0.5 (n = 3); RacC WT(+), 38.7 ± 2.4 (n = 3).
To ensure that the latex beads were actually being internalized into cells and were not just sticking to their surface, control experiments were carried out. Latex beads were added to cells at 4°C, a temperature at which beads will bind to the outside of cells but will not be internalized. In this case, we observed no difference in the number of beads attached to the outside of RacC WT(+) cells compared with control Ax2 cells, indicating that overexpression of RacC does not simply make cells more “sticky” for beads (unpublished results). Furthermore, we also performed the phagocytosis assay in the presence of both latex beads and the fluid-phase marker lucifer yellow (LY). At the end of the assay, the cells were washed in fresh HL5 media, seeded on coverslips, and viewed by phase-contrast and fluorescence microscopy. For both control Ax2 and RacC WT(+) cells, >90% of the latex beads associated with the cells were ringed with the fluid-phase marker LY, indicating that these beads were internalized and located on the inside of the cell (unpublished results). Finally, RacC WT(+) cells also internalized a natural food source, E. coli, faster than control cells, indicating that this response may be physiologically relevant (Figure 5F).
Surprisingly, although LY294002 treatment of RacC WT(+) cells appeared to reverse the formation of petalopodia, phagocytosis rates in these cells were not affected significantly (Figure 5B), indicating that PI 3-kinase activity and petalopodia structures might not be important for most of the RacC-induced stimulation of phagocytosis seen in RacC WT(+) cells.
A Diacylglycerol-binding Protein May Be Required for Phagocytosis
It has been demonstrated that protein kinase C (PKC) can transduce signals that lead to a rearrangement of the actin cytoskeleton and increases in phagocytosis in macrophages (Hartwig et al., 1992; Allen and Aderem, 1996). Because RacC overexpression appeared to stimulate phagocytosis in Dictyostelium, it seemed plausible that PKC might be signaling upstream or downstream of RacC to mediate one or more of its effects. To test whether PKC or other proteins containing diacylglycerol (DAG)-binding motifs were important for RacC-mediated phagocytosis, we measured phagocytosis in RacC WT(+) cells in the presence of several PKC inhibitors. When wild-type or RacC WT(+) cells were preincubated with calphostin C (0.75 μM) for 20 min before latex beads were added to growing cells, phagocytosis appeared to be almost completely abrogated (Figure 5C). Because calphostin C competes with phorbol esters for binding to PKC enzymes (Kobayashi et al., 1989), this result indicated that proteins containing DAG-binding motifs might be necessary for phagocytosis in Dictyostelium. To examine whether PKC was one of these proteins, we performed additional experiments using two specific inhibitors of PKC activity: bis-indolylmaleimide I and chelerythrine, both of which compete with ATP for binding to the active site of PKC. As shown in Figure 5, D and E, pretreatment with bis-indolylmaleimide I or chelerythrine had no effect on the rates of phagocytosis in control Ax2 or RacC WT(+) cells, suggesting that a DAG-binding protein, perhaps lacking PKC activity, was mediating RacC-induced phagocytosis in Dictyostelium.
Macropinocytosis and Exocytosis of Fluid and Hydrolases Are Inhibited by RacC Overexpression
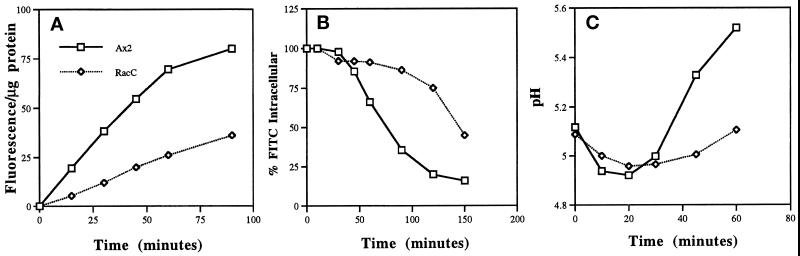
RacC WT(+) cells internalized latex beads and bacteria at a faster rate than control Ax2 cells, perhaps because of a general stimulation of plasma membrane internalization, which might also result in an increase in pinocytosis. Therefore, we determined the rate of fluid-phase pinocytosis by measuring the ability of control Ax2 or RacC WT(+) cells to internalize the fluid-phase marker FITC–dextran (Figure 6A). Unexpectedly, RacC WT(+) cells internalized FITC–dextran fluid phase at a rate three times slower than that of control cells. This observation indicated that 1) the RacC-mediated positive effect on phagocytosis was specific for particles, and not the result of a general stimulation of all endocytic processes, and 2) fluid-phase pinocytosis and phagocytosis may independently regulate processes in D. discoideum.
Figure 6.
RacC WT(+) cells are defective in both fluid-phase endocytosis and exocytosis. (A) Control and RacC WT(+) cells were incubated with FITC–dextran for the times indicated, collected by centrifugation, washed, and prepared for spectrofluorimetry (see MATERIALS AND METHODS). The rates are reported as fluorescence per micrograms of protein to correct for any differences in cell size among the strains examined. RacC WT(+) cells internalized FITC–dextran at a rate two to three times slower than that of control Ax2 cells. Data from the 30-min time point: Ax2, 37.0 ± 1.5 (n = 3); RacC WT(+), 11.6 ± 1.3 (n = 3). (B) Cells were pulsed with FITC–dextran for 10 min to label the early compartments of the endolysosomal system, washed, and resuspended at the original titer in fresh medium. The cells were then collected by centrifugation at the indicated times, and the percentage of FITC–dextran that remained inside the cell was calculated as the ratio of the fluorescence at the indicated time compared with the fluorescence at time = 0. Shown is a representative experiment of three independent ones performed that indicated that RacC WT(+) cells displayed a delay in the release of fluid phase from the cell. In control Ax2 cells, fluid phase began to be released from the endolysosomal system after 45 min, whereas RacC WT(+) cells did not begin to release fluid phase until after 90 min. (C) Cells were pulsed with FITC–dextran for 10 min, chased in fresh media, and treated as described in B. The cells were then collected by centrifugation at the indicated times, and the intracellular pH was calculated as described in MATERIALS AND METHODS. There appeared to be a delay in the transport of fluid phase from acidic to nonacidic compartments, because FITC–dextran was released from an acidic compartment much more slowly in RacC WT(+) cells than in control Ax2 cells. This experiment was repeated two more times with the same results.
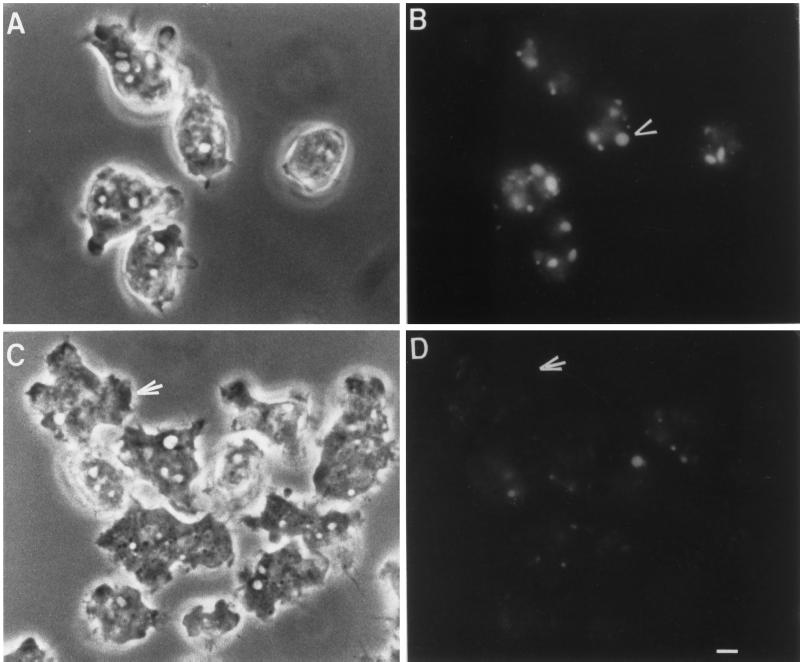
To determine whether RacC overexpression decreased macropinocytosis, one of the major pathways for fluid-phase internalization in Dictyostelium (Hacker et al., 1997), cells were allowed to adhere to coverslips and were then incubated with the fluid-phase marker LY for 5 min in growth medium. After the cells were washed in fresh medium, they were viewed using phase-contrast and fluorescence microscopy. The results of this experiment are shown in Figure 7. Few (<10%) of the RacC WT(+) cells internalized LY into large vesicles (Figure 7, C and D). In contrast, in a single field, 70% of control Ax2 cells formed at least one large vesicle that we consider to be a macropinosome (Figure 7, A and B).
Figure 7.
Macropinocytosis is inhibited in RacC WT(+) cells. Control Ax2 cells (A and B) and RacC WT(+) cells (C and D) were incubated with the fluid-phase marker lucifer yellow for 5 min to load up macropinosomes and then washed in fresh media, placed on a coverslip, and examined using phase-contrast (A and C) and fluorescence (B and D) microscopy. Because the size of micropinosomes is below the level of detection, only larger macropinosomes can be visualized by fluorescence microscopy. RacC WT(+) cells fail to form macropinosomes [arrow points to a RacC WT(+) cell], whereas control Ax2 cells contain three to four macropinosomes per cell (arrowhead). Bar, 2 μm.
In D. discoideum, fluid-phase markers are normally ingested, transferred to acidic lysosomal vesicles, and then transported to large nonacidic organelles termed postlysosomes before their release from the cell (Aubry et al., 1993b; Padh et al., 1993); no rapid recycling fluid-phase compartment has been observed. Interestingly, transport from acidic lysosomes to postlysosomes is blocked by cytochalasin A treatment, which disrupts the actin cytoskeleton. To determine whether RacC overexpression, demonstrated above to regulate actin cytoskeleton distribution, also affected fluid-phase exocytosis, we performed fluid-phase flux experiments. Control Ax2 cells, pulsed for 10 min with FITC–dextran in growth medium and then resuspended in fresh media, began to efflux their fluid phase after 45–60 min (Figure 6B); in contrast, RacC WT (+) cells did not start exocytosing FITC–dextran until after 60–90 min. Furthermore, after 2.5 h control Ax2 cells had exocytosed almost all of the FITC–dextran into the supernatant, whereas RacC WT(+) cells still retained 40% of the FITC–dextran internalized during the pulse period. These data suggest that the movement of fluid phase through the endolysosomal system was being delayed in RacC WT(+) cells. To determine the step at which the movement of fluid phase was being delayed, the endosomal pH of RacC WT(+) cells was measured by a dual excitation ratio method using FITC–dextran as a pH probe (see MATERIALS AND METHODS). As indicated in Figure 6C, in wild-type cells, FITC–dextran moved from a slightly acidic compartment to more acidic lysosomes within 20 min of entering the cell. From these acidic compartments, fluid phase then moved to less acidic postlysosomes within 60 min; however, in RacC WT(+) cells, the fluid phase reached acidic compartments at a slightly reduced rate and only slowly entered less acidic compartments, presumably postlysosomes. These data indicate that RacC overexpression leads to a delay at two different steps along the endolysosomal pathway: 1) in the internalization of fluid phase, via macropinocytosis, into the cell, and 2) in the movement of fluid phase from acidic to less acidic compartments.
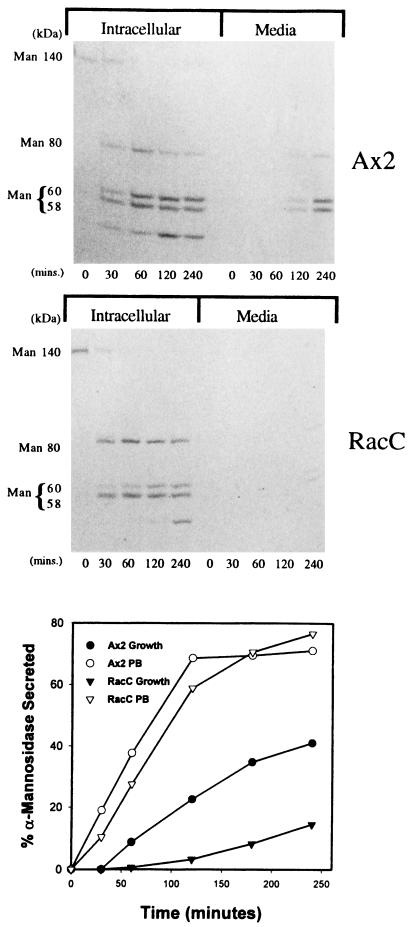
Next, we examined the rate of secretion of the lysosomal hydrolase α-mannosidase in growth medium to determine whether RacC overexpression affected lysosomal hydrolase secretion as well as the movement of internalized fluid phase markers through the endolysosomal system of D. discoideum. Not surprisingly, our experiments indicated that RacC WT(+) cells secreted α-mannosidase at a much slower rate than the control Ax2 cell line when suspended in growth medium (Figure 8, bottom panel). In response to hypo-osmotic shock or starvation, D. discoideum cells more rapidly secrete lysosomal hydrolases into the extracellular environment (Cardelli, 1993). Because RacC WT(+) cells were defective in the secretion of α-mannosidase in HL5 growth media, we predicted that they might be defective in the secretion of lysosomal enzymes induced by starvation conditions. To test this, secretion experiments were performed in the same manner as described above, except that cells were suspended in phosphate buffer to induce secretion. As predicted, the starved control Ax2 cells secreted α-mannosidase at a higher rate than cells suspended in growth medium (Figure 8, bottom panel); however, in contrast to secretion rates observed in growth medium, the starved RacC WT(+) cell lines secreted α-mannosidase at the same rate as control cells. This indicates that RacC may play a negative role in the secretion of α-mannosidase under growth conditions but does not appear to play a significant role in regulating the secretion of lysosomal hydrolases from cells responding to starvation.
Figure 8.
RacC overexpression does not affect the processing, targeting, or secretion of lysosomal α-mannosidase. (Top two panels) Cells were pulsed with [35S]methionine in HL5 growth medium for 20 min, washed, and placed in fresh medium. At the indicated times, cells and supernatants were collected by centrifugation, and α-mannosidase was immunoprecipitated from the samples. The resultant immunoprecipitates were fractionated using SDS-PAGE and then subjected to autoradiography. The processing and sorting of α-mannosidase is normal in both control Ax2 and RacC WT(+) cells. α-Mannosidase is synthesized as a 140-kDa precursor protein, which is processed to a mature 58- and 60-kDa form through an 80-kDa intermediate protein. There is a defect in the secretion of mature α-mannosidase from the postlysosomal compartment of RacC WT(+) cells, however. In control Ax2 cells, the mature form of α-mannosidase (and some of the 80-kDa intermediate) is secreted into the extracellular milieu after a 120-min chase. RacC WT(+) cells did not begin to secrete α-mannosidase until 240 min after chase. (Bottom panel) Control Ax2 and RacC WT(+) cells were incubated in HL5 growth medium or phosphate buffer and were harvested at the indicated times. The samples were separated into cell pellets and supernatants by centrifugation, and the amount of α-mannosidase activity in these samples was calculated. RacC WT(+) cells were defective in the secretion of α-mannosidase compared with control cells in growth medium; however, under starvation conditions, control Ax2 and RacC WT(+) cells secrete the lysosomal hydrolase α-mannosidase at equivalent rates. This experiment was repeated two more times with identical results.
Because RacC overexpression negatively affected the secretion of α-mannosidase and fluid phase from cells suspended in growth medium, we suspected that overexpression of this G protein might also affect other lysosomal hydrolase trafficking events, such as proteolytic processing and sorting. To examine the kinetics of processing and the efficiency of sorting of lysosomal α-mannosidase, cells were subjected to a radiolabel pulse–chase protocol followed by immunoprecipitation of cellular and extracellular radiolabeled α-mannosidase. The immunoprecipitated protein was subjected to SDS-PAGE followed by autoradiography. The top two panels of Figure 8 indicate that RacC overexpression did not appear to have an effect on the processing rates of the 140-kDa precursor to the mature 58- and 60-kDa forms or the targeting efficiency of α-mannosidase to lysosomes, as evidenced by the low level of secretion of radiolabeled 140-kDa precursor polypeptides; however, consistent with the secretion assays described above, there was an apparent defect in the rate of secretion of radiolabeled α-mannosidase from RacC WT(+) D. discoideum cells. In control cells, the mature form of α-mannosidase (and some of the 80-kDa intermediate) began to be secreted into the extracellular milieu after a 120-min chase; however, RacC WT(+) cells did not begin to secrete α-mannosidase until after a 240-min chase.
DISCUSSION
In studies reported here, we demonstrate that modest overexpression of a Rho family GTPase, RacC, results in changes in cell morphology and actin cytoskeleton organization in the simple eukaryote D. discoideum and leads to a significant increase in phagocytosis rates. Cells overexpressing HA-tagged RacC at a level three- to fourfold higher than endogenous RacC contained F-actin–rich “membrane blebs” that were organized into dynamic dorsal and lateral surface structures that we term petalopodia, because they resemble the petals of a flower. The petalopodia structures described here are not to be confused with earlier defined structures termed “petaloid coelomocytes,” from the sea urchin Strongylocentrotus droebachiensis (Edds, 1977), which spontaneously change into filopodia to induce the clotting process in the celom of this organism. Although the petalopodia appearance is reminiscent of coronin-containing “crown-like” structures described previously by Hacker et al. (1997), we believe that they are distinct from one another. First of all, crown-like structures are predominantly located on the dorsal surface of the cell, whereas we observed petalopodia forming on both the lateral edges and the dorsal surfaces of RacC WT(+) cells. In addition, crown-like structures were only infrequently observed in RacC WT(+) cells. Finally, crown-like structures are associated with the engulfment of both fluid-phase and particulate matter and open up as a cup-like structure to bring material into the cell. Using video microscopy to examine GFP-labeled ABP-120 in the RacC WT(+) cells, we observed that the cellular F-actin never formed into open crown-like structures (unpublished results). Because the petalopodia are also morphologically different from other previously identified actin-based structures such as lamellipodia or filopodia, they may represent a new form of actin-based structure.
The biochemical mechanisms regulating petalopodia formation are not known, although data presented here suggest that their formation involves RacC and one or more wortmannin- or LY294002-sensitive PI 3-kinase(s). The fact that PI 3-kinase activity appeared necessary for the formation of petalopodia was not unexpected because previous studies have indicated that these enzymes were also necessary for PDGF-induced membrane ruffling in porcine aortic endothelial cells (Wennstrom et al., 1994) and the Ras-mediated signal transduction pathway that leads to actin cytoskeleton changes (Rodriguez-Viciana et al., 1997). Rac can also interact with PI 3-kinases, and growth factors can activate Rac through activation of PI 3-kinases (Hawkins et al., 1995; Bokoch et al., 1996); however, our results do not distinguish between PI 3-kinase and RacC operating in the same or parallel pathways to regulate the formation of petalopodia, although we favor the first possibility.
RacC WT(+) cells internalized both latex beads and bacteria at three times the rate of control cells. This same phenotype was observed for cells overexpressing non-epitope–tagged RacC, suggesting that the HA epitope does not influence phagocytosis (unpublished results). Interestingly, treating cells with the PI 3-kinase–specific inhibitors Wortmannin and LY294002, which inhibited the formation of petalopodia, only slightly and insignificantly impaired RacC WT(+) cells in their ability to internalize latex beads and bacteria (Figure 6B). These drugs also did not inhibit phagocytosis in control cells and, in fact, caused a slight stimulation in bead uptake. These results indicate that, although PI 3-kinases may be necessary for RacC-induced morphological and actin cytoskeletal changes, they do not play a major role in RacC-induced phagocytosis, and that RacC-induced actin cytoskeleton changes (petalopodia formation) may not play an important role in the stimulation of phagocytosis. These results are in contrast to other studies that suggested PI 3-kinases were necessary for phagocytosis in macrophages (Araki et al., 1996). Although our results were unexpected, they are consistent with previous observations indicating that membrane ruffling (dependent on PI 3-kinase activity) could be functionally uncoupled from a stimulation of pinocytosis in mammalian cells. (Kotani et al., 1994; Li et al., 1997).
Interestingly, RacC WT(+) cells were inhibited in their ability to internalize FITC–dextran markers via macropinocytosis, although they internalized particles at rates higher than control cells. This is consistent with previous experiments that indicated that Rac1 and Rho may negatively regulate receptor-mediated endocytosis (Lamaze et al., 1996), although Rho proteins have been implicated in positively regulating bulk fluid-phase pinocytosis (Schmalzing et al., 1995). This is an important observation, because it has been proposed previously that phagocytosis and macropinocytosis are biochemically similar processes in macrophages (Araki et al., 1996) and D. discoideum (Hacker et al., 1997). In contrast, our data suggest that these processes are mechanistically distinct in D. discoideum. Because the formation of crown-like structures is associated with macropinocytosis (Hacker et al., 1997), this evidence also supports our hypothesis that the actin-based petalopodia structures are functionally distinct from crown-like structures; however, our results presented here do not distinguish between RacC acting as a negative regulator of macropinocytosis or as an inducer of the formation of F-actin structures that are incompatible with macropinocytosis.
Several lines of evidence indicate that PKC may play a role in the regulation of the actin cytoskeleton and/or phagocytosis in mammalian cells such as macrophages (Allen and Aderem, 1996). For instance, PKC is activated in response to ligation of the Fc receptor in human monocytes (Zheleznyak and Brown, 1992), suggesting that it may be involved in signal transduction leading to phagocytosis; however, our studies presented here indicated that PKC per se might not play a major role in regulating phagocytosis in Dictyostelium, because treatment of cells with the PKC inhibitors bis-indolylmaleimide I and chelerythrine did not reduce phagocytosis. Importantly, it has been shown previously that bis-indolylmaleimide I–sensitive forms of PKC do exist in D. discoideum (Phillips et al., 1997); however, bacteria and latex bead uptake were significantly inhibited when cells were treated with the inhibitor calphostin C (a drug that competes with phorbol esters for binding to DAG-binding domains), suggesting that a non-PKC protein containing a DAG-binding motif was mediating phagocytosis in Dictyostelium. Also, calphostin C treatment effectively reversed the formation of petalopodia in RacC WT(+) cells. Whether this unidentified protein is signaling upstream or downstream of RacC remains to be determined at this time and will require the generation of a Dictyostelium cell line that overexpresses a constitutively active form of RacC. Interestingly, many Rho family GEFs contain DAG-binding domains (Cerione and Zheng, 1996), suggesting that the target of calphostin C may be an upstream activator of RacC.
In addition to inhibiting macropinocytosis, RacC overexpression also led to a decrease in the rate of efflux of fluid-phase and lysosomal enzymes from the endolysosomal system of D. discoideum. This block appeared to be imposed at the level of fluid-phase movement from acidic to nonacidic compartments. Although yet to be demonstrated conclusively, this result suggests that RacC overexpression may reduce the transport of fluid phase from lysosomes to postlysosomes, a process that may depend on proper F-actin regulation and the function of other GTPases and lipid kinases. For instance, in addition to RacC, we (and others) have demonstrated that Rab7, a Rab4-like GTPase (RabD), the PI 3-kinases DdPIK1 and DdPIK2, vacuolin B, and actin regulate this step (Bush et al., 1996; Temesvari et al., 1996; Buczynski et al., 1997a,b; Hacker et al., 1997; Jenne et al., 1998). It will be important to determine whether (and how) these proteins functionally interact and, if so, the order of interaction required for the formation of postlysosomes. Why RacC overexpression mediates disparate effects on phagocytosis and fluid-phase pinocytosis and trafficking is unclear at this time, but it raises the question that we are currently experimentally attempting to answer: are the effects mediated by RacC on the movement of fluid-phase matter throughout the endolysosomal system direct, or are they indirect effects caused by a recruitment of F-actin that leads to a stimulation of phagocytosis?
We and others have demonstrated recently that other GTPases, not belonging to the Rho subfamily, can also regulate the distribution of the cellular actin cytoskeleton and endolysosomal processes. For example, overexpressing wild-type or constitutively activated (GTP-bound) forms of a Rap1-like GTPase induced the formation of lamellipodia (Rebstein et al., 1997) and stimulated phagocytosis in D. discoideum, whereas overexpressing dominant negative (GDP-bound) forms of Rap1 inhibited phagocytosis (unpublished results). Taken together, these experiments suggest a possible model in which small GTPases may interact in the signal transduction pathway regulating phagocytosis in Dictyostelium. Defining the biochemical nature of these interactions is a subject of future investigations.
ACKNOWLEDGMENTS
We thank Dr. Arturo DeLozanne for the RacE-overexpressing cell line and anti-RacE antibodies. We also thank Linyi Zhang for critical technical assistance. This work was supported by grants from National Institutes of Health to J.A.C. (DK3923205) and D.A.K. (GM40599). We acknowledge the support from The Feist-Weiller Cancer Center and the Center for Excellence in Arthritis and Rheumatology Research.
REFERENCES
- Allen LA, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40. doi: 10.1016/s0952-7915(96)80102-6. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Klein G, Martiel JL, Satre M. Kinetics of endosomal pH evolution in Dictyostelium discoideum amoebae. Study by fluorescence spectroscopy. J Cell Sci. 1993a;105:861–866. doi: 10.1242/jcs.105.3.861. [DOI] [PubMed] [Google Scholar]
- Aubry L, Klein G, Satre M. Endo-lysosomal acidification in Dictyostelium discoideum amoebae. Effects of two endocytosis inhibitors: caffeine and cycloheximide. Eur J Cell Biol. 1993b;61:225–228. [PubMed] [Google Scholar]
- Bokoch GM. Regulation of the phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 1995;5:109–113. doi: 10.1016/s0962-8924(00)88960-6. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997a;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski G, Grove B, Nomura A, Kleve M, Bush J, Firtel RA, Cardelli J. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to post-lysosome transport, and actin cytoskeleton organization. J Cell Biol. 1997b;136:1271–1286. doi: 10.1083/jcb.136.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J, Franek K, Cardelli J. Cloning and characterization of seven novel Dictyostelium discoideum rac-related genes belonging to the rho family of GTPases. Gene. 1993;136:61–68. doi: 10.1016/0378-1119(93)90448-c. [DOI] [PubMed] [Google Scholar]
- Bush J, Temesvari L, Rodriguez-Paris J, Buczynski G, Cardelli J. A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol Biol Cell. 1996;7:1623–1638. doi: 10.1091/mbc.7.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli JA. Regulation of lysosomal trafficking and function during growth and development of Dictyostelium discoideum. In: Storie B, Murphy R, editors. Advances in Cell and Molecular Biology of Membranes. Greenwich, CT: JAI Press; 1993. pp. 341–390. [Google Scholar]
- Cardelli JA, Richardson J, Miears D. Role of acidic intracellular compartments in the biosynthesis of Dictyostelium lysosomal enzymes. The weak bases ammonium chloride and chloroquine differentially affect proteolytic processing and sorting. J Biol Chem. 1989;264:3454–3463. [PubMed] [Google Scholar]
- Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- Early AE, Williams JG. Two vectors which facilitate gene manipulation and a simplified transformation procedure for Dictyostelium discoideum. Gene. 1987;59:99–106. doi: 10.1016/0378-1119(87)90270-8. [DOI] [PubMed] [Google Scholar]
- Edds KT. Dynamic aspects of filopodial formation by reorganization of microfilaments. J Cell Biol. 1977;73:479–491. doi: 10.1083/jcb.73.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haataja L, Groffen J, Heisterkamp N. Characterization of RAC3, a novel member of the Rho family. J Biol Chem. 1997;272:20384–20388. doi: 10.1074/jbc.272.33.20384. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Schreiber A, Zhang W, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn C, Aderem A. MARCKS is an actin filament cross-linking protein regulated by protein kinase C and calcium calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Jenne N, Rauchenberger R, Hacker U, Kast T, Maniak M. Targeted gene disruption reveals a role for vacuolin B in the late endocytic pathway and exocytosis. J Cell Sci. 1998;111:61–70. doi: 10.1242/jcs.111.1.61. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kotani K, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- Larochelle DA, Vithalani KK, De Lozanne A. A novel member of the rho family of small GTP-binding proteins is specifically required for cytokinesis. J Cell Biol. 1996;133:1321–1329. doi: 10.1083/jcb.133.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle DA, Vithalani KK, De Lozanne A. Role of Dictyostelium racE in cytokinesis: mutational analysis and localization studies by use of green fluorescent protein. Mol Biol Cell. 1997;8:935–944. doi: 10.1091/mbc.8.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, D’Souza-Schorey C, Barbieri MA, Cooper JA, Stahl PD. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J Biol Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell. 1995;83:915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Mierendorf RCJ, Dimond RL. Functional heterogeneity of monoclonal antibodies obtained using different screening assays. Anal Biochem. 1983;135:221–229. doi: 10.1016/0003-2697(83)90754-6. [DOI] [PubMed] [Google Scholar]
- Niewohner J, Weber I, Maniak M, Muller-Taubenberger A, Gerisch G. Talin-null cells of Dictyostelium are strongly defective in adhesion to particle and substrate surfaces and slightly impaired in cytokinesis. J Cell Biol. 1997;138:349–361. doi: 10.1083/jcb.138.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Padh H, Ha J, Lavasa M, Steck TL. A post-lysosomal compartment in Dictyostelium discoideum. J Biol Chem. 1993;268:6742–6747. [PubMed] [Google Scholar]
- Phillips P, Thio M, Pears C. A protein kinase C-like activity involved in the chemotactic response of Dictyostelium discoideum. Biochim Biophys Acta. 1997;1349:72–80. doi: 10.1016/s0005-2760(97)00084-2. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Rathi A, Kayman SC, Clarke M. Induction of gene expression in Dictyostelium by prestarvation factor, a factor secreted by growing cells. Dev Genet. 1991;12:82–87. doi: 10.1002/dvg.1020120115. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev. 1995;5:24–30. doi: 10.1016/s0959-437x(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Rose G, Pomerat C, Shindler T, Trunnell J. A cellophane strip technique for culturing tissue in multipurpose culture chambers. J Biophys Biochem Cytol. 1958;4:761–764. doi: 10.1083/jcb.4.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Gauthier-Rouviere C, Doucet-Brutin S, Fort P. The small GTPases Cdc42Hs, rac1 and RhoG delineate raf-independent pathways that cooperate to transform NIH3T3 cells. Curr Biol. 1997;7:629–637. doi: 10.1016/s0960-9822(06)00289-2. [DOI] [PubMed] [Google Scholar]
- Schmalzing G, Richter HP, Hansen A, Schwarz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J Cell Biol. 1995;130:1319–1332. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesvari L, Zhang L, Cardelli JA. The role of the actin cytoskeleton in the endolysosomal system of Dictyostelium discoideum. Mol Biol Cell. 1996;7:451a. (abstract). [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznyak A, Brown EJ. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation. Evidence for protein kinase C translocation to phagosomes. J Biol Chem. 1992;267:12042–12048. [PubMed] [Google Scholar]
- Zhou K, Pandol S, Traynor-Kaplan G. Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J Cell Sci. 1998;111:283–294. doi: 10.1242/jcs.111.2.283. [DOI] [PubMed] [Google Scholar]