Abstract
Background
Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy in current use were defined using autopsy results or echocardiography; criteria defined using mortality might be more clinically meaningful.
Methods
Using data from NHANES III, we selected electrocardiographic measures that best differentiated those surviving at five years from those who did not. We identified voltage thresholds using regression techniques, and then compared survival for subjects above and below the thresholds.
Results
Cornell voltage, Cornell product, and Novacode estimate of left ventricular mass index were discriminative for mortality and had identifiable thresholds present in their relationships with mortality. Independent of systolic blood pressure, there were significant associations with five-year mortality for Novacode index above threshold; hazard ratios were 1.58 for women and 1.27 for men, and for five-year cardiovascular mortality were 1.78 for women and 2.34 for men.
Conclusions
Electrocardiographic criteria for left ventricular hypertrophy validated against mortality might be clinically useful.
Left ventricular hypertrophy (LVH) is a significant risk factor for cardiovascular disease independent of blood pressure (1,2), and reductions in left ventricular mass are associated with lower cardiovascular event rates independent of reductions in blood pressure. In the Losartan Intervention for Endpoint reduction (LIFE) study, the risk of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke was reduced by 22% for each one standard deviation decrement in left ventricular mass index measured by echocardiography, independent of systolic blood pressure or treatment assigned (3). In the Heart Outcomes Prevention Evaluation (HOPE) trial, patients who had resolution of electrocardiographic LVH findings or who did not develop new LVH findings had significantly fewer cardiovascular deaths than did patients who did not have resolution or who developed new LVH (4). In the Appropriate Blood Pressure Control in Diabetes (ABCD) trial, the change in a QRS voltage measure of LVH predicted time to cardiovascular events, after controlling for change in blood pressure (5). These findings imply that hypertension treatment that leads to both regression of LVH and blood pressure reduction to goal may decrease cardiovascular event rates more than treatment to a blood pressure goal alone.
To be clinically meaningful, a definition of LVH should be linked to a mortality reduction and make use of readily available technology. The most readily available technologies used to define LVH in research and in clinical practice are echocardiography and electrocardiography. Echocardiography has better sensitivity and specificity for detection of LVH than electrocardiography, but is expensive and is technically inadequate in 10–20% of unselected subjects (1) and in over 30% in of older subjects (6). In contrast, electrocardiography is inexpensive, is readily available, and can be performed in nearly all patients and interpreted with relatively little advanced skill.
We hypothesized that electrocardiographic voltage measures of LVH treated as continuous variables have relationships with mortality that can be modeled to define a threshold above which mortality rises progressively, independent of blood pressure.
Methods
Data Sources
The data sources for the study were the Third National Health and Nutrition Examination Survey (NHANES III) (7) database and the NHANES III Linked Mortality File (8).
NHANES III was conducted in 81 counties in the United States between 1988 and 1994; subjects were selected to obtain a sample representative of the civilian, non-institutionalized population of the United States greater than 2 months old. Electrocardiograms were obtained in NHANES III only for subjects older than 40 years. Twelve-lead tracings were recorded during examination at a mobile examination center by trained technicians using a Marquette MAC 12 system. Electrocardiograms were transferred to the National Center for Health Statistics for electronic analysis. Baseline blood pressure was measured as the average of the three recordings performed at the mobile examination center by a physician. Examiners used a mercury manometer and were instructed to record the blood pressure from the right arm with the subject seated, choosing the appropriate cuff size from among four available.
The NHANES III Linked Mortality File contains the results of linking NHANES III subject identifiers with data in the National Death Index (9) as of December 31, 2000. Date of death and cause of death available in the National Death Index are from death certificates.
Definitions
We chose to examine electrocardiographic measures of LVH that had been validated by echocardiography and that could be analyzed as continuous variables. Using these criteria we selected six measures:
The Cornell voltage (10) is the sum of the R wave dimension in lead aVL and the S wave dimension in lead V3; left ventricular hypertrophy is said to be present if this voltage is greater than 20 mm in women and 28 mm in men. The Cornell product (11) is the product of the Cornell voltage and the QRS duration; left ventricular hypertrophy is said to be present if this product is greater than 2436 mm-msec (adjusted by the addition of 8 mm for female gender). The adjusted Cornell voltage is defined by the regression equations of Norman and Levy (12):
For males: (Cornell voltage + 0.0174*age + 0.1914*BMI – 4.0)/2
For females: (Cornell voltage + 0.0387*age + 0.1778*BMI – 4.9)/3
where BMI is the body mass index.
The Sokolow-Lyon voltage (13) has been defined variously in the literature; we chose to define it as the sum of the greatest S wave dimension in leads V1 or V2 and the greatest R wave dimension in leads V5 or V6; left ventricular hypertrophy is said to be present if this voltage is greater than 35 mm. Finally, we used the Novacode estimates of left ventricular mass and left ventricular mass index calculated from the regression equations of Rautaharju et al (14). The variables and coefficients for these equations are available in the NHANES III Electrocardiography Data File documentation (15) and in the Appendix.
We also examined the indpendent effect of the ST-T changes associated with LVH (the so-called “strain pattern”) on outcomes, both separately and in combination with Cornell voltage and Cornell product. A strain pattern was judged to be present if there was horizontal or downsloping ST depression associated with T wave inversion in lead V5 and/or V6 (16).
We defined an analysis set by including those subjects with all the necessary data available to calculate the electrocardiographic measures and whose vital status was known. We excluded subjects with QRS duration greater than 0.12 sec since validity in the presence of bundle branch block has not been established for all of the electrocardiographic measures.
We defined five-year mortality as the primary outcome of interest. All-cause mortality is less subject to ascertainment bias than is cardiovascular mortality, and the five year time frame has been commonly used in cardiovascular epidemiology as a compromise between the longer times necessary for effects of the exposure (in this case LVH) to accrue and the shorter times that exclude the accrual of competing causes of mortality.
We defined cardiovascular death as those for which the principal cause of death was listed on the death certificate as ICD-9 codes 390.0–459.9 (for deaths before or during 1999) or ICD-10 codes I10-15, I20-25, I50-51, I60-69, I70-73 (for deaths after 1999).
In assessing the independent contribution of electrocardiographic LVH measures to mortality risk, we defined the relevant covariates as the Framingham risk factors (17) of age, gender, smoking status, presence or absence of diabetes, systolic blood pressure, and total and high density lipoprotein cholesterol. Diabetes was defined in NHANES III by self-identification.
Statistical Analysis
We assessed for colinearity among the six measures using Pearsons’s correlation coefficients.
We selected the electrocardiographic measures best able to discriminate those at risk for mortality from those not at risk using receiver operating characteristic (ROC) curves. We divided the sample into deciles (with an equal number of subjects in each group), plotted five year all-cause mortality by decile, and calculated sensitivity and specificity for five-year mortality by decile. We constructed a separate ROC curve for each of the six measures and calculated the area under the ROC curve. Measures were considered discriminative if the lower bound of the 95% confidence intervals around the areas under the curve was greater than 0.50.
For the discriminative measures, we sought thresholds above which mortality rose above a baseline level. Because left ventricular mass and left ventricular mass index vary systematically by gender, we sought separate thresholds for men and women. We selected at random two thirds of the analysis sample for a development sample. To enhance interpretability, we recoded the data for this set by ten-unit ranges for the measure, except for the smallest and largest of the ranges which included the remaining values on either end, for a total of ten ranges. We applied piecewise regression to this set with five-year mortality as the outcome, modeling the data as a spline function with two distinct slopes. We took as our thresholds the midpoint of the ranges for which risk was significantly greater than for values below that category.
We tested the performance of the thresholds we found in four ways:
First, we compared five-year survival curves for male and female subjects with baseline voltage measures above and below the thresholds using the log-rank test.
Second, we used Cox proportional hazards models to assess for an independent contribution of voltage, first controlling for systolic blood pressure alone, and then controlling for all the Framingham risk factors, to the primary outcome of five-year all-cause mortality.
Third, we created the same Cox proportional hazards models for three secondary outcomes: five-year cardiovascular mortality, ten-year all-cause mortality, and ten-year cardiovascular mortality.
Finally, we repeated the Cox models in the one third of the sample not used to establish the thresholds.
Results
Of subjects in the NHANES III sample, 8561 had an electrocardiogram performed. We excluded from our analysis sample 641 subjects with QRS duration > 0.12 sec, 64 subjects because of incomplete electrocardiographic data, and 4 subjects because vital status was not known, leaving 7852 subjects with data available for analysis. In addition, 379 subjects were excluded from the adjusted analyses in the validation set because of missing Framingham risk factors.
The characteristics of the study sample are shown in Table 1 and reflect a middle aged and elderly population. The mean age of the sample was approximately 60 years and was slightly female predominant. Approximately 10% of subjects had diabetes and approximately 40% had systolic hypertension.
Table 1.
Characteristics of study population
| Variable: | Total | Males | Females |
|---|---|---|---|
| Age, years (mean, sd) | 59.9 (13.4) | 60 (13.4) | 59.7 (13.5) |
| Gender (% female) | 53% | - | - |
| Current smoker at baseline (%) | 23% | 27% | 18% |
| Total cholesterol, mmol/l (mean, sd) | 5.64 (1.14) | 5.51 (1.10) | 5.78 (1.16) |
| HDL* cholesterol, mmol/l (mean, sd) | 1.32 (0.42) | 1.19 (0.38) | 1.42 (0.42) |
| Diabetes (%) | 11% | 11% | 11% |
| Systolic blood pressure, mmHg | 132.9 (19.9) | 134.0 (18.5) | 132.4 (21.0) |
| Cornell voltage, mm | 13.1 (5.5); | 14.0 (5.7); | 12.0 (5.0); |
| (mean, sd; median, IQR) | 12.7 (9.20,16.5) | 14.0 (10.3,17.9) | 11.6 (8.38,15.1) |
| Cornell product, mm-msec | 1272 (580); | 1428 (610); | 1132 (512); |
| (mean, sd; median, IQR) | 1205 (858,1611) | 1368 (999,1788) | 1076 (761,1432) |
| Adjusted Cornell voltage | 1.5 (0.6); | 1.8 (0.6); | 1.2 (0.5); |
| (mean, sd; median, IQR) | 1.5 (1.08,1.90) | 1.8 (1.44,2.19) | 1.2 (0.88,1.50) |
| Sokolow-Lyons voltage, mm | 25.9 (7.8); | 27 (8.3); | 24.8 (7.0); |
| (mean, sd; median, IQR) | 25.0 (20.6,30.2) | 26.3 (21.5,32.1) | 23.8 (20.0,28.7) |
| Novacode mass, gms | 154 (29.4); | 172 (25.5); | 138 (22.6); |
| (mean, sd; median, IQR) | 153 (133,174) | 171 (156,188) | 136 (123,152) |
| Novacode mass index, gms/m2 | 105 (21.7); | 112 (20.5); | 98.4 (20.8); |
| (mean, sd; median, IQR) | 103 (90,118) | 110 (99,123) | 96.0 (85,110) |
HDL = high density lipoprotein
Correlation coefficients among the voltage measures are shown in Table 2; with the possible exception of the Cornell voltage and Cornell product, the measures are sufficiently different from one another to justify examination of each of them.
Table 2.
Pearson correlation coefficient matrix
| Cornell voltage | Cornell product | Adjusted Cornell | Sokolow-Lyons | Novacode LVM | Novacode LVMI | |
|---|---|---|---|---|---|---|
| Cornell voltage | 1.0 | |||||
| Cornell product | 0.942 | 1.0 | ||||
| adjusted Cornell | 0.628 | 0.621 | 1.0 | |||
| Sokolow-Lyons | 0.397 | 0.357 | 0.093 | 1.0 | ||
| Novacode LVM* | 0.566 | 0.631 | 0.855 | 0.095 | 1.0 | |
| Novacode LVMI† | 0.465 | 0.585 | 0.393 | 0.401 | 0.471 | 1.0 |
LVM = left ventricular mass
LVMI = left ventricular mass index
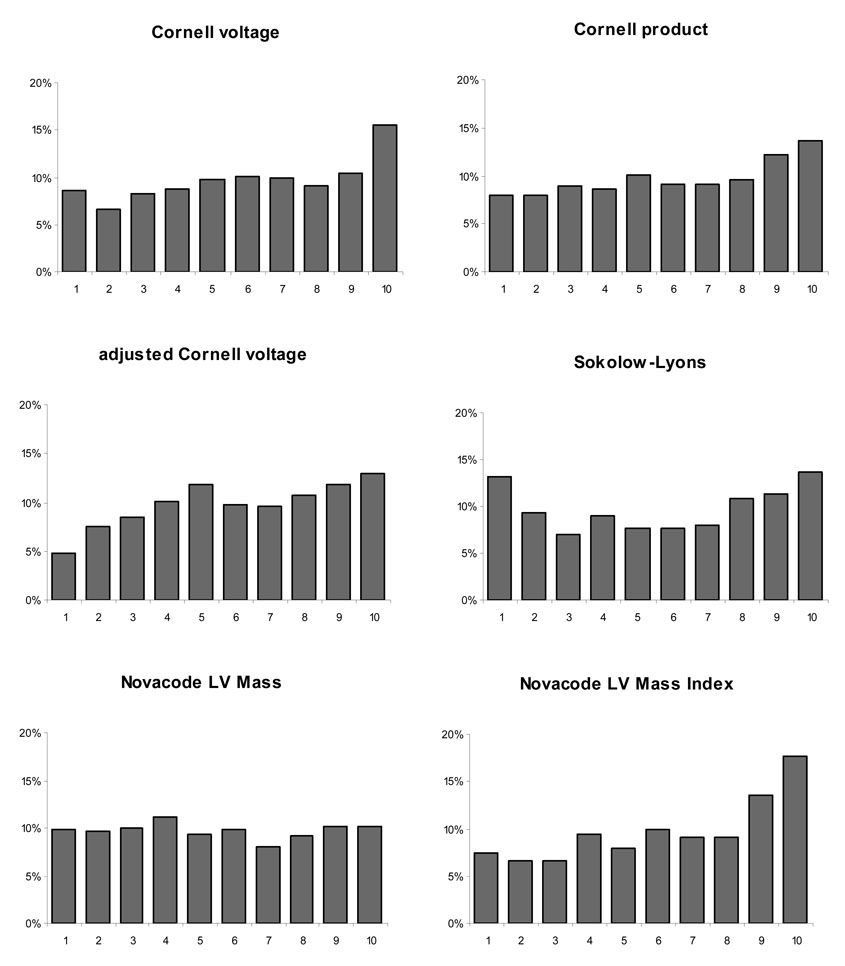
Figure 1 shows five-year all-cause mortality by decile for each of the six electrocardiographic measures, and Table 3 shows the area under the ROC curve (C-statistic) for each of the six measures. Four measures, Cornell voltage, Cornell product, adjusted Cornell voltage, and Novacode estimate of left ventricular mass index were discriminative for mortality, with C-statistics significantly greater than 0.5. Because we examined LVH as a univariate predictor of mortality, the C-statistics are much lower than that typically described for multivariable models.
Figure 1.
Five year all-cause mortality by decile for each of the voltage criteria tested
Table 3.
C-statistics and gender-specific thresholds for each of the voltage criteria
| Voltage Measure | C-statistic (95% CI) | Threshold- Females | Threshold- Males |
|---|---|---|---|
| Cornell voltage | 0.55 (0.53–0.57) | 19 mm | 20 mm |
| Cornell product | 0.55 (0.56–0.57) | 1700 mm-msec | 2150 mm-msec |
| Adjusted Cornell voltage | 0.56 (0.54–0.59) | Not applicable* | Not applicable |
| Sokolow-Lyons voltage | 0.52 (0.49–0.54) | Not applicable | Not applicable |
| Novacode LV† mass | 0.50 (0.48–0.52) | Not applicable | Not applicable |
| Novacode LV mass index | 0.59 (0.57–0.61) | 115 gms/m2 | 130 gms/m2 |
piecewise regression demonstrated there was no threshold for the adjusted Cornell voltage
LV= left ventricular
By piecewise regression analysis, there were statistically identifiable thresholds in the mortality relationship for three of the discriminative measures: Cornell voltage, Cornell product, and Novacode left ventricular mass index; these thresholds are shown in Table 3. In men 16.7% of the sample had a Novacode left ventricular mass index above threshold, 16.1% had a Cornell voltage above threshold, and 13.3% had Cornell product above threshold. Comparable figures for women were 19.3%, 9.2% and 13.2%.
As described in the Methods section, we tested the thresholds in four ways.
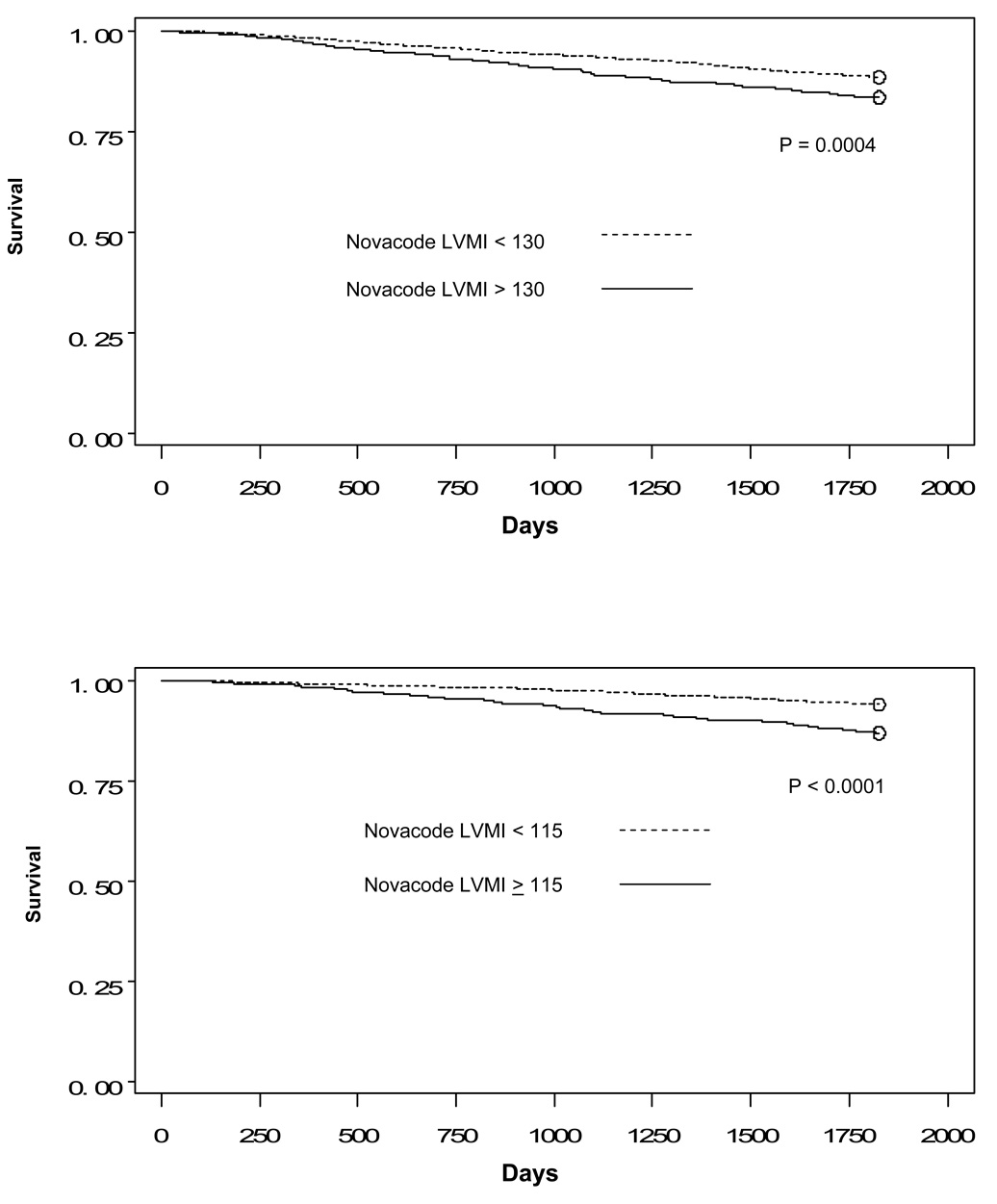
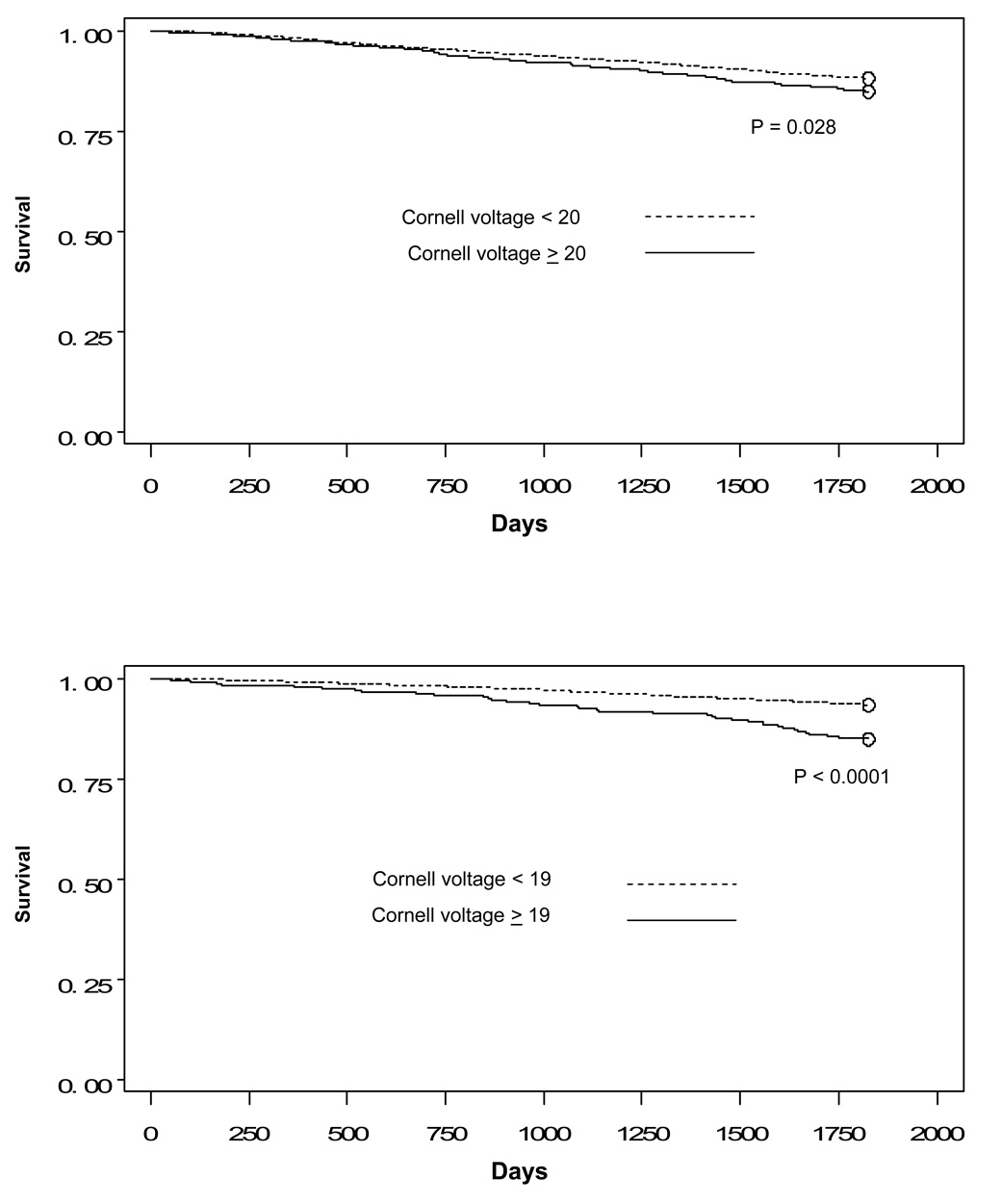
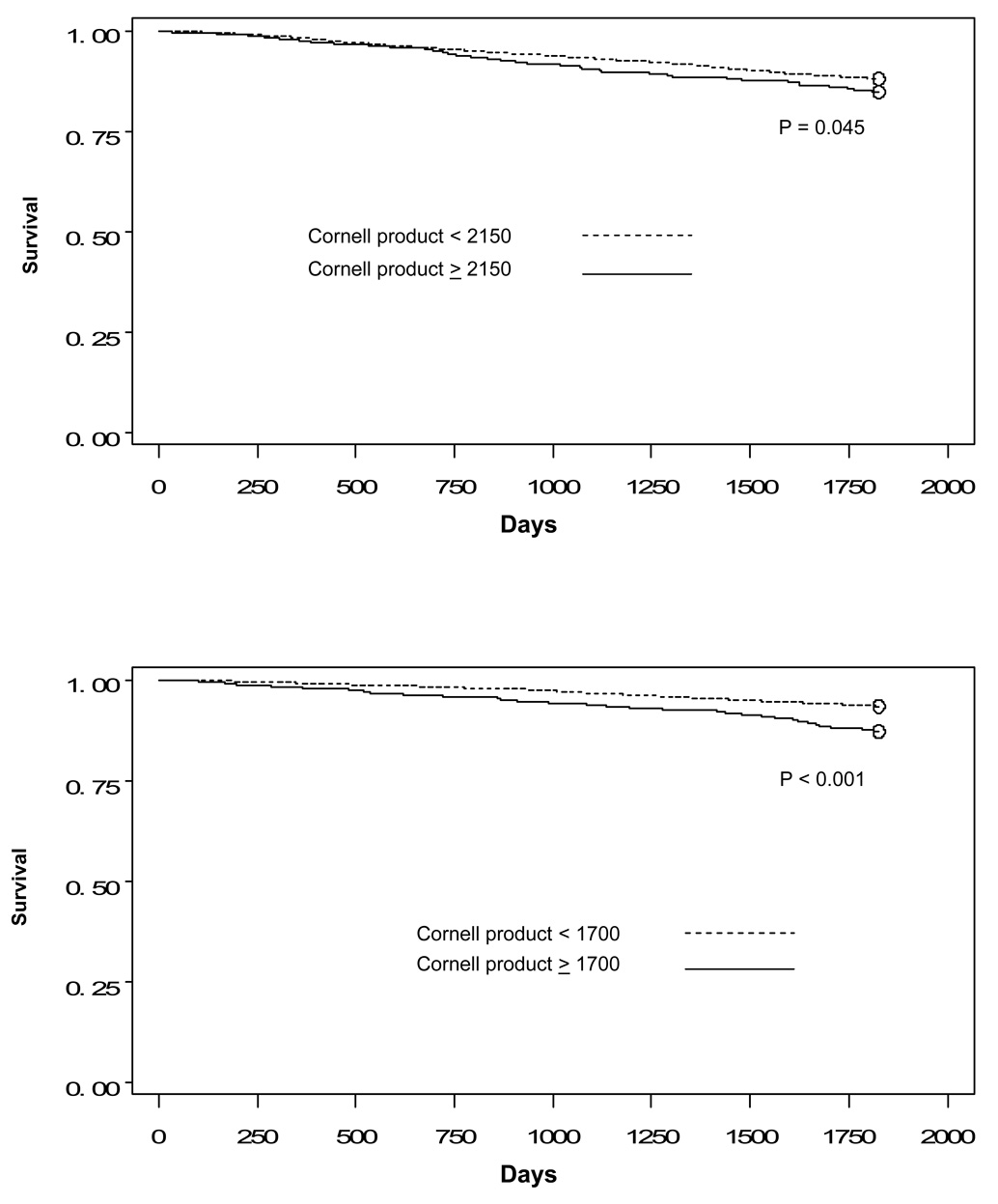
First, we compared five-year survival for women and for men by voltage above and below the thresholds. As shown in Figure 2, Figure 3, and Figure 4, there are statistically significant differences for all three sets of thresholds.
Figure 2.
Figure 2a: Kaplan-Meier curves comparing survival for men by Novacode left ventricular mass index above and below threshold
Figure 2b: Kaplan-Meier curves comparing survival for women by Novacode left ventricular mass index above and below threshold
Figure 3.
Figure 3a: Kaplan-Meier curves comparing survival for men by Cornell voltage above and below threshold
Figure 3b: Kaplan-Meier curves comparing survival for women by Cornell voltage above and below threshold
Figure 4.
Figure 4a: Kaplan-Meier curves comparing survival for men by Cornell product above and below threshold
Figure 4b: Kaplan-Meier curves comparing survival for women by Cornell product above and below threshold
Second, we assessed for an independent contribution of the voltages to five-year mortality by adjusting first for systolic blood pressure alone and then for all the Framingham risk factors. As shown in Table 4, statistically significant relationships for men are present with adjustment for all the Framingham risk factors but not for blood pressure alone. For women, there are statistically significant relationships adjusted for blood pressure but not for all the Framingham risk factors.
Table 4.
Associations between 5-year total mortality and electrocardiographic indices above threshold for the development set
| Cornell voltage | Cornell product | Novacode LVMI* | ||||
|---|---|---|---|---|---|---|
| Males (309 deaths) | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Unadjusted | 1.42 | (1.08–1.87) | 1.38 | (1.03–1.86) | 1.52 | (1.16–1.98) |
| Adjusted for systolic BP | 1.19 | (0.89–1.58) | 1.16 | (0.85–1.57) | 1.27 | (0.99–1.68) |
| Adjusted for all Framingham risk factors† | 1.34 | (1.01–1.79) | 1.34 | (0.98–1.82) | 1.40 | (1.05–1.85) |
| Females (201 deaths) | ||||||
| Unadjusted | 2.66 | (1.89–3.75) | 2.33 | (1.70–3.20) | 2.33 | (1.74–3.12) |
| Adjusted for systolic BP | 1.65 | (1.15–2.24) | 1.60 | (1.15–2.24) | 1.58 | (1.16–2.16) |
| Adjusted for all Framingham risk factors | 1.34 | (0.91–1.98) | 1.41 | (0.99–2.01) | 1.12 | (0.81–1.54) |
left ventricular mass index
age, smoking status, diabetes, systolic blood pressure, total cholesterol, HDL cholesterol
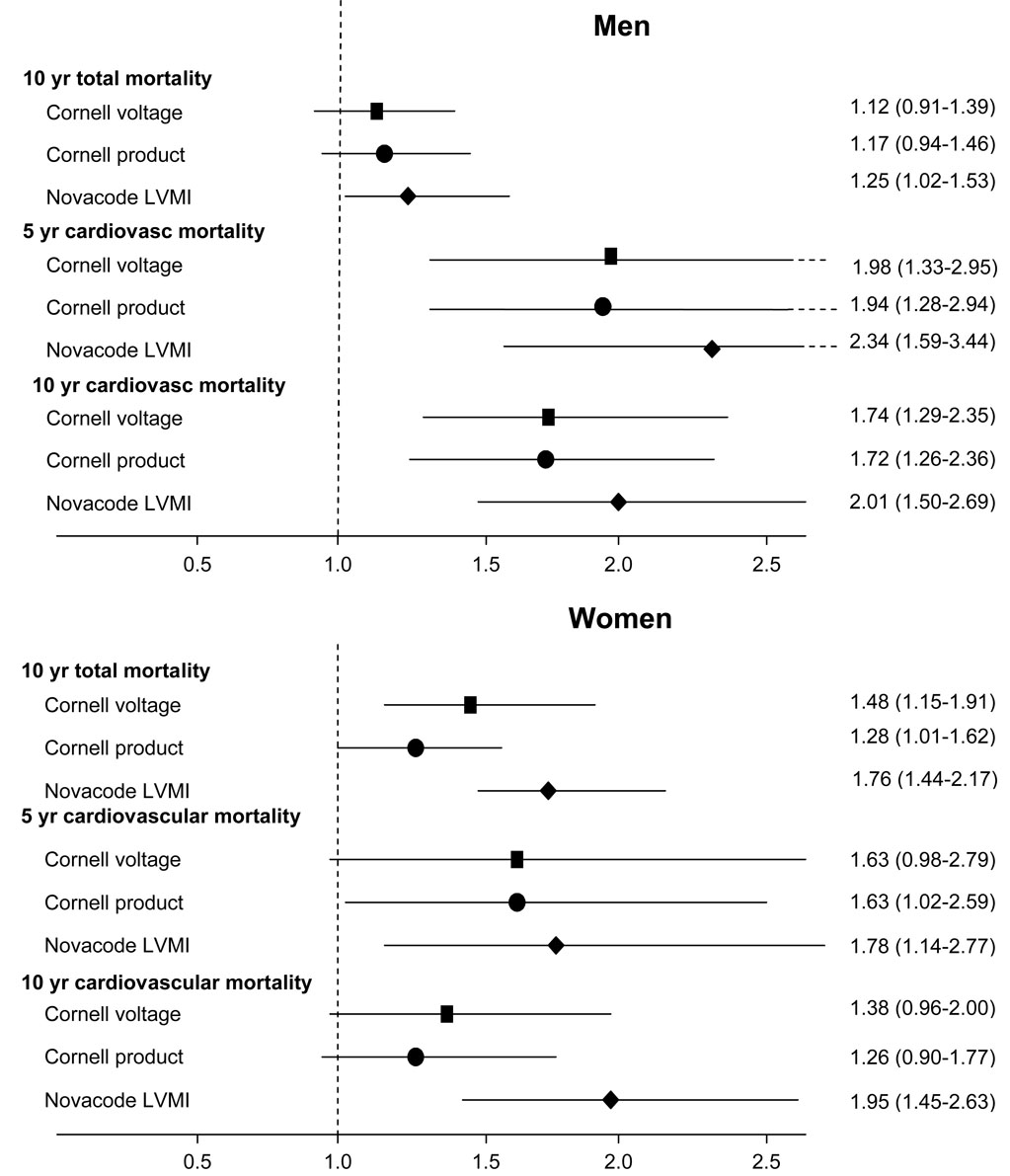
Third, we created the same Cox proportional hazards models for the three secondary outcome measures of five-year cardiovascular mortality, ten-year all-cause mortality, and ten-year cardiovascular mortality. Adjusted relationships were stronger for cardiovascular mortality than for all-cause mortality. Figure 4 shows the results for the adjustment for systolic blood pressure alone. The Novacode left ventricular mass index predicted mortality independent of blood pressure for all three secondary outcome measures; results with the other two measures were less consistent. With adjustment for all Framingham risk factors, in men the thresholds for all three measures predicted mortality independently for all three secondary outcomes. For women, the thresholds predicted mortality independent of all Framingham risk factors only for Novacode left ventricular mass index and ten-year cardiovascular mortality.
Finally, we tested these thresholds in the one third of the sample not used to develop the thresholds. As shown in Table 5, hazard ratios were consistent with those found in the two thirds of the sample used to develop the thresholds for Novacode left ventricular mass index but not for the Cornell voltage and Cornell product. Results were similar for the secondary outcomes in the validation set.
Table 5.
Associations between 5-year total mortality and electrocardiographic indices above threshold for the validation set
| Cornell voltage | Cornell product | Novacode LVMI* | ||||
|---|---|---|---|---|---|---|
| Males (153 deaths) | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Unadjusted | 1.02 | (0.64–1.62) | 1.08 | (0.65–1.80) | 1.43 | (0.97–2.11) |
| Adjusted for systolic BP | 0.82 | (0.51–1.31) | 0.88 | (0.53–1.46) | 1.14 | (0.76–1.70) |
| Adjusted for all Framingham risk factors† | 0.86 | (0.53–1.40) | 0.91 | (0.53–1.55) | 1.29 | (0.86–1.92) |
| Females (118 deaths) | ||||||
| Unadjusted | 1.83 | (1.04–3.21) | 1.45 | (0.87–2.42) | 2.19 | (1.44–3.32) |
| Adjusted for systolic BP | 1.47 | (0.82–2.64) | 1.18 | (0.70–2.00) | 1.84 | (1.17–2.88) |
| Adjusted for all Framingham risk factors | 1.03 | (0.53–1.97) | 0.84 | (0.48–1.49) | 1.21 | (0.76–1.92) |
age, smoking status, diabetes, systolic blood pressure, total cholesterol, HDL cholesterol
We performed additional analysis to assess whether the ST-T changes accounted for in the Novacode left ventricular mass index were responsible for the superior performance of this measure. First, we assessed the independent contribution of the strain pattern alone. In males in the development set, the hazard ratio associated with strain for five-year all-cause mortality was 5.72 (3.67–8.92) unadjusted, 4.56 (2.88–7.23) adjusted for systolic blood pressure, and 2.56 (1.59–4.13) adjusted for all Framingham risk factors; comparable hazard ratios for women were 4.84 (2.28–10.29), 2.44 (1.13–5.29), and 2.11 (0.93–4.81). Results in the validation set confirmed these findings. The value of this strong association between the strain pattern and subsequent mortality was tempered by its relatively low prevalence. A strain pattern was found on the electrocardiograms of only 5.0% of the 760 subjects who had died at five years, whereas 18.5% of subjects with Cornell voltage above threshold, 18.2% of subjects with Cornell product above threshold, and 26.8% of subjects with Novacode left ventricular mass index above threshold had died at five years.
We therefore tested association between presence of a strain pattern in combination with Cornell voltage and Cornell product. In males in the development set, the hazard ratio associated with strain combined with Cornell voltage for five-year all-cause mortality was 1.68 (1.30–2.18) unadjusted, 1.42 (1.09–1.86) adjusted for systolic blood pressure, and 1.47 (1.12–1.94) adjusted for all Framingham risk factors; comparable hazard ratios for women were 2.85 (2.04–3.97), 1.76 (1.23–2.52), and 1.44 (0.99–2.10). These results were marginally better than the Novacode measure in men and marginally worse than the Novacode measure in women, and were nearly identical for the Cornell product. Results in the validation set confirmed these findings. Of the 760 subjects who died, 21.4% had strain or Cornell voltage criteria present and 21.2% had strain or Cornell product present.
Discussion
Our study confirmed the relationship between greater electrocardiographic voltage measures of LVH and higher mortality, and identified the Cornell voltage, Cornell product, and Novacode estimate of left ventricular mass index as having discriminative capacity for mortality and having demonstrable thresholds above which mortality rises progressively. The Novacode left ventricular mass index performed slightly better in multivariable models. The Cornell voltage and Cornell product also demonstrated significant threshold relationships, but the thresholds showed fewer significant relationships with mortality after adjustment for other risk factors. Novacode left ventricular mass index above threshold was present in 26.8% of subjects who had died at five years; for Cornell voltage and Cornell product these figures were 18.5% and 18.2% respectively. There were fewer statistically significant relationships in a validation sample comprising one third of the full analytic sample probably because of the smaller number of observations in the validation sample.
These results suggest that electrocardiography might be used to establish regression of left ventricular hypertrophy in the management of hypertension, which in turn might be expected to reduce mortality. Even small increases in voltages above the thresholds appear to be associated with significant increases in mortality.
Comparison with prior literature
Electrocardiographic measures of left ventricular hypertrophy have been associated with elevated risk of mortality in a number of prior studies, though few have treated these measures as continuous variable. Prineas and colleagues (18) showed the Sokolow-Lyons voltage, Cornell voltage, Cornell product, and Novacode score used as continuous variables were better predictors of mortality in patients in the Multiple Risk Factor Intervention Trial than were the same parameters dichotomized as the presence or absence of LVH.
The electrocardiographic measure that performed best in our study, the Novacode estimate of left ventricular mass index, is probably the least studied of the measures we investigated. In one study (19) this score had lower sensitivity and specificity for the detection of echocardiographic LVH than the Sokolow-Lyons voltage, the Cornell voltage, and the Cornell product. In a study of subjects in the Cardiovascular Health Study (6), the Novacode equations consistently overestimated left ventricular mass. The superior sensitivity and specificity for detection of mortality despite its limitations for detection of echocardiographic hypertrophy may be a result of the unique use of T wave inversion in the Novacode score. Inversion of the T wave is a feature of the “strain” pattern that is an independent predictor of poor prognosis (20). This explanation is supported by the strong association between the presence of a strain pattern and subsequent mortality in our data set, and by the fact that combination of the strain pattern with either Cornell voltage or Cornell product made the performance of these two measures comparable to that of the Novacode index. Further development and validation of such combinations are worthy of future study.
The thresholds we identified for the Novacode left ventricular mass index are strikingly comparable to the upper limits of normal for echocardiographic left ventricular mass index determined by Devereux and colleagues (21) (132–136 gms/m2 for men and 109–112 gms/m2 for women). In a paper using data from the Framingham study and the same echocardiographic conventions as Devereux et al, the thresholds identified were similar for men (131 gms/m2) and slightly lower for women (100 gms/m2) (22).
The hazard ratios for mortality in the current study are similar to the results of studies based on echocardiography for men but not for women. In a report from the Framingham study (1), Levy et al found relative risk for all-cause mortality adjusted for age and risk factors in men of 1.49 and for cardiovascular mortality of 1.73; for women the comparable figures were 2.01 and 2.12. Liao and colleagues (23) studied the relationship between echocardiographically determined left ventricular mass and subsequent mortality, and after adjusting for age and hypertension also found a stronger relationship between LVH and mortality in women compared with men. In the current study, the opposite is the case, with relationships stronger in men than in women. This suggests that further refinement of the Novacode equations for women is possible, and might further improve the discriminative capacity of the thresholds.
Although the Novacode left ventricular mass index threshold was more clearly predictive of mortality than the Cornell voltage or the Cornell product thresholds, both of these latter measures were discriminative for mortality and had identifiable thresholds. For men, we found an increase in mortality at lower levels than the established criteria for diagnosis of LVH for both measures (20 vs. 28 mm for the Cornell voltage and 2150 vs. 2436 mm-msec for the Cornell product). For women, we found thresholds at comparable levels (19 vs. 20 mm for the Cornell voltage and 1700 vs. approximately 1740 mm-msec for the Cornell product). The reasons for this gender difference in the relationship between electrocardiographic voltage and outcome are uncertain. One possibility is that the partition values for Cornell voltage and Cornell product were set too high and that partitions with lower sensitivities would be more clinically useful. The Cornell voltage and the Cornell product are simple to calculate, might therefore be more useful in routine clinical practice and are worthy of further study.
The poor performance of the Sokolow-Lyons voltage is in part due to its low sensitivity and specificity for the detection of LVH (24–28), but other factors may be at play. A study by Velury and Spodick (29) suggested that low R voltage (<10 mm) in leads V5 and V6 has a high sensitivity for the diagnosis of LVH. This may explain the trend toward higher mortality in patients with low Sokolow-Lyons voltage seen in Figure 1, since low Sokolow-Lyon voltage might also be a marker of LVH.
Study limitations
The greatest limitation to the current study is that it relies on data taken at a single point in time. We are thus unable to assess whether decrements in the electrocardiographically estimated left ventricular mass index are associated with diminished risk of mortality. Data from other studies (30–32), however, suggests an association between decrements in electrocardiographic voltage and diminished mortality. Information about potentially significant cofactors, such as treatment during follow-up, was not available. A third limitation is that we relied on cardiovascular causes of death determined from death certificates, which may be unreliable. However, we found significant relationships with all-cause mortality, an outcome less subject to ascertainment bias.
We chose not to consider race or ethnicity as possible covariates. Although the prevalence of LVH assessed by Sokolow-Lyon or Cornell voltage criteria is greater in African-Americans, there are no racial differences when assessed by echocardiography (33,34) and the diminished specificity of the electrocardiographic criteria are greatly attenuated when the higher prevalence of obesity in African-Americans is accounted for (35,36). The Novacode estimate of left ventricular mass index not only accounts for differences in body size, but employs separate regression equations for black and white women. Other potential ethnic differences, particularly for Latinos, have generally not been explored in the literature. The relationship among race and ethnicity, electrocardiographic measures of LVH, and mortality is deserving of future research.
Conclusions and Implications
Electrocardiographic definitions of LVH based on relationships with mortality are feasible. Such definitions might be clinically useful in the routine management of patients with hypertension as goals for therapy in addition to lowering blood pressure below goal; independent prospective validation is required.
Figure 5.
Hazard ratios with 95% confidence intervals for the secondary outcome measures, adjusted for systolic blood pressure, for men (top panel) and for women (bottom panel)
Acknowledgments
Financial support:
NHLBI Grant U01 HL079160
HRSA Administrative Unit Grant HP00054 5 D12
Appendix
| Men: | ||
| Coefficient | ||
| Variable | LV Mass | LV Mass Index |
| R in amplitude in V5 | 0.0217 | 0.0100 |
| Q or S amplitude in V1 | 0.0338 | 0.0203 |
| Q or S amplitude in III | 0.0600 | 0.0287 |
| Negative T amplitude in V6 | 0.3158 | 0.1819 |
| Positive T amplitude in aVR | −0.2958 | −0.1482 |
| QRS duration | 1.8204 | 1.0485 |
| Intercept | −58.5098 | −36.4290 |
| White women: | ||
| Coefficient | ||
| Variable | LV Mass | LV Mass Index |
| R in amplitude in aVL | 0.0320 | - |
| R amplitude in V5 | 0.0233 | 0.0178 |
| Q or S amplitude in V5 | 0.0693 | 0.0528 |
| Q or S amplitude in I | −0.1545 | −0.1128 |
| Positive T amplitude in V1 | 0.1122 | 0.1075 |
| Negative T amplitude in aVF | - | 0.1701 |
| Positive T amplitude in V6 | −0.1236 | −0.0939 |
| Intercept | 134.7722 | 88.4357 |
| Black women: | ||
| Coefficient | ||
| Variable | LV Mass | LV Mass Index |
| R in amplitude in aVL | - | 0.0216 |
| R amplitude in I | 0.0498 | - |
| R amplitude V6 + S amplitude V2 | 0.0235 | 0.0184 |
| R amplitude in V1 | −0.0507 | - |
| R amplitude in V2 | - | −0.0143 |
| Larger of Q or S amplitude in V6 | −0.0980 | −0.0693 |
| Negative T amplitude in aVL | - | 0.199 |
| Negative T amplitude in I | 0.5225 | - |
| QRS duration | 1.8478 | 0.7460 |
| Intercept | −90.7136 | −22.3064 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Casatelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 4.Matthew J, Sleight P, Lonn E, Johnstone K, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of ECG markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 5.Havranek EP, Esler A, Mehler PS, Estacio RO, Schrier RW. Differential effects of anti-hypertensive agents on ECG voltage: results from the Appropriate Blood Pressure Control in Diabetes (ABCD) Trial. Am Heart J. 2003;145:993–998. doi: 10.1016/S0002-8703(02)94780-0. [DOI] [PubMed] [Google Scholar]
- 6.Rautaharju PM, Manolio TA, Siscovick D, Zhou SH, Gardin JM, Kronmal R, Furberg CD, Borhani NO, Newman A Cardiovascular Health Study Collaborative Research Group. Utility of new ECG models for left ventricular mass in older adults. Hypertension. 1996;28:8–15. doi: 10.1161/01.hyp.28.1.8. [DOI] [PubMed] [Google Scholar]
- 7. [accessed June 1, 2007]; http://www.cdc.gov/nchs/nhanes.htm.
- 8. [accessed June 1, 2007]; http://www.cdc.gov/nchs/r&d/nchs_datalinkage/nhanes_data_linkage_activities.htm.
- 9. [accessed June 1, 2007]; http://www.cdc.gov/nchs/ndi.htm.
- 10.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiograhic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 11.Molloy TJ, Okin PM, Devereux RB, Kligfield P. ECG detection of echocardiographic left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180–1186. doi: 10.1016/0735-1097(92)90376-x. [DOI] [PubMed] [Google Scholar]
- 12.Norman JE, Levy D. Improved ECG detection of echocardiographic left ventricular hypertrophy: results of a correlated data base approach. J Am Coll Cardiol. 1995;26:1022–1029. doi: 10.1016/0735-1097(95)00269-5. [DOI] [PubMed] [Google Scholar]
- 13.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 14.Rautaharju PM, Lacroix AZ, Savage DD, Haynes SG, Madans JH, Wolf HK, Hadden W, Keller J, Cornoni-Huntley J. ECG estimate of left ventricular mass versus radiographic cardiac size and the risk of cardiovascular disease mortality in the epidemiologic Follow-Up Study of the First National Health and Nutrition Examination Survey. Am J Cardiol. 1988;62:59–66. doi: 10.1016/0002-9149(88)91365-3. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed June 1, 2007]; Available at http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm#Data%20Files%202a.
- 16.Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Julius S, Dahlof B LIFE Study Investigators. Relationship of the electrocardiographic strain pattern to left ventricular structure and function in hypertensive patients: The LIFE Study. J Am Coll Cardiol. 2001;38:514–520. doi: 10.1016/s0735-1097(01)01378-x. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KM, Wilson PWF, Odell PM, Kannel WB. An updated coronary risk profile: a statement for health professionals. Circulation. 1991;83:357–363. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 18.Prineas RJ, Rautaharju PM, Grandits G, Crow R MRFIT Research Group. Independent risk for cardiovascular disease predicted by modified continuous score ECG criteria for 6-year incidence and regression of left ventricular hypertrophy among clinically disease free men: 16-year follow-up for the Multiple Risk Factor Intervention Trial. J Electrocardiol. 2001;34:91–101. doi: 10.1054/jelc.2001.23360. [DOI] [PubMed] [Google Scholar]
- 19.Okin PM, Roman MJ, Devereux RB, Kligfield P. ECG identification of left ventricular hypertrophy: test performance in relation to definition of hypertrophy and presence of obesity. J Am Coll Cardiol. 1996;27:124–131. doi: 10.1016/0735-1097(95)00421-1. [DOI] [PubMed] [Google Scholar]
- 20.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Porcellati C. Prognostic value of a new ECG method for diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 1998;31:383–390. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, Miller DH, Reis G, Alderman MH, Laragh JH. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol. 1984:1222–1230. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 22.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: The Framingham Heart Study. Am J Cardiol. 1987;59:956–960. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y, Cooper RS, Mensah GA, McGee DL. Left ventricular hypertrophy has a greater impact on survival in women than in men. Circulation. 1995;92:805–810. doi: 10.1161/01.cir.92.4.805. [DOI] [PubMed] [Google Scholar]
- 24.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhury BS, Phillips MC. ECG detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 25.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 26.Vijan SG, Manning G, Millar-Craig MW. How reliable is the electrocardiogram in detecting left ventricular hypertrophy in hypertension? Postgrad Med J. 1991;67:646–648. doi: 10.1136/pgmj.67.789.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abergel E, Tase M, Menard J, Chatellier Influence of obesity on the diagnostic value of ECG criteria for detecting left ventricular hypertrophy. Am J Cardiol. 1996;77:739–744. doi: 10.1016/s0002-9149(97)89209-0. [DOI] [PubMed] [Google Scholar]
- 28.Okin PM, Jern S, Devereux RB, Kjeldsen SE, Dahlof B LIFE Study Group. Effect of obesity on ECG left ventricular hypertrophy in hypertensive patients: The Losartan Intervention For Endpoint (LIFE) Reduction in Hypertension Study. Hypertension. 2000;35:13–18. doi: 10.1161/01.hyp.35.1.13. [DOI] [PubMed] [Google Scholar]
- 29.Velury S, Spodick DH. Increased left ventricular mass in patients with lateral low-voltage electrocardiograms. Am J Cardiol. 1992;69:707–708. doi: 10.1016/0002-9149(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 30.Levy D, Salomon M, D’Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline ECG features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 31.Fagard RH, Staessen JA, Thijs, Celis H, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Leonetti G, Sarti C, Tuomilehto J, Webster J, Yodfat Y Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Prognostic significance of ECG voltages and their serial changes in elderly with systolic hypertension. Hypertension. 2004;44:459–464. doi: 10.1161/01.HYP.0000142169.17298.54. [DOI] [PubMed] [Google Scholar]
- 32.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snappin S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B LIFE Study Investigators. Regression of ECG left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 33.Lee DK, Marantz PR, Devereux RB, Kligfield P, Alderman MH. Left ventricular hypertrophy in black and white hypertensives: Standard ECG criteria overestimate racial differences in prevalence. JAMA. 1992;267:3294–3299. [PubMed] [Google Scholar]
- 34.Arnett DK, Rautaharju P, Crow R, Folsom AR, Ekelund LG, Hutchinson R, Tyroler HA, Heiss G ARIC Investigators. Black-white differences in ECG left ventricular mass and its association with blood pressure (the ARIC Study) Am J Cardiol. 1994;74:247–252. doi: 10.1016/0002-9149(94)90365-4. [DOI] [PubMed] [Google Scholar]
- 35.Rautaharju PM, Park LP, Gottdiener JS, Siscovick D, Boineau R, Smith V, Powe NR. Race- and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African-Americans. J Electrocardiol. 2000;33:205–218. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
- 36.Okin PM, Wright JT, Nieminen MS, Jern S, Taylor AL, Phillips R, Papademetriou V, Clark LT, Ofili EO, Randall OS, Oikarinen L, Viitasalo M, Toivonen L, Julius S, Dahlof B, Devereux RB LIFE Study Investigators. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE Study. American Journal of Hypertension. 2002;15:663–671. doi: 10.1016/s0895-7061(02)02945-x. [DOI] [PubMed] [Google Scholar]