Abstract
Previously, we showed that protein kinase B (Akt) activation increases intracellular ATP levels and decreases necrosis in renal proximal tubular cells (RPTC) injured by the nephrotoxicant S-(1, 2-dichlorovinyl)-L-cysteine (DCVC) (Shaik ZP, Fifer EK, Nowak G. Am J Physiol Renal Physiol 292: F292–F303, 2007). This study examined the role of Akt in improving mitochondrial function in DCVC-injured RPTC. Our data show a novel observation that phosphorylated (active) Akt is localized in mitochondria of noninjured RPTC, both in mitoplasts and the mitochondrial outer membrane. Mitochondrial levels of active Akt decreased in nephrotoxicant-injured RPTC, and this decrease was associated with mitochondrial dysfunction. DCVC decreased basal, uncoupled, and state 3 respirations; ATP production; activities of complexes I, II, and III; the mitochondrial membrane potential (ΔΨm); and F0F1-ATPase activity. Expressing constitutively active Akt in DCVC-injured RPTC increased the levels of phosphorylated Akt in mitochondria, reduced the decreases in basal and uncoupled respirations, increased complex I-coupled state 3 respiration and ATP production, enhanced activities of complex I, complex III, and F0F1-ATPase, and improved ΔΨm. In contrast, inhibiting Akt activation by expressing dominant negative (inactive) Akt or using 20 μM LY294002 exacerbated decreases in electron transport rate, state 3 respiration, ATP production, ΔΨm, and activities of complex I, complex III, and F0F1-ATPase. In conclusion, our data show that Akt activation promotes mitochondrial respiration and ATP production in toxicant-injured RPTC by 1) improving integrity of the respiratory chain and maintaining activities of complex I and complex III, 2) reducing decreases in ΔΨm, and 3) restoring F0F1-ATPase activity.
Keywords: mitochondria; respiratory complexes; ATP; S-(1,2-dichlorovinyl)-L-cysteine; mitochondrial membrane potential
Stress stimuli, including ischemia, hypoxia, drugs, and toxicants, induce mitochondrial injury in a variety of cell types (2, 18). Mitochondrial dysfunction and the resulting energy deficits are common mechanisms of cell death (16, 22, 30, 31). Cells that require high energy input and are dependent on oxidative phosphorylation for ATP production, such as cardiac myocytes and renal cortical tubules, are particularly susceptible to injury and death caused by inhibition of mitochondrial energy metabolism (16, 18, 30). Mitochondrial dysfunction and ATP deficits result in renal proximal tubular cell (RPTC) injury and necrosis, and are common mechanisms of acute renal failure caused by ischemia and nephrotoxicants (26, 30, 31).
Mitochondrial dysfunction is also a major mechanism of RPTC injury and death caused by the exposure to S-(1,2-dichlorovinyl)-L-cysteine (DCVC), a nephrotoxic metabolite of common environmental contaminants trichloroethylene and dichloroacetylene (28). DCVC selectively accumulates in RPTC, causing mitochondrial damage and cell death. Mitochondrial dysfunction produced by DCVC is associated with the reduction in substrate oxidation by dehydrogenases of the citric acid cycle, the decreases in activities of respiratory, complexes and F0F1-ATPase, and a decline in mitochondrial membrane potential (ΔΨm), ATP production, and intracellular ATP levels (11, 25–28, 32, 51, 52). Prolonged decreases in oxidative phosphorylation and ATP levels lead to decreases in Na+-K+-ATPase activity and active Na+ transport, resulting in the disruption of intracellular ion homeostasis and sublethal and lethal injury in RPTC (38, 41).
Recent evidence shows that protein kinases play an important role in the regulation of mitochondrial functions. PKA, PKC (isoforms α, ε, and δ), ERK, JNK/SAPK, and p38 MAPK regulate mitochondrial respiration, activities of respiratory complexes, the mitochondrial permeability transition (MPT), ΔΨm, and overall mitochondrial integrity (5, 19). PKA enhances the activity of complex I in myoblasts and decreases activity of the cytochrome oxidase (complex IV) in renal and heart mitochondria (19). Another kinase, c-Src, increases activity of complex IV in osteoclasts (5, 19). In RPTC, PKC-α activation decreases the activity of ATP synthase following DCVC injury (32). In oxidant-injured RPTC, ERK1/2 activation decreases the activity of respiratory complex I (40).
Protein kinase B (Akt) promotes cell survival and protects against apoptosis initiated by the mitochondrial pathway through phosphorylation and inhibition of the mitochondrial proapoptotic proteins Bad, Bax, and caspase-9 (33). Akt-mediated phosphorylation also activates antiapoptotic proteins Bcl-xL and Bcl-2 and blocks the release of cytochrome c from mitochondria (33). However, little is known about the role of Akt in necrosis and regulation of mitochondrial respiration and oxidative phosphorylation. It has been shown that the activation of Akt maintains ΔΨm, the driving force for ATP production, increases intracellular ATP levels, and inhibits cell death in neuronal cells, cardiac myocytes, and hepatocytes (9, 10, 15, 21, 23, 36, 53). Akt activation inhibits opening of the mitochondrial permeability transition pore (MPTP) through the inactivation of glycogen synthase kinase 3-β (GSK-3β) (23,43). Akt activation induces translocation of hexokinase II to mitochondria, where it binds to the voltage-dependent anion channel (VDAC) and prevents GSK-3β-mediated phosphorylation of VDAC and mitochondrial permeability transition (MPT) (15, 44). Previous studies also showed that Akt activation is associated with its translocation to mitochondria and phosphorylation of the catalytic β-subunit of F0F1-ATPase in neuronal cells (7). Our recent study demonstrated that in RPTC, DCVC exposure initially induces transient activation of Akt that is followed by Akt inactivation (48). Blocking Akt activity decreases intracellular ATP levels and increases RPTC death, whereas increasing Akt activation maintains intracellular ATP levels and decreases DCVC-injured RPTC necrosis (48). The goal of the present study was to determine the role of Akt in the regulation of oxidative phosphorylation and ATP production in RPTC following DCVC injury.
MATERIALS AND METHODS
Materials
Female New Zealand White rabbits (2.0–2.5 kg) were purchased from Myrtle’s Rabbitry (Thompson Station, TN). The cell culture media, DMEM, Eagle’s MEM, a 50:50 mixture of DMEM and Ham’s F-12 nutrient mix without phenol red, pyruvate, and glucose, and FBS were purchased from MediaTech Cellgro (Herndon, VA). Phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002), Akt activity assay kit, anti-Akt, and anti-phospho-Akt (Ser473) antibodies were supplied by Cell Signaling Technologies (Beverly, MA). Lysosomal-associated membrane protein-1 (LAMP-1), and monoamine oxidase A/B antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and antibody against calreticulin was supplied by Abcam (Cambridge, MA). Antibody against β-subunit of F0F1-ATPase was purchased from Molecular Probes/Invitrogen (Carlsbad, CA). Decyl ubiquinone was purchased from Sigma (St. Louis, MO) Adenoviral vectors encoding dominant negative (inactive) Akt [HA-Akt (K179M)] and constitutively active Akt (HA-myr Akt) were constructed and aliquots provided by Dr. Junichi Sadoshima (University of Medicine and Dentistry of New Jersey, Newark, NJ). Adenovirus carrying an empty pShuttle vector was obtained from BD Biosciences Clontech (Palo Alto, CA). The sources of other reagents were described previously (40, 42, 48). DCVC was synthesized using the method of McKinney et al. (34) as described previously (48). The purity of DCVC was analyzed by determining the melting point and by TLC. Characterization of DCVC was carried out using nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and mass spectroscopy.
Isolation and culture of RPTC
All procedures in animals were carried out in accordance with federal guidelines and approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Renal proximal tubules were isolated from female New Zealand White rabbit kidneys by the iron-oxide perfusion method and grown under improved culture conditions as previously described (42). The culture medium used was a 50:50 mixture of DMEM and Ham’s F-12 nutrient mix without phenol red, glucose, and pyruvate and supplemented with 15 mM HEPES, 15 mM NaHCO3, 2.5 mM glutamine, and 6 mM lactate (pH 7.4, 295 mosmol/kgH2O). Human transferrin (5 μg/ml), selenium (5 ng/ml), hydrocortisone (50 nM), bovine insulin (10 nM), and L-ascorbic acid-2-phosphate (50 μM) were added before daily media change.
DCVC treatment of RPTC monolayers
Because insulin activates Akt in RPTC (data not shown), insulin was withdrawn from the media 24 h before and following DCVC exposure. Confluent quiescent RPTC monolayers were exposed to 240 μM DCVC for 90 min. DCVC exposure was terminated by aspirating the culture media and adding fresh warm culture media containing all hormones except for insulin. Akt activation was inhibited by adding 20 μM LY294002, the inhibitor of PI3K, or expressing dominant negative (inactive) Akt using adenoviral vector encoding a mutant form of Akt rendered inactive by replacing the phosphorylatable serine and threonine residues with nonphosphorylatable alanine residue [multiplicity of infection (MOI) = 25]. Akt activation was increased by expressing constitutively active Akt using adenoviral vector encoding the NH2 terminus myristylated form of Akt with enhanced basal kinase activity (MOI = 25). Infection with adenoviral particles encoding an empty pShuttle vector (MOI = 25) was used as a negative control. Adenoviral infection of confluent RPTC was carried out 48 h before DCVC exposure. A second infection was carried out immediately after DCVC exposure to maintain RPTC levels of active or inactive Akt until the end of experiment. At the concentrations used, adenoviral particles did not produce any cytotoxic effect in RPTC (data not shown).
Adenoviral amplification
Adenoviral particles were amplified in AD293 cells followed by second amplification in HEK293 cells as we described previously (48).
Immunoblot analysis
Phosphorylation and protein levels of Akt in mitochondria isolated from RPTC were assessed by immunoblot analysis as described previously (48).
Immunoprecipitation and measurement of Akt activity
Akt activity in RPTC mitochondria was determined by immunoprecipitation followed by kinase assay using a nonradioactive Akt kinase assay kit and manufacturer’s protocol as described previously (48).
Oxygen consumption
Oxygen consumption (QO2) was measured polarographically at 37°C using a Clark-type electrode as described previously (37, 40, 42). Uncoupled QO2 (a marker of electron transfer rate through the respiratory chain) was measured in the presence of FCCP (1 μM). Oligomycin-sensitive QO2 (a marker of oxidative phosphorylation) was measured in the presence of oligomycin (inhibitor of F0F1-ATPase; 0.6 μg/ml). State 3 respiration was determined by measuring QO2 in the presence of 0.4 mM ADP and excess oxidative substrates: 10 mM citrate (complex I-linked state 3 respiration) and 10 mM succinate (complex II-linked state 3 respiration)+0.1 μM rotenone. RPTC were resuspended in a buffer containing 120 mM KCl, 5 mM KH2PO4, 10 mM HEPES, 2 mM EGTA, 1 mM MgSO4, 0.01% digitonin, and 10 mM potassium citrate or 10 mM potassium succinate plus 0.1 μM rotenone; pH 7.4. State 3 respiration was initiated by adding 0.4 mM ADP.
ATP production rate
ATP production rate was measured as described previously (32, 40) using 10 mM citrate or 10 mM succinate plus 0.1 μM rotenone as the oxidative substrates (pH 7.4). The reaction was initiated by adding ADP (2 mM final concentration), carried out for 5 min at 37°C and terminated by adding an aliquot of ice-cold perchloric acid (3% final concentration). The suspension was spun down, the supernatant was neutralized to pH 7.5, and ATP content was measured using the ATP bioluminescence assay as described previously (32).
ΔΨm
ΔΨm was assessed by flow cytometry using JC-1 dye as described previously (37). RPTC monolayers were loaded with JC-1 (10 μM) and incubated for 30 min at 37°C. Following incubation, media were aspirated, monolayers washed twice with ice-cold PBS, scraped off culture dishes, and resuspended in PBS. Fluorescence was determined by flow cytometry (FACS Calibur, Beckton Dickinson), using excitation by a 488-nm argon-ion laser. The fluorescence of JC-1 monomer (green) and the J-aggregates (red) were detected separately in FL1 (emission, 525 nm) and FL2 (emission, 590 nm) channels, respectively.
Isolation of RPTC mitochondria
Mitochondria were isolated from RPTC as described previously (39). Isolated mitochondria were purified by centrifugation in discontinuous Percoll gradient. Mitochondria were suspended in 50 μl of 10 mM HEPES, 395 mM sucrose, and 0.1 mM EDTA (pH 7.4), layered on the top of the Percoll gradient (70, 40, and 30%) and centrifuged at 68,000 g for 5 min at 4°C. Purified mitochondria were collected from the interphase between 40 and 70% Percoll and washed twice in the suspension buffer. The final mitochondrial pellet was resuspended either in a hypotonic buffer containing 25 mM potassium phosphate and 5 mM MgCl2 (pH 7.2) to determine activity of the respiratory complexes or in 10 mM Tris · HCl containing 200 mM KCl and 2 mM MgCl2 (pH 8.2) to determine F1F0-ATPase activity. For immunoblot analysis, purified mitochondria were solubilized in lysis buffer (50 mM Tris · HCl, 150 mM NaCl, 1 mM EGTA, 2% Triton X-100, 1 mM Na3VO4, 1 mM NaF, the protease and phosphatase inhibitor cocktails; pH 7.4) and detection of phosphorylated and total Akt carried out as described previously (44). Purity of mitochondrial fractions was tested by probing with an antibody recognizing endoplasmic reticulum marker protein calreticulin and lysosomal protein LAMP-1, and mitochondrial inner membrane protein β-subunit of F0F1-ATPase.
Fractionation of mitochondria
Fractionation of purified mitochondria was carried out by the swelling-shrinking method as described by Hovius et al. (20). Percoll-purified mitochondria were suspended and incubated for 20 min in a swelling buffer (10 mM KH2PO4, pH 7.4, containing protease and phosphatase inhibitors) at 4°C followed by incubation (20 min) in a shrinking buffer (10 mM KH2PO4 buffer, pH 7.4, containing 32% sucrose, 30% glycerol, 10 mM MgCl2, and protease and phosphatase inhibitors). The suspension was then centrifuged at 10,000 g for 10 min at 4°C. The pellet containing the mitoplasts was washed three times in the 1:1 mixture of the swelling and shrinking buffers, resuspended in a lysis buffer (50 mM Tris · HCl, pH 7.4, containing 150 mM NaCl, 1 mM EGTA, 2% Triton X-100, 1 mM Na3VO4, 1 mM NaF, and protease and phosphatase inhibitor cocktails), and processed for immunoblot analysis. The supernatant containing the outer mitochondrial membrane and the intermembrane-space components was collected and centrifuged at 68,000 g for 30 min at 4°C. The pellet containing the outer membrane fraction was solubilized in the lysis buffer and processed for immunoblot analysis of Akt and monoamine oxidase (MAO-A/B, the mitochondrial outer membrane marker protein).
Activity of respiratory complexes
The activities of respiratory complexes I and II were measured in isolated mitochondria according to the method of Birch-Machin et al. (8) as described previously (39). Freshly isolated mitochondria were suspended in the hypotonic assay buffer (25 mM potassium phosphate buffer containing 5 mM MgCl2, pH 7.2) and freeze-thawed in liquid nitrogen. Complex I (NADH: ubiquinone oxidoreductase) activity was assayed spectrophotometrically by following the oxidation of NADH (0.25 mM) at 340 nm at 30°C in the assay buffer containing 62.5 μM ubiquinone, 0.25% BSA, antimycin A (2 μg/ml), and mitochondria in the absence and presence of rotenone (10 μg/ml). Complex I activity was calculated as the rotenone-sensitive NADH:ubiquinone oxidoreductase activity. Complex II (succinate:ubiquinone oxidoreductase) activity was assayed spectrophotometrically by following the reduction of dichlorophenolindophenol (0.25 mM) at 590 nm at 30°C in the assay buffer containing succinate (20 mM), antimycin A (2 μg/ml), rotenone (10 μg/ml), 0.25% BSA, and ubiquinone (62.5 μM). Complex III (ubiquinol:cytochrome c oxidoreductase) activity was assessed by following the reduction of cytochrome c (60 μM) at 550 nm at 30°C in the assay buffer containing rotenone (10 μg/ml), 10% BSA, decyl ubiquinol (50μM), and KCN (0.24 mM) as described by Barrientos et al. (6). Decyl ubiquinone was reduced to decyl ubiquinol according to the method described by Barrientos et al. (6). The increase in absorbance (reduction of cytochrome c) was recorded in the absence of antimycin A and then in the presence of antimycin A (2 μg/ml). Complex III activity was calculated as the antimycin A-sensitive ubiquinol:cytochrome c oxidoreductase activity. Complex IV (cytochrome oxidase) activity was assessed by following the oxidation of reduced cytochrome c (90 μM) at 550 nm at 30°C in the assay buffer containing 10% BSA, antimycin A (2 μg/ml) in the presence and absence of KCN (0.24 mM) as described by Barrientos et al. (6). Complex IV activity was calculated as KCN-sensitive cytochrome oxidase activity.
F0F1-ATPase activity
ATPase activity of the ATP synthase was determined in freshly isolated mitochondria by measuring the release of Pi from ATP by the method of Law et al. (28) as described previously (31). Each sample was run in the absence and presence of oligomycin (10 μg/ml), and the oligomycin-sensitive ATPase activity of F0F1-ATPase was calculated.
All results were normalized to cellular protein, which was measured by bicinchoninic acid (BCA) assay using BSA as the standard.
Statistical analysis
Data are presented as averages ± SE and were analyzed for significance by one-way ANOVA. Multiple averages were compared using Student-Newman-Keuls test. The level of significance was set at P < 0.05. RPTC isolated from an individual rabbit represented one experiment (n = 1).
RESULTS
Akt in RPTC mitochondria
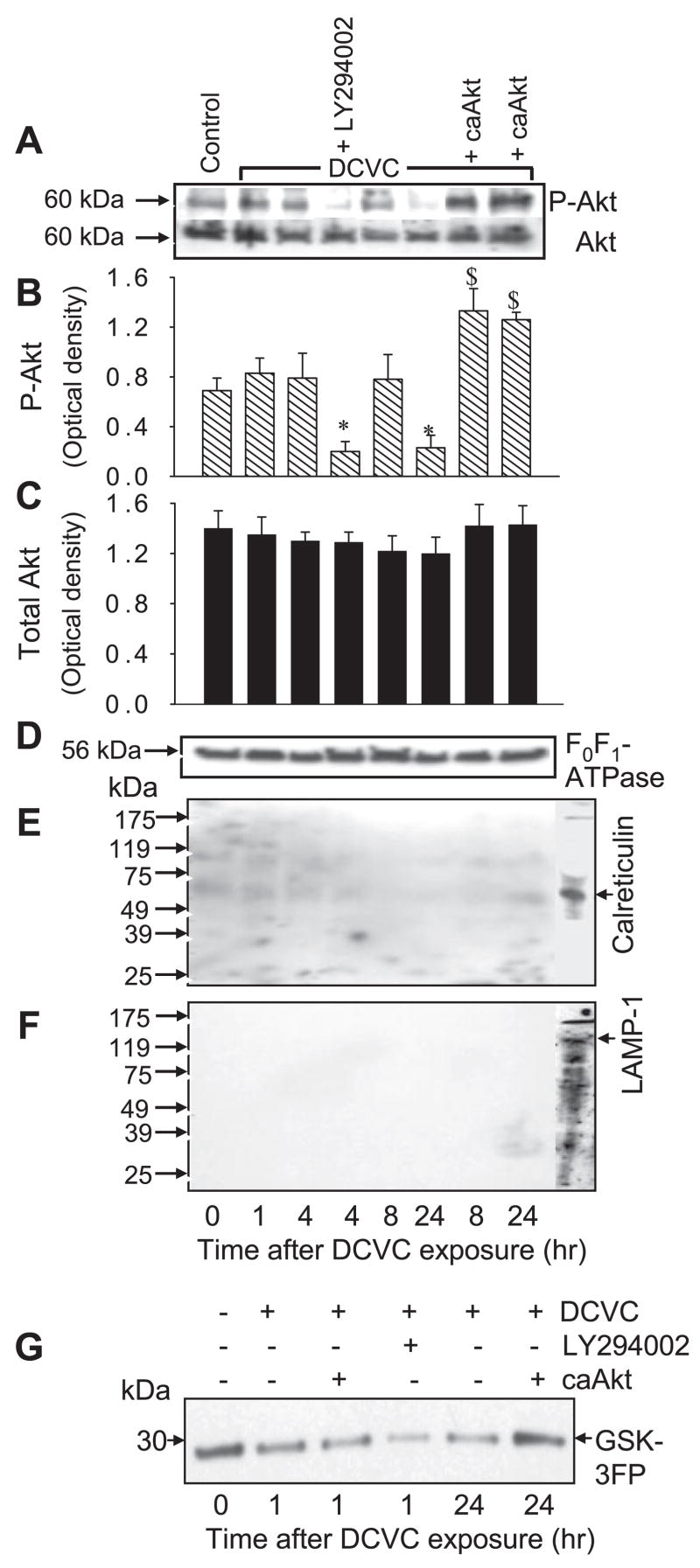
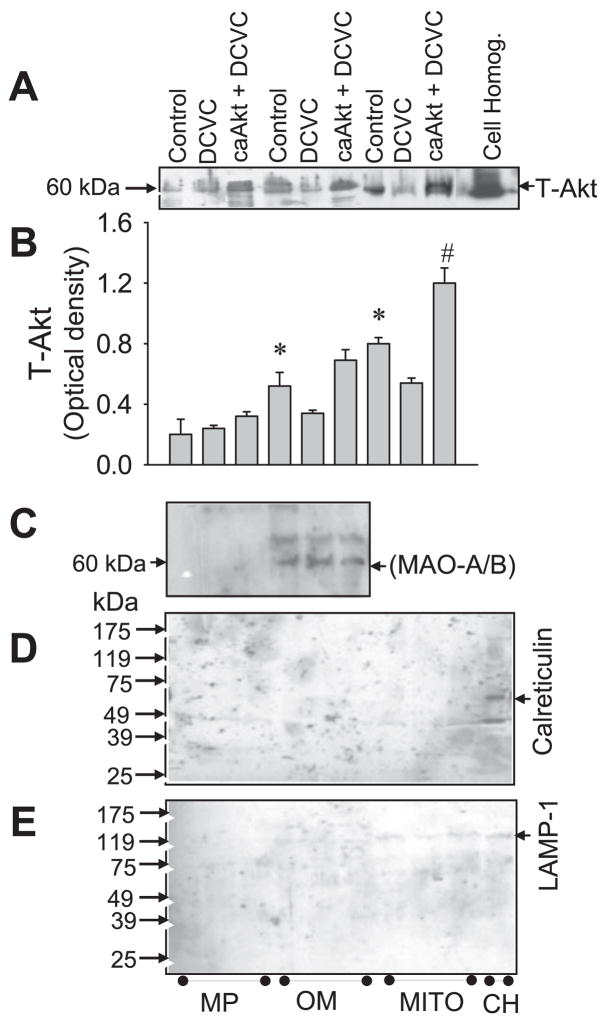
Figure 1A shows that Akt is localized in RPTC mitochondria. The levels of phosphorylated (active) Akt in mitochondria were decreased at 24 h following DCVC exposure (Fig. 1, A and B). Blocking Akt activation, using LY294002, decreased the levels of phosphorylated (active) Akt in RPTC mitochondria (Fig. 1, A and B). In contrast, expressing active Akt maintained the levels of phosphorylated Akt in mitochondria isolated from DCVC injured RPTC at 8 and 24 h following the exposure (Fig. 1, A and B). Akt kinase assay demonstrated Akt activity in mitochondria of noninjured RPTC (Fig. 1G). Akt activity was decreased at 24 h following DCVC exposure. Presence of LY294002 inhibited Akt activity in mitochondria of injured RPTC (Fig. 1G). In contrast, overexpressing active Akt (caAkt) in RPTC maintained Akt activity in mitochondria at 24 h following DCVC injury (Fig. 1G). DCVC-induced injury did not change mitochondrial protein levels of Akt (Fig. 1, A and C). Immunoblot analysis of mitochondrial subfractions demonstrated that the majority of mitochondrial Akt is present in the mitochondrial outer membrane. Less Akt is present in the mitoplasts (Fig. 2, A and B). Therefore, we conclude that Akt localizes in RPTC mitochondria and that DCVC injury decreases the levels of phosphorylated Akt and Akt activity in mitochondria.
Fig. 1.
Presence of Akt in renal proximal tubular cells (RPTC) mitochondria. A: protein levels of phosphorylated (Ser473) and total Akt in RPTC mitochondria at various time points following S-(1, 2-dichlorovinyl)-L-cysteine (DCVC; 240 μM) exposure. B and C: levels of phosphorylated (B) and total (C) levels of Akt in RPTC mitochondria at different time points following DCVC exposure. The results quantified by densitometry are the average ± SE (n = 3). *, $: Significantly (P < 0.05) different from controls. D: protein levels of the β-subunit (56.6 kDa) of F0F1-ATPase (mitochondrial marker) were used as the loading control. E: lack of endoplasmic reticulum (ER) contamination (calreticulin, 60 kDa) in Percoll-purified mitochondria. F: lack of lysosomal contamination [lysosomal-associated membrane protein (LAMP-1), 120 kDa] in Percoll-purified mitochondria. G: Akt activity [measured using phosphorylation of GSK-3 fusion protein (GSK-3FP) as the substrate] in mitochondria from control and DCVC-injured RPTC at 1 and 24 h following DCVC injury. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment. To activate Akt, RPTC were infected with adenovirus carrying caAkt [multiplicity of infection (MOI) = 25].
Fig. 2.
Presence of Akt in mitoplasts and the mitochondrial outer membrane, A: protein levels of total Akt in mitoplasts (MP) and mitochondrial outer membrane (OM) isolated from purified mitochondria (MITO) and in the whole-cell homogenates (CH) of RPTC. To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). B: optical densities of total Akt in subfractions of mitochondria and purified mitochondria. The results quantified by densitometry are the average ± SE (n = 3). *, $, #: Significantly (P < 0.05) different from controls. C and D: absence of calreticulin (C) and LAMP-1 (D) in purified mitochondrial fractions. E: lack of the mitochondrial outer membrane marker [monoamine oxidase (MAO-A/B), 60 kDa] in mitoplasts. Blots are representative of the results obtained from 3 different experiments.
Figures 1, E and F, and 2, D and E, show that purified mitochondria and mitochondrial subfractions had no detectable levels of calreticulin (an endoplasmic reticulum marker) and LAMP-1 (a lysosomal marker), demonstrating that mitochondria were not significantly contaminated by the endoplasmic reticulum and lysosomes. As shown in Fig. 2C, mitoplasts were not contaminated by the outer mitochondrial membrane (no detectable levels of MAO, the mitochondrial outer membrane marker).
Akt regulates integrity of the respiratory chain
To determine whether Akt regulates the rate of electron transport through the respiratory chain, the levels of active and inactive Akt were increased in RPTC and basal and uncoupled QO2 was measured at 24 h following DCVC exposure. DCVC decreased basal and uncoupled QO2s to 48 and 45% of controls, respectively. Akt activation increased basal and uncoupled QO2s in injured RPTC to 75 and 60% of controls (Figs. 3, A and B). In contrast, inhibition of Akt activation by LY294002 or expressing dnAkt exacerbated DCVC-induced decreases in basal QO2 to 30 and uncoupled QO2 to 30 and 22% of controls, respectively (Fig. 3, A and B). These data demonstrate that Akt activation improves electron transfer rate through the respiratory chain in injured RPTC and suggest that Akt is involved in the regulation of activities of respiratory complexes.
Fig. 3.
Effect of modulation of Akt activity on basal oxygen consumption (QO2; A) and uncoupled QO2 (B) in RPTC at 24 h following DCVC (240 μM) exposure. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv, RPTC infected with adenovirus carrying an empty plasmid vector (MOI = 25). The results are the average ± SE (n = 7). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
Akt regulates functions of respiratory complexes
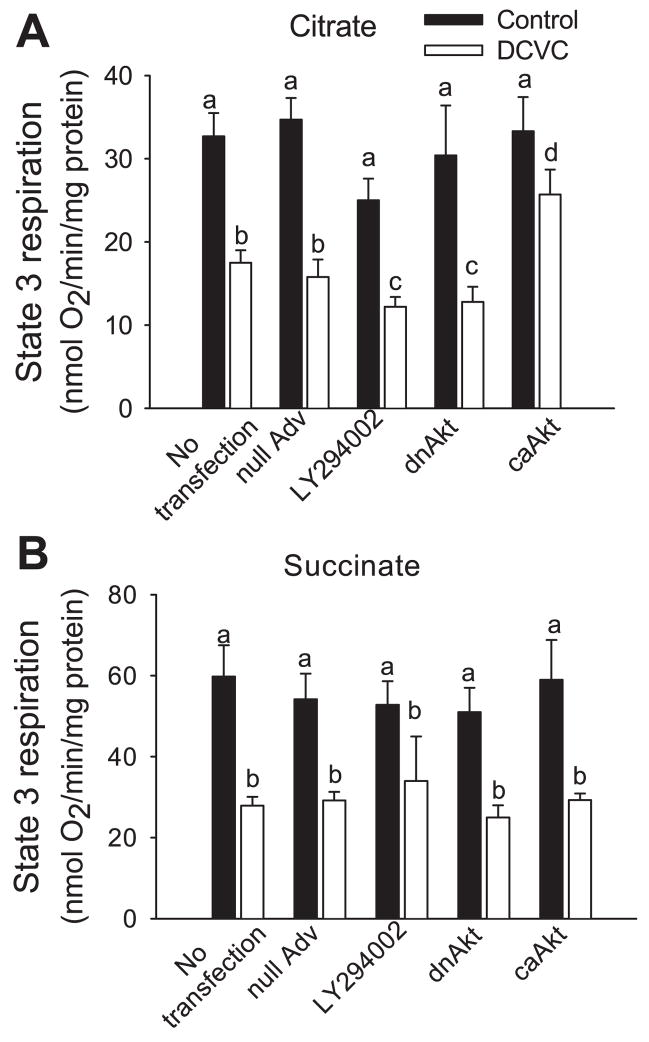
The functions of complex I and complex II were differentiated by using different respiratory substrates that energize mitochondria through these two complexes. Citrate was used to measure substrate oxidation through complex I and succinate (+0.1 μM rotenone to block complex I) to measure oxidation through complex II. State 3 respiration (maximum rate of mitochondrial respiration) coupled to complex I decreased to 68% of controls at 24 h after DCVC exposure (Fig. 4A). The inhibition of Akt in DCVC-injured RPTC exacerbated the decreases in complex I-coupled state 3 respiration (42% of controls) whereas the overexpression of active Akt increased complex I-coupled state 3 respiration to 77% of controls (Fig. 4A). State 3 respiration coupled to complex II was decreased 55% in DCVC-injured RPTC (Fig. 4B). Neither Akt activation nor Akt inhibition had any effect on the decreases in complex II-coupled state 3 respiration (Fig. 4B). These results demonstrate that Akt activation regulates mitochondrial respiration in DCVC-injured RPTC by promoting oxidation of substrates through complex I but has no effect on oxidation of substrates through complex II.
Fig. 4.
Effect of modulation of Akt activity on state 3 respiration coupled to complex I (A) and complex II (B) at 24 h following DCVC (240 μM) exposure in RPTC. Complex I- coupled state 3 respiration was measured in the presence of 10 mM citrate and 0.4 mM ADP as described in MATERIALS AND METHODS. Complex II-coupled state 3 respiration was measured in the presence of 10 mM succinate+0.1 μM rotenone and 0.4 mM ADP as described in MATERIALS AND METHODS. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv is defined as in Fig. 3. Values are the average ± SE (n = 5). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
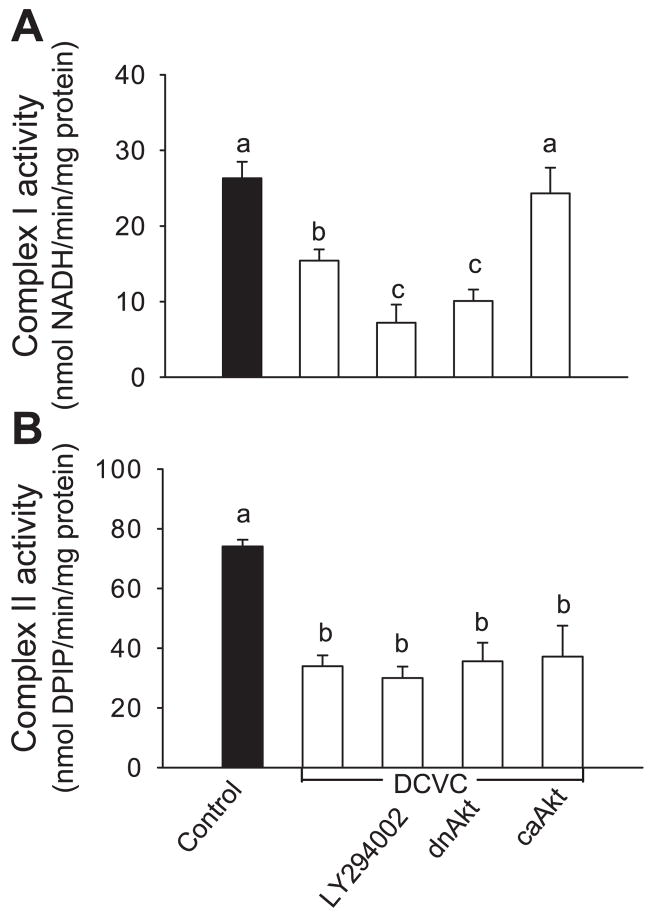
To test a hypothesis that Akt regulates state 3 respiration by improving the function of respiratory complex(es), the activities of all four complexes were measured in mitochondria isolated from injured and noninjured RPTC. DCVC exposure decreased the activities of complex I and complex II to 55 and 50% of controls, respectively (Fig. 5). Blocking Akt activation using LY294002 or expressing dnAkt exacerbated the decreases in complex I activity in DCVC-injured RPTC to 28 and 40% of controls, respectively (Fig. 5A). In contrast, expressing the constitutively active Akt maintained complex I activity in injured RPTC (89% of controls) (Fig. 5A). Neither Akt activation nor Akt inhibition had any effect on DCVC-induced decreases in complex II activity (Fig. 5B).
Fig. 5.
Effect of blocking and increasing Akt activity on the activities of complex I (A) and complex II (B) in mitochondria isolated from RPTC at 24 h following DCVC (240 μM) exposure. Complex I activity was measured by following the oxidation of 0.25 mM NADH. Complex II activity was measured by following the reduction of 0.25 mM dichlorophenolindophenol (DPIP) in the presence of rotenone (10 μg/ml). To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h prior to and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv is defined as in Fig. 3. Values are the average ± SE (n = 5). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
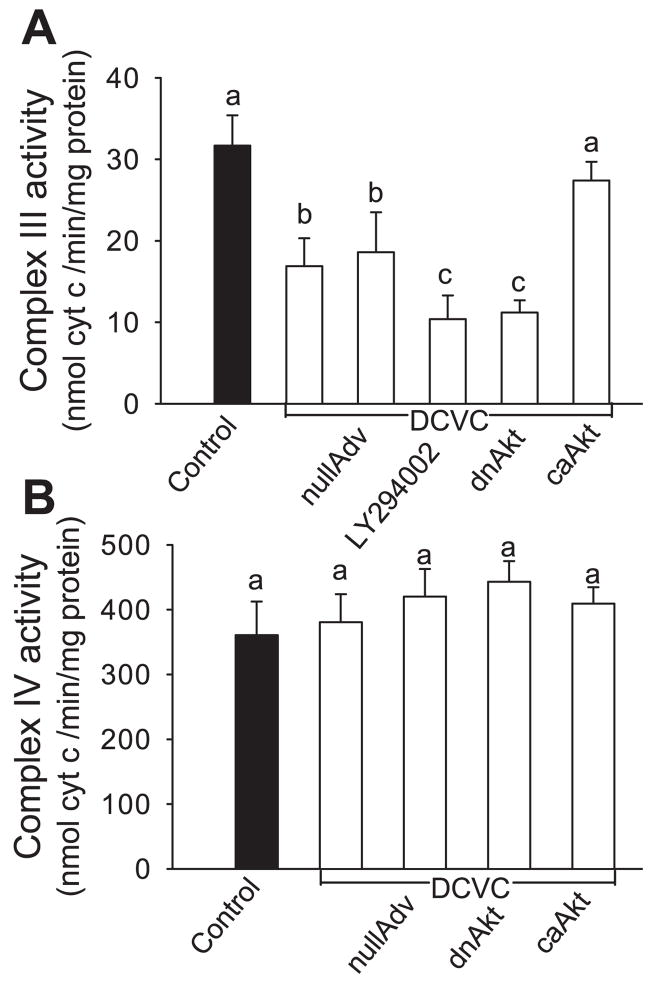
Complex III activity was decreased in DCVC-injured RPTC to 54% of controls (Fig. 6A). Inhibition of Akt activation exacerbated the decrease in activity of complex III to 33% of controls (Fig. 6A). In contrast, activation of Akt maintained activity of complex III (87% of controls) (Fig. 6A). Neither DCVC injury nor changes in Akt activity had any effect on complex IV activity in RPTC (Fig. 6B). Infection of RPTC with adenovirus carrying cDNA coding constitutively active Akt and adenovirus carrying cDNA coding inactive Akt had no effect on the activity of respiratory complexes in noninjured RPTC (data not shown). Similarly, adenovirus carrying an empty vector and LY294002 had no effect on the activities of respiratory complexes in noninjured RPTC (data not shown).
Fig. 6.
Effect of modulation of Akt activity on the activities of complex III (A) and complex IV (B) in RPTC at 24 h following DCVC exposure. Complex III activity was measured by following the reduction of cytochrome c in the presence of 50 μM decyl ubiquinol as a substrate. Complex IV activity was measured as the oxidation of 90 μM reduced cytochrome c. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv is defined as in Fig. 3. Values are the average ± SE (n = 5). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
These data demonstrate that Akt activation increases the activities of complex I and complex III, but has no effect on the activities of complex II and complex IV in DCVC-injured RPTC. Thus our results show for the first time that Akt activation promotes the functions of complex I and complex III and improves mitochondrial respiration in RPTC following toxicant injury.
Akt regulates ΔΨm in injured RPTC
ΔΨm was assessed by incubating RPTC monolayers with JC-1 followed by the flow cytometric analysis of green and red fluorescence. Green fluorescence of JC-1 increased 31% in DCVC-injured RPTC, which demonstrates increased accumulation of JC-1 monomers in the cytosol (Fig. 7, A and B). Concomitantly, red fluorescence decreased 33% in DCVC-injured RPTC, which demonstrated decreased accumulation of J-1 aggregates in mitochondria. DCVC exposure also decreased the ratio of J-1 red aggregates to JC-1 green monomers to 48% of controls at 24 h following the injury (Fig. 7C). These data show that DCVC decreased ΔΨm in RPTC. Akt inhibition exacerbated DCVC-induced decrease in ΔΨm as indicated by 1) increased green fluorescence, 2) decreased red fluorescence, and 3) decreased red/green fluorescence ratio to 30% of controls (Fig. 7). In contrast, Akt activation decreased accumulation of JC-1 monomers in the cytosol, increased accumulation of J-1 aggregates in the mitochondria, and increased red/green fluorescence ratio in injured RPTC to 60% of controls (Fig. 7). These results demonstrate that Akt activation improves ΔΨm in DCVC-injured RPTC.
Fig. 7.
Effect of modulation of Akt activity on mitochondrial membrane potential (ΔΨm) in RPTC at 24 h following DCVC (240 μM) exposure. A: green fluorescence of JC-1 monomers in RPTC. B: red fluorescence of J-1 aggregates in mitochondrial matrix of RPTC. C: ratio of J-1 red aggregate fluorescence to JC-1 green monomer fluorescence. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv is defined as in Fig. 3. Values are the average ± SE (n = 5). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
Akt regulates ATP production in RPTC following DCVC injury
Figure 8 shows that DCVC induces a decrease in ATP production coupled to substrate oxidation through complex I and complex II (66 and 75% of controls at 24 h after the exposure). Activation of Akt by expressing constitutively active Akt in injured RPTC increased complex I (but not complex II) linked ATP production to 81% of controls (Fig. 8, A and B). In contrast, inhibition of Akt activation exacerbated the decreases in complex I-linked synthesis of ATP to 50% of controls (Fig. 8A). These data show that Akt activation improves ATP production coupled to substrate oxidation through complex I.
Fig. 8.
Effect of modulation of Akt activity on ATP production rate coupled to oxidation of substrates through complex I (A) and complex II (B) at 24 h following DCVC (240 μM) exposure in RPTC. Complex I-linked ATP production rate was measured in the presence of 10 mM citrate and 2 mM ADP. Complex II-coupled rate of ATP production was measured in the presence of 10 mM succinate, 0.1 μM rotenone, and 2 mM ADP. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv is defined as in Fig. 3. Values are the average ± SE (n = 5). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
Akt regulates F0F1-ATPase activity in RPTC following DCVC injury
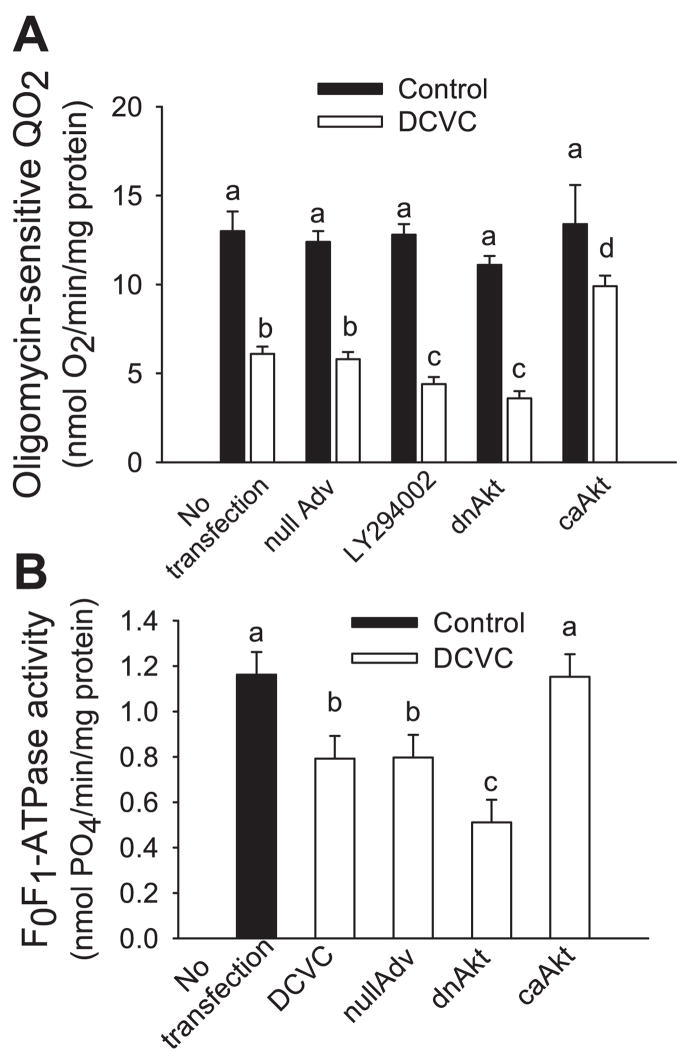
Two approaches were used to determine whether Akt regulates F0F1-ATPase activity in RPTC. First, we used oligomycin (an inhibitor of F0F1-ATPase) to assess oligomycin-sensitive QO2 as an indirect measure of F0F1-ATPase function and oxidative phosphorylation in the whole RPTC. Figure 9A shows that DCVC exposure decreases oligomycin-sensitive QO2 to 47% of controls at 24 h following the injury. Akt activation in DCVC-injured RPTC increased oligomycin-sensitive QO2 to 63% of controls (Fig. 9A). Blocking Akt activation using LY294002 or expressing inactive Akt, exacerbated decreases in oligomycin-sensitive QO2 to 34 and 28% of controls in DCVC-injured RPTC (Fig. 9A). Direct measurements of F0F1-ATPase activity in isolated mitochondria showed that DCVC decreased F0F1-ATPase activity to 65% of controls at 24 h following the exposure (Fig. 9B). Akt activation prevented the decrease in F0F1-ATPase activity whereas overexpression of the inactive Akt (dnAkt) exacerbated DCVC-induced decreases in F0F1-ATPase activity to 44% of controls (Fig. 9B). These results demonstrate that Akt activation maintains the activity of F0F1-ATPase and improves oxidative phosphorylation in RPTC following DCVC injury and suggest that F0F1-ATPase is a target of Akt in RPTC.
Fig. 9.
Effect of modulation of Akt activity on oxidative phosphorylation in RPTC at 24 h following DCVC (240 μM) exposure. A: oligomycin-sensitive oxygen consumption (QO2). B: F0F1-ATPase activity in isolated mitochondria. To inhibit Akt activation, RPTC were exposed to 20 μM LY294002 for 1 h before and immediately after DCVC treatment or infected with adenovirus carrying dnAkt (MOI = 25). To activate Akt, RPTC were infected with adenovirus carrying caAkt (MOI = 25). nullAdv is defined as in Fig. 3. Values are the average ± SE (n = 5–7). Values with dissimilar superscripts are significantly (P < 0.05) different from each other.
DISCUSSION
Our present study demonstrates that Akt plays a protective role against mitochondrial dysfunction induced by the nephrotoxicant, DCVC, in RPTC. Our data show that the phosphorylated (active) Akt is expressed in mitochondria of noninjured RPTC and that there is activation of Akt in mitochondria of control RPTC. A database (Mitoprot) search for the presence of mitochondrial-targeting sequences in Akt (probability for Akt to be translocated to mitochondria), showed that there is 0.25% chance for Akt to be translocated to mitochondria. Mookherjee and colleagues (35) have shown that the overexpression of Akt increases Akt activity in mitochondria of neuroblastoma cells. In addition, overexpression of Akt using Ad-Mito-HA-Akt (adenovirus encoding Akt cDNA composed of the mitochondrial-targeting sequence MSVLTPLLLRGLTGSARRLPVPRAKIHSL) further increases Akt activity in mitochondria (35). Our data show that overexpression of Akt in RPTC increases both protein levels of phosphorylated (active) Akt and Akt activity in mitochondria of injured RPTC.
Previous studies also demonstrated that cytosolic Akt translocates to mitochondria upon exposure to IGF-1 in human neuroblastoma and embryonic kidney cells and following treatment of cardiomyocytes with mitochondrial K+-ATP channel opener BMS-191095 (1, 7). Our data show that Akt localizes predominantly in the mitochondrial outer membrane and to a lesser extent in the mitoplasts. Bijur and colleagues (7) also demonstrated that the cytosolic Akt (activated by IGF-1) translocates to the outer and inner membrane of mitochondria in neuroblastoma cells. Active Akt phosphorylates GSK-3β in the mitochondrial outer membrane (7). Active GSK-3β phosphorylates MPTP, which leads to MPTP opening (23). Phosphorylation of GSK-3β by Akt inhibits GSK-3β and prevents MPTP opening (23). Akt also associates with hexokinases (HK-I and HK-II) present in the mitochondrial outer membrane to inhibit MPTP opening (47). In cardiac cells, mitochondrial Akt regulates mitochondrial K+-ATP channels present in the mitochondrial inner membrane (1).
The studies of Turkseven et al. (50) and Asija et al. (4) demonstrated that Akt phosphorylation in the kidney is controlled by heme oxygenase-1 (HO-1) and heme oxygenase-2 (HO-2), respectively (4, 50). Di Noia et al. (13) showed that overexpression of HO-1 increases the levels of phosphorylated Akt in the kidneys of diabetic rats. Activation of Akt by HO-1 inhibits the loss of ΔΨm in aortic smooth muscle cells (9). Therefore, these studies suggest that HO-1-mediated Akt activation plays an important role in the regulation of ΔΨm. HO-1 is localized in the inner mitochondrial membrane and plays an important role in the protection against apoptosis mediated by the mitochondrial pathway (50). Furthermore, overexpression of HO-1 increases protein levels of mitochondrial carriers (citrate, carnitine/acylcarnitine, deoxynucleotides, dicarboxylic acids, inorganic phosphate, and ADP/ATP) and the activity of cytochrome c oxidase in the diabetic kidney (13). Thus it is likely that HO-1-mediated Akt activation protects against mitochondrial dysfunction.
Our study shows that Akt is constitutively present and is active in the mitochondria of RPTC grown in the improved primary culture conditions that stimulate mitochondrial functions and block glycolysis (42). The presence of active Akt in RPTC mitochondria suggests that Akt plays a role in regulating mitochondrial function in these cells. No decreases in mitochondrial respiration were observed when Akt was active in both the cytosolic and mitochondrial fractions (state 3 respiration coupled to complex I: 20.8 ± 2.4 vs. 22.4 ± 1.5 nmol O2 · min−1 · mg protein−1 in DCVC-treated vs. control RPTC, respectively). In contrast to glycolytic cancer (human leukemia and lymphoma) cells in which the decrease in mitochondrial respiration activates Akt (12, 45), the loss of Akt activity in RPTC is associated with the decreases in mitochondrial respiration and the decline in ATP production.
To determine the role of Akt in protection against mitochondrial dysfunction in injured RPTC, Akt activity was increased or decreased and mitochondrial functions were assessed at 24 h following the DCVC injury. Activation of Akt is protective in DCVC-injured RPTC and improves mitochondrial functions such as respiration, ΔΨm, and ATP production, resulting in the maintenance of intracellular ATP levels. Previous studies have demonstrated that the activation of ERK1/2, PKC-ε, and PKC-α decreases mitochondrial respiration in toxicant-injured RPTC (37, 39, 40). This is the first study that demonstrates that Akt activation improves mitochondrial respiration and oxidative phosphorylation following injury in RPTC. Our data show that the mechanisms responsible for increasing ATP production and intracellular ATP levels by active Akt (48) are increased functions of the respiratory chain and improved ΔΨm. Furthermore, we determined specific mitochondrial targets whose function is regulated by Akt in RPTC mitochondria. We hypothesized that Akt improves the flow of electrons through the respiratory chain by regulating the activity of one or more of the respiratory complexes. Decreases in the activities of respiratory complexes are associated with many pathological conditions such as neurodegeneration, diabetes, aging, and cardiac failure (13, 18, 31). Hypoxia/reoxygenation, cisplatin, DCVC, and oxidant injury also decrease activities of some or all of the complexes in proximal tubular cells (14, 24, 27, 40, 52). Our present data show that DCVC decreases activities of complexes I, II and III, but has no effect on complex IV in RPTC. Oxidation of NADH and FADH2 involves the transfer of electrons through complexes of the respiratory chain and generation of the proton-motive force, which serves as a driving force for the rotation of the F1 portion of the ATP synthase. Therefore, the reduction in activities of complexes ultimately decreases ATP production in mitochondria. Activation of Akt increases whereas Akt inhibition decreases activities of complex I and complex III in injured RPTC, which demonstrates that complex I and complex III are regulated by Akt in injured RPTC. Increases in activities of complex I and complex III result in increased oxidation of NADH, and this change may contribute to improvement of ΔΨm and the driving force for ATP synthesis. Indeed, our data show that Akt activation improves ΔΨm and ATP production in injured RPTC. Thus our study is the first to demonstrate the protective role of active Akt against the decreases in activities of complex I and complex III and the loss of ΔΨm in mitochondria of injured cells.
The activities of mitochondrial complexes are regulated by different mechanisms including phosphorylation (5, 19). Previously, it has been shown that PKA increases the activity of complex I in cardiac myoblasts by phosphorylating the 18-kDa subunit of complex I (5, 19). Several subunits of complex I (NDUFA10 and NDUFA1) are phosphorylated at serine residues in a cAMP-dependent manner (5, 19). A study by He and collaborators (17) suggests that the dephosphorylation of Rieske iron-sulfur protein (RISP) of complex III is involved in MPT in rat liver mitochondria. Another study demonstrated that in myocardial tissue, increased Akt activity is associated with decreased activity of complex III, but the direct mechanism of this decrease has not been delineated (3). Suliman et al. (49) demonstrated that in cardiac tissue, carbon monoxide-mediated Akt activation increases protein levels of all four mitochondrial complexes. Therefore, it is possible that in DCVC-injured RPTC, active Akt increases complex I and III activities by phosphorylating the subunits of and/or increasing the protein levels of these complexes. However, further studies are necessary to determine the exact mechanism by which active Akt increases the functions of complexes I and III in DCVC-injured RPTC.
It is also likely that Akt phosphorylates one or more components of the MPTP. Previously, Akt activation has been shown to maintain ΔΨm by inhibiting MPT and blocking the MPTP in many cell types during toxicant or ischemic insult (9, 10, 13, 23, 44, 47). We speculate that, in addition to regulating the activity of mitochondrial complexes, Akt present in the mitochondrial outer membrane phosphorylates and regulates MPTP opening in injured RPTC. In cardiac myocytes and monocytes, Akt forms a complex with PKC-ε and eNOS, which leads to phosphorylation of Akt and results in maintaining ΔΨm (46, 54). Our results show that expressing active Akt reduced the decreases in ΔΨm DCVC-injured RPTC. However, Akt activation did not restore ΔΨm as efficiently as it restored activities of complex I and complex III, suggesting an existence of a proton leak that is not regulated by Akt in injured RPTC. It is also possible that NADH (the substrate for complex I) levels are decreased in DCVC-injured mitochondria but the levels of mitochondrial NADH (generated in the citric acid cycle) are not controlled by Akt.
The α-, β-, and γ-subunits of F1 portion of the ATP synthase are also regulated by phosphorylation (5, 7). Mitochondrial Akt forms a complex with the catalytic β-subunit of F0F1-ATPase in human neuroblastoma cells and embryonic kidney mitochondria (7). In myocardial cells, Akt activation decreases F0F1-ATPase activity, downregulates oxidative phosphorylation, and stimulates the uptake and utilization of glucose in glycolysis (3). In contrast, our data show that in DCVC-injured RPTC, the activation of Akt restores the activity of F0F1-ATPase and oxidative phosphorylation. Because RPTC are not glycolytic in vivo and, in our model, are grown in glucose-free media that stimulate mitochondrial ATP production, Akt does not stimulate oxidative phosphorylation by decreasing glycolysis, but by increasing oxidative metabolism. Therefore, we conclude that, in addition to increasing ΔΨm, active Akt also promotes oxidative phosphorylation and ATP synthesis by increasing F0F1-ATPase activity in DCVC-injured RPTC.
In conclusion, this report shows a novel observation that the activation of Akt protects against nephrotoxicant-induced decreases in oxidative phosphorylation and ATP levels in RPTC. Active Akt diminishes mitochondrial dysfunction and improves oxidative phosphorylation following the DCVC injury by preventing the decreases in functions of complex I and complex III, improving ΔΨm, and restoring F0F1-ATPase activity. Improvement of oxidative phosphorylation and ATP production by Akt activation leads to increases in intracellular ATP levels and inhibition of necrosis in DCVC-injured RPTC. This study suggests that the activation of Akt could serve as a means of protection against renal injury by promoting mitochondrial function in RPTC.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-59558 (to G. Nowak.), a Predoctoral Fellowship (0510051Z and 0710159Z) from the American Heart Association, Heartland Affiliate (to Z. P. Shaik), and a grant from the Committee for Allocation of Graduate Student Research Funds at the University of Arkansas for Medical Sciences, Little Rock, AR (to Z. P. Shaik).
References
- 1.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 2.Amacher DE. Drug-associated mitochondrial toxicity and its detection. Curr Med Chem. 2005;12:1829–1839. doi: 10.2174/0929867054546663. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan R, Moe GW, Goldenthal MJ, Marin-Garcia J. Akt signaling pathway in pacing-induced heart failure. Mol Cell Biochem. 2005;268:103–110. doi: 10.1007/s11010-005-3699-3. [DOI] [PubMed] [Google Scholar]
- 4.Asija A, Peterson SJ, Stec DE, Abraham NG. Targeting endothelial cells with heme oxygenase-1 gene using VE-cadherin promoter attenuates hyperglycemia-mediated cell injury and apoptosis. Antioxid Redox Signal. 2007;9:2065–2074. doi: 10.1089/ars.2007.1804. [DOI] [PubMed] [Google Scholar]
- 5.Augereau O, Claverol S, Boudes N, Basurko MJ, Bonneu M, Rossignol R, Mazat JP, Letellier T, Dachary-Prigent J. Identification of tyrosine-phosphorylated proteins of the mitochondrial oxidative phosphorylation machinery. Cell Mol Life Sci. 2005;62:1478–1488. doi: 10.1007/s00018-005-5005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrientos A. In vivo and in organello assessment of OXPHOS activities. Methods. 2002;26:307–316. doi: 10.1016/S1046-2023(02)00036-1. [DOI] [PubMed] [Google Scholar]
- 7.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- 9.Brunt KR, Fenrich KK, Kiani G, Tse MY, Pang SC, Ward CA, Melo LG. Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler Thromb Vasc Biol. 2006;26:2027–2034. doi: 10.1161/01.ATV.0000236204.37119.8d. [DOI] [PubMed] [Google Scholar]
- 10.Caro AA, Cederbaum AI. Role of phosphatidylinositol 3-kinase/AKT as a survival pathway against CYP2E1-dependent toxicity. J Pharmacol Exp Ther. 2006;318:360–372. doi: 10.1124/jpet.106.102921. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Cai J, Anders MW, Stevens JL, Jones DP. Role of mitochondrial dysfunction in S-(1,2-dichlorovinyl)-L-cysteine-induced apoptosis. Toxicol Appl Pharmacol. 2001;170:172–180. doi: 10.1006/taap.2000.9107. [DOI] [PubMed] [Google Scholar]
- 12.Coloff JL, Rathmell JC. Metabolic regulation of Akt: roles reversed. J Cell Biol. 2006;175:845–847. doi: 10.1083/jcb.200610119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Noia MA, Van Driesche S, Palmieri F, Yang LM, Quan S, Goodman AI, Abraham NG. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome c oxidase activity in experimental diabetes. J Biol Chem. 2006;281:15687–15693. doi: 10.1074/jbc.M510595200. [DOI] [PubMed] [Google Scholar]
- 14.Feldkamp T, Kribben A, Roeser NF, Senter RA, Kemner S, Venkatachalam MA, Nissim I, Weinberg JM. Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules. Am J Physiol Renal Physiol. 2004;286:F749–F759. doi: 10.1152/ajprenal.00276.2003. [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3β. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Hall AM, Unwin RJ. The not so ’mighty chondrion’: emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiol. 2007;105:p1–p10. doi: 10.1159/000096860. [DOI] [PubMed] [Google Scholar]
- 17.He L, Lemasters JJ. Dephosphorylation of the Rieske iron-sulfur protein after induction of the mitochondrial permeability transition. Biochem Biophys Res Commun. 2005;334:829–837. doi: 10.1016/j.bbrc.2005.06.170. [DOI] [PubMed] [Google Scholar]
- 18.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann NY Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 19.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Hovius R, Lambrechts H, Nicolay K, de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- 21.Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194:279–283. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997;280:638–649. [PubMed] [Google Scholar]
- 25.Lash LH, Anders MW. Mechanism of S-(1,2-dichlorovinyl)-L-cysteine-and S-(1,2-dichlorovinyl)-L-homocysteine-induced renal mitochondrial toxicity. Mol Pharmacol. 1987;32:549–556. [PubMed] [Google Scholar]
- 26.Lash LH, Hueni SE, Putt DA. Apoptosis, necrosis, and cell proliferation induced by S-(1,2-dichlorovinyl)-L-cysteine in primary cultures of human proximal tubular cells. Toxicol Appl Pharmacol. 2001;177:1–16. doi: 10.1006/taap.2001.9295. [DOI] [PubMed] [Google Scholar]
- 27.Lash LH, Putt DA, Hueni SE, Krause RJ, Elfarra AA. Roles of necrosis, Apoptosis, and mitochondrial dysfunction in S-(1,2-dichlorovinyl)-L-cysteine sulfoxide-induced cytotoxicity in primary cultures of human renal proximal tubular cells. J Pharmacol Exp Ther. 2003;305:1163–1172. doi: 10.1124/jpet.102.046185. [DOI] [PubMed] [Google Scholar]
- 28.Lash LH, Xu Y, Elfarra AA, Duescher RJ, Parker JC. Glutathione-dependent metabolism of trichloroethylene in isolated liver and kidney cells of rats and its role in mitochondrial and cellular toxicity. Drug Metab Dispos. 1995;23:846–853. [PubMed] [Google Scholar]
- 29.Law RH, Manon S, Devenish RJ, Nagley P. ATP synthase from Saccharomyces cerevisiae. Methods Enzymol. 1995;260:133–163. doi: 10.1016/0076-6879(95)60135-x. [DOI] [PubMed] [Google Scholar]
- 30.Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin Nephrol. 1998;18:505–518. [PubMed] [Google Scholar]
- 31.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Godwin ML, Nowak G. Protein kinase C-α inhibits the repair of oxidative phosphorylation after S-(1,2-dichlorovinyl)-L-cysteine injury in renal cells. Am J Physiol Renal Physiol. 2004;287:F64–F73. doi: 10.1152/ajprenal.00216.2003. [DOI] [PubMed] [Google Scholar]
- 33.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinney LL, Picken JC, Weakley FB, Eldridge AC, Campbell RE, Cowan JC, Biester HE. Possible toxic factor of trichloroethylene-extracted soybean oil meal3. J Am Chem Soc. 1959;81:909–915. [Google Scholar]
- 35.Mookherjee P, Quintanilla R, Roh MS, Zmijewska AA, Jope RS, Johnson GV. Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. J Cell Biochem. 2007;102:196–210. doi: 10.1002/jcb.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller C, Dunschede F, Koch E, Vollmar AM, Kiemer AK. Alphalipoic acid preconditioning reduces ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G769–G778. doi: 10.1152/ajpgi.00009.2003. [DOI] [PubMed] [Google Scholar]
- 37.Nowak G. Protein kinase C-α and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem. 2002;277:43377–43388. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak G, Aleo MD, Morgan JA, Schnellmann RG. Recovery of cellular functions following oxidant injury. Am J Physiol Renal Physiol. 1998;274:F509–F515. doi: 10.1152/ajprenal.1998.274.3.F509. [DOI] [PubMed] [Google Scholar]
- 39.Nowak G, Bakajsova D, Clifton GL. Protein kinase C-ε modulates mitochondrial function and active Na+ transport after oxidant injury in renal cells. Am J Physiol Renal Physiol. 2004;286:F307–F316. doi: 10.1152/ajprenal.00275.2003. [DOI] [PubMed] [Google Scholar]
- 40.Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–F855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak G, Keasler KB, McKeller DE, Schnellmann RG. Differential effects of EGF on repair of cellular functions after dichlorovinyl-L-cysteine-induced injury. Am J Physiol Renal Physiol. 1999;276:F228–F236. doi: 10.1152/ajprenal.1999.276.2.F228. [DOI] [PubMed] [Google Scholar]
- 42.Nowak G, Schnellmann RG. L-Ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol Cell Physiol. 1996;271:C2072–C2080. 2. doi: 10.1152/ajpcell.1996.271.6.C2072. [DOI] [PubMed] [Google Scholar]
- 43.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3β. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3β disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 45.Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preiss S, Namgaladze D, Brune B. Critical role for classical PKC in activating Akt by phospholipase A2-modified LDL in monocytic cells. Cardiovasc Res. 2007;73:833–840. doi: 10.1016/j.cardiores.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 48.Shaik ZP, Fifer EK, Nowak G. Protein kinase B/Akt modulates nephrotoxicant-induced necrosis in renal cells. Am J Physiol Renal Physiol. 2007;292:F292–F303. doi: 10.1152/ajprenal.00082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 50.Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S, Abraham NG. Impact of silencing HO-2 on EC-SOD and the mitochondrial signaling pathway. J Cell Biochem. 2007;100:815–823. doi: 10.1002/jcb.21138. [DOI] [PubMed] [Google Scholar]
- 51.van de Water B, Zoeteweij JP, de Bont HJ, Mulder GJ, Nagelkerke JF. Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential. Studies in isolated proximal tubular cells using the nephrotoxin 1,2-dichlorovinyl-L-cysteine. J Biol Chem. 1994;269:14546–14552. [PubMed] [Google Scholar]
- 52.van de Water B, Zoeteweij JP, de Bont HJ, Nagelkerke JF. Inhibition of succinate:ubiquinone reductase and decrease of ubiquinol in nephrotoxic cysteine S-conjugate-induced oxidative cell injury. Mol Pharmacol. 1995;48:928–937. [PubMed] [Google Scholar]
- 53.Xu R, Chen J, Cong X, Hu S, Chen X. Lovastatin protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt and ERK1/2. J Cell Biochem. doi: 10.1002/jcb.21402. In press. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Baines CP, Zong C, Cardwell EM, Wang G, Vondriska TM, Ping P. Functional proteomic analysis of a three-tier PKCε-Akt-eNOS signaling module in cardiac protection. Am J Physiol Heart Circ Physiol. 2005;288:H954–H961. doi: 10.1152/ajpheart.00756.2004. [DOI] [PubMed] [Google Scholar]