Abstract
Recently, a classification system was proposed for rotaviruses in which all the 11 genomic RNA segments are used (Matthijnssens et al., 2008; J. Virol. 82:3204-3219). Based on nucleotide identity cut-off percentages, different genotypes were defined for each genome segment. A nomenclature for the comparison of complete rotavirus genomes was considered in which the notations Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx are used for the VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5/6 encoding genes, respectively. This classification system is an extension of the previously applied, genotype-based system which made use of the rotavirus gene segments encoding VP4, VP7, VP6, and NSP4. In order to assign rotavirus strains to one of the established genotypes or a new genotype, a standard procedure is proposed in this report. As more human and animal rotavirus genomes will be completely sequenced, new genotypes for each of the eleven gene segments may be identified. A Rotavirus Classification Working Group (RCWG) including specialists in molecular virology, infectious diseases, epidemiology, and public health was formed, which can assist in the appropriate delineation of new genotypes, thus avoiding duplications and helping minimize errors. Scientists discovering a potentially new rotavirus genotype for any of the 11 gene segments are invited to send the novel sequence to the RCWG, where the sequence will be analyzed, and a new nomenclature will be advised as appropriate. The RCWG will update the list of classified strains regularly and make this accessible on a website. Close collaboration with the Study Group Reoviridae of the International Committee on the Taxonomy of Viruses will be maintained.
Introduction
Rotaviruses are a common cause of severe, acute gastroenteritis in infants and young children worldwide. By the age of 5 years, 95% of children will have experienced at least one rotavirus infection, with or without evidence of gastroenteritis symptoms. It is estimated that, globally, 1 in 5 cases will be presented to a doctor, 1 in 65 will require hospitalization, and 1 in 293 will die [27, 28, 39].
Rotaviruses have a genome of 11 segments of double stranded RNA encoding 6 structural viral proteins [VP] (VP1, VP2, VP3, VP4, VP6, VP7) and 6 nonstructural [NS] proteins (NSP1-NSP6). With the exception of genome segment 11 which encodes 2 proteins (NSP5 and NSP6) the genome segments are monocistronic. Full gene-protein assignments have been achieved for several rotavirus strains ([8], http://www.iah.bbsrc.ac.uk/dsRNA_virus_proteins/Rotavirus.htm.). The genome is enclosed in a triple layered particle: the inner layer consisting of VP2 encloses the genome and two minor structural proteins, VP1 and VP3, thus forming the ‘core’; the middle layer consisting of VP6 surrounds the core, forming a double layered particle (DLP); the outer layer, consisting of VP7 and spike-like projections of VP4, enwraps the DLP to form the triple-layered particle (TLP) or infectious virion [8].
Rotaviruses (Rotavirus genus) are classified into the Reoviridae family, along with the genera: Orthoreovirus, Orbivirus, Rotavirus, Coltivirus, Aquareovirus, Cypovirus, Fijivirus, Phytoreovirus, Oryzavirus, Seadornavirus, Idnoreovirus and Mycoreovirus [21]. The Rotavirus genus includes at least seven serogroups (A to G) that may be distinguished on the basis of their antigenic relationships and the pattern of migration of the dsRNA segments in polyacrylamide gel electrophoresis [4]. Group A rotaviruses are important enteric pathogens in humans and in a wide range of mammalian and avian species [8].
Historically, VP6 was the first rotavirus protein used for classification. Both VP2 and VP6 are highly immunogenic protein in the virion [4, 41]. After infection, antibodies to VP6 are easily detected [4, 41], and the most sensitive immunologic diagnostic assays are based on detection of this protein. VP6 bears different epitopes which allow to differentiate different subgroup (SG) specificities of group A rotaviruses: SG I, SG II, SG I + II, or SG non-I, non-II viruses can be distinguished according to reactivities with two monoclonal antibodies (MAbs) [4]. More recently, based on molecular characterization, only 2 groups (termed genogroups) were distinguished among group A human rotaviruses (genogroup I: SGI; genogroup II: SGII, SGI + II , and SG non-I, non-II.) [13]. In 1985, a classification scheme for rotaviruses was proposed to allow for the presence of multiple “groups” of rotaviruses and for the existence of “serotypes” which crossed species [10] and later in 1989, a binary classification system reminiscent of the one used for the classification of influenza viruses was established, derived from immunological reactivities and gene structures of the two outer capsid proteins, VP4 and VP7, that independently elicit neutralizing antibodies [4]. Thus, rotavirus strains are classified into VP4 or P serotypes (P for protease-sensitive) and VP7 or G (G for glycoprotein) serotypes [8]. Classification of rotaviruses into VP4 or VP7 serotypes is performed by cross-neutralization assays using hyperimmune sera raised to prototype viruses and/or to laboratory-engineered mono-reassortants. Since antigenic characterization is time-consuming and requires virus collections and proper immunological reagents that are not available in all the laboratories, and due to the increasing ease of sequencing, the antigenic classification has slowly been replaced by a classification system of rotaviruses into VP4 or VP7 genotypes, performed by sequence analyses and based on identities between sequences of cognate rotavirus gene segments. So far, 19 G genotypes (14 G serotypes) and 27 different P genotypes (14 P serotypes, 1A, 1B, and 2 to 14) have been identified [19]. G serotype designations largely coincide with G genotype designations. In contrast, a dual nomenclature has been adopted for the VP4 antigenic and genetic classification [8]. The P serotype (when known) is denoted by an arabic number (sometimes followed by a capital letter) and the P genotype is denoted immediately after the P serotype number by an arabic number within squared brackets. Rotavirus strains belonging to 11 G types (G1-G6, G8-G12) and 12 P types (P1A[8], P1B[4], P2A[6], P2C[6], P3[9], P4[10], P5A[3], P6[1], P8[11], P11[14], P12[19], P[25]) have been isolated from humans [4, 7, 9, 19, 25, 33, 36]. During the late 1990s, sequence analyses of the rotavirus enterotoxin NSP4 gene from human and animal rotavirus strains revealed the presence of six (A to F) distinct NSP4 genotypes [3, 5, 8, 11, 15, 22].
The complete ORF sequences of all eleven genome segments of 53 rotavirus strains have been determined (human Wa, DS-1, AU-1, D, KU, P, TB-Chen, ST3, IAL28, Se584, 69M, WI61, A64, L26, T152, B1711, B3458, B10925-97, 111/05-27, B4633-03, RV161-00, RV176-00, N26-02, Dhaka12-03, Dhaka16-03, Matlab13-03, Dhaka25-02, Dhaka6, DRC86, DRC88, Hun5, MG6, PA169, KTM368, B4106, simian SA11, RRV, TUCH, bovine B383, UK, RF, WC3, BRV033, porcine A131, A253, YM, OSU, Gottfried, avian PO-13, ovine OVR762, guanaco Chubut, Río Negro, and lapine 30/96) [12, 17-19, 34, 38].
The simian rotavirus SA11 strain is considered the type species of group A rotaviruses, and its genome segments range in size from 3,302 (segment 1) to 667 (segment 11) base pairs (bp) [8, 35]. Sequences from different rotavirus strains show that each RNA segment starts with two 5′guanines followed by the 5′end non-coding sequences [= untranslated region, UTR] (9-49 nucleotides [nt]), an open reading frame (or two in the case of genome segment 11), the 3′ UTRs (17-182 nt), and ends with two 3′ cytidines. In addition, the plus-stranded RNA is capped at the 5′ end with m7GpppG(m)Gpy, but there are no polyadenylated sequences at the 3′ end [8].
The overall genetic relatedness among homologues genome segments has been assessed by RNA-RNA hybridizations performed under high stringency conditions [23] and, more recently, by direct sequence comparisons. RNA-RNA hybridization has provided molecular evidence to show close interspecies relationships between human and animal strains, or confirms the existence of naturally occurring rotavirus reassortant strains. Three human genogroups, represented by reference rotavirus strains Wa, DS-1, and AU-1 have been established. Several animal genogroups have also been identified but the extent of their relationship among each other and to human strains has not been completely elucidated [24]. However, inter-genogroup reassortments may generate chimeric viruses with mixed constellations of dsRNA segments that are not easily assignable to a defined genogroup. In addition, viruses partially or completely divergent in their genome constellation from the prototype viruses may not be readily characterized. Sequence analysis of human and animal rotavirus strains has revealed the existence of a number of unusual rotavirus strains and has revealed that some strain have a puzzling genome composition derived from repeated reassortment events that could not be tracked effectively using nucleic acids hybridization techniques [2, 14, 16, 18].
Sequence-based studies targeting all the rotavirus gene segments appear more appropriate to generate conclusive data and support studies on rotavirus evolution. When rotavirus strains are analyzed and compared to one another by partial or complete sequences of all 11 gene segments, as initiated by Maunula and von Bonsdorff [20], the genetic relationships among all rotavirus strains can be determined [12, 17-19, 29, 34, 38]. Recently, using comparison of sequenced genes, evidence was presented that the human rotavirus strains belonging to the Wa-like genogroup might have a common origin with porcine rotaviruses, while the human DS-1-like genogroup might have a common origin with bovine rotaviruses [19]. Sequence comparison of rotavirus genomes is critical to the assignment of genotypes and elucidation of rotavirus evolutionary patterns [19]. One method that is used to study typical evolutionary distances between virus strains is pairwise sequence identity profiles [1], and this method resolves virus genotypes as well distinguished frequency peaks and on this biological basis provides the rationale for classification. This approach has been used successfully to classify astroviruses [26], sapoviruses [37], noroviruses [42] and papillomaviruses [6].
Accordingly, the establishment of a classification system encompassing all rotavirus gene segments and sorting individual genes into defined clusters/genotypes based on reliable percentage identity cut-off values is required in order to adopt a universal, standardized nomenclature suitable for reliable data analysis and to exchange readily and unequivocally information on the various rotavirus strains. Recently, phylogenetic analyses were performed and pairwise sequence identity profiles constructed for each of the 11 genome segments of many group A rotaviruses. Based on these analyses, a modified classification system was proposed for VP4, VP7, and NSP4, and a novel classification system for VP1, VP2, VP3, VP6, NSP1, NSP2, NSP3, and NSP5/6 [19] to be used for international standardization and implementation. The system provides an excellent framework to further analyze rotavirus interspecies evolutionary relationships [19], gene reassortment events (genetic shift), functional gene linkage in reassortant progeny, and emergence of new rotaviruses by interspecies transmission.
Review of Nomenclature System
A summary of the calculated cut-off values for the 11 rotavirus RNA segments is shown in Table 1 [19]. The nucleotide cut-off percentages define different genotypes for all genes. To designate the complete genetic make up of a virus, the schematic nomenclature was proposed: Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx, representing the genotypes of respectively the VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5/6 encoding gene segments, with x indicating the number of the genotype. A nomenclature overview of a large number of strains is provided in Table 2. It should be noted that no classification was obtained for NSP6, since the NSP6 ORF does completely fall into the NSP5 ORF. The phylogenetic analysis (at the nucleotide level) of the NSP6 ORF would be nearly identical to the NSP5 phylogenetic tree. Moreover, not all strains possess a NSP6 ORF.
Table 1.
Nucleotide percentage identity cut-off values defining genotypes for 11 rotavirus gene segments.
| Gene product | Percentage identity cut-off values |
Genotypes | Name of genotypes |
|---|---|---|---|
| VP7 | 80% | 19G | Glycosylated |
| VP4 | 80% | 27P | Protease sensitive |
| VP6 | 85% | 11I | Inner capsid |
| VP1 | 83% | 4R | RNA-dependent RNA polymerase |
| VP2 | 84% | 5C | Core protein |
| VP3 | 81% | 6M | Methyltransferase |
| NSP1 | 79% | 14A | Interferon Antagonist |
| NSP2 | 85% | 5N | NTPase |
| NSP3 | 85% | 7T | Translation enhancer |
| NSP4 | 85% | 11E | Enterotoxin |
| NSP5 | 91% | 6H | pHosphoprotein |
Table 2.
Application of the proposed classification and nomenclature to the structural and non-structural protein encoding genes for known human, bovine, porcine, simian, avian, equine, lapine, murine and ovine rotavirus strains. Green, red, and orange were used for Wa-like, DS-1-like and AU-like gene segments, respectively, while yellow, blue, and purple were used for the avian PO-13-like rotavirus gene segments, some typical porcine VP4, VP7, and VP6 genotypes, and the SA-11-like gene segments, respectively. It should be noted that the genotypes of more than 60% of the isolates in this table are partial, requiring additional work.
| Genotypes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain name | Host | VP7 | VP4 | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP4 | NSP5 |
| M37 | Human | G1 | P[6] | A1 | E1 | |||||||
| Wa | Human | G1 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| KU | Human | G1 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| Dhaka16-03 | Human | G1 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| D | Human | G1 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| K8 | Human | G1 | P[9] | A1 | H3 | |||||||
| RV4 | Human | G1 | N1 | E1 | ||||||||
| DS-1 | Human | G2 | P[4] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 |
| TB-Chen | Human | G2 | P[4] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 |
| NR1 | Human | G2 | P[4] | I2 | A2 | N2 | T2 | E2 | H2 | |||
| S2 | Human | G2 | P[4] | I2 | R2 | C2 | M2 | T2 | E2 | |||
| RV5 | Human | G2 | P[4] | N2 | E2 | |||||||
| RMC/G66 | Human | G2 | P[4] | I1 | E2 | H2 | ||||||
| KUN | Human | G2 | P[4] | E2 | H2 | |||||||
| E210 | Human | G2 | P[4] | I1 | E2 | |||||||
| AU85 | Human | G2 | P[4] | E2 | H1 | |||||||
| AU102 | Human | G2 | P[4] | E2 | H1 | |||||||
| 1076 | Human | G2 | P[6] | I2 | E2 | |||||||
| IS2 | Human | G2 | I2 | N2 | T2 | H2 | ||||||
| PA260/97 | Human | G3 | P[3] | E8 | ||||||||
| RV3 | Human | G3 | P[6] | I1 | E1 | |||||||
| YO | Human | G3 | P[8] | I1 | R1 | C1 | M1 | E1 | ||||
| RMC437 | Human | G3 | P[8] | I1 | E1 | H1 | ||||||
| P(rice?) | Human | G3 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| Ai-75 | Human | G3 | P[8] | E1 | ||||||||
| CH32 | Human | G3 | P[8] | E1 | ||||||||
| CHW2 | Human | G3 | P[8] | E1 | ||||||||
| MO | Human | G3 | P[8] | E1 | ||||||||
| M | Human | G3 | T1 | E1 | H1 | |||||||
| Au-1 | Human | G3 | P[9] | I3 | R3 | C3 | M3 | A3 | N3 | T3 | E3 | H3 |
| “02-92” | Human | G3 | P[9] | E3 | ||||||||
| B4106 | Human | G3 | P[14] | I2 | R2 | C2 | M3 | A9 | N2 | T6 | E5 | H3 |
| RMC61 | Human | G4 | P[4] | I1 | E1 | H1 | ||||||
| ST3 | Human | G4 | P[6] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| Hosokawa | Human | G4 | P[8] | I1 | R1 | C1 | M1 | |||||
| RMC100 | Human | G4 | P[8] | I1 | A1 | N1 | T1 | E1 | H1 | |||
| Hochi | Human | G4 | P[8] | M1 | A1 | E1 | ||||||
| VA70 | Human | G4 | P[8] | E1 | ||||||||
| Odelia | Human | G4 | P[8] | E1 | ||||||||
| IAL28 | Human | G5 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| B1711 | Human | G6 | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 |
| Se584 | Human | G6 | P[9] | I2 | R2 | C2 | M2 | A3 | N2 | T1 | E2 | H3 |
| Hun5 | Human | G6 | P[14] | I2 | R2 | C2 | M2 | A11 | N2 | T6 | E2 | H3 |
| MG6 | Human | G6 | P[14] | I2 | R2 | C2 | M2 | A11 | N2 | T6 | E2 | H3 |
| PA169 | Human | G6 | P[14] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| 111/05-27 | Human | G6 | P[14] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| B10925-97 | Human | G6 | P[14] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| MP409 | Human | G8 | P[1] | A11 | T6 | E2 | ||||||
| DRC86 | Human | G8 | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 |
| DRC88 | Human | G8 | P[8] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 |
| 69M | Human | G8 | P[10] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 |
| B37 | Human | G8 | N2 | H2 | ||||||||
| US1205 | Human | G9 | P[6] | I2 | E2 | |||||||
| WI61 | Human | G9 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| B3458 | Human | G9 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| F45 | Human | G9 | P[8] | N2 | ||||||||
| 116E | Human | G9 | P[11] | I1 | A1 | E1 | ||||||
| RMC321 | Human | G9 | P[19] | I5 | A1 | N1 | T1 | E1 | H1 | |||
| RMC/G7 | Human | G9 | P[19] | I5 | A1 | E1 | H1 | |||||
| Mc323 | Human | G9 | P[19] | H1 | ||||||||
| Mc345 | Human | G9 | P[19] | H1 | ||||||||
| I321 | Human | G10 | P[11] | I2 | A1 | N2 | T1 | E2 | ||||
| A64 | Human | G10 | P[14] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| Dhaka6 | Human | G11 | P[25] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| KTM368 | Human | G11 | P[25] | I5 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| L26 | Human | G12 | P[4] | I2 | R2 | C2 | M1/M2 | A2 | N1 | T2 | E2 | H1 |
| Dhaka12-03 | Human | G12 | P[6] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| Matlab13-03 | Human | G12 | P[6] | I1 | R1 | C1 | M1 | A1 | N1 | T2 | E1 | H1 |
| N26-02 | Human | G12 | P[6] | I2 | R2 | C2 | M2 | A2 | N1 | T2 | E6 | H2 |
| RV176-00 | Human | G12 | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E6 | H2 |
| RV161-00 | Human | G12 | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E1 | H2 |
| B4633-03 | Human | G12 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| Dhaka25-02 | Human | G12 | P[8] | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| T152 | Human | G12 | P[9] | I3 | R3 | C3 | M3 | A12 | N3 | T3 | E3 | H6 |
| RUBV3 | Bovine | G3 | P[3] | I2 | E2 | H3 | ||||||
| KJ44 | Bovine | G5 | P[1] | A1 | N1 | T1 | E1 | H1 | ||||
| KJ75 | Bovine | G5 | P[5] | A1 | N1 | T1 | E1 | H1 | ||||
| NCDV-Lincoln | Bovine | G6 | P[1] | I2 | R2 | C2 | M2 | N2 | T6 | E2 | ||
| BRV033 | Bovine | G6 | P[1] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| RF | Bovine | G6 | P[1] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| Uk(tc) | Bovine | G6 | P[5] | I2 | R2 | C2 | M2 | A3 | N2 | T7 | E2 | H3 |
| WC3 | Bovine | G6 | P[5] | I2 | R2 | C2 | M2 | A3 | N2 | T6 | E2 | H3 |
| B641 | Bovine | G6 | P[5] | A3 | ||||||||
| O | Bovine | G8 | P[1] | A11 | N2 | |||||||
| A5 | Bovine | G8 | P[1] | A14 | ||||||||
| B223 | Bovine | G10 | P[11] | I2 | A13 | E2 | ||||||
| A44 | Bovine | G10 | P[11] | A3 | ||||||||
| Hg18 | Bovine | G15 | P[21] | E2 | ||||||||
| 993-83 | Bovine | G18 | P[17] | I4 | ||||||||
| Ty-1 | Avian | G7 | P[17] | I4 | N4 | E4 | ||||||
| Ty-3 | Avian | G7 | P[17] | I4 | H11 | |||||||
| PO-13 | Avian | G18 | P[17] | I4 | R4 | C4 | M4 | A4 | N4 | T4 | E4 | H4 |
| Ch-1 | Avian | G19 | P[17] | I10 | H10 | |||||||
| P21-5 | Porcine | G1 | P[27] | E9 | ||||||||
| 34461-4 | Porcine | G2 | P[23] | I5 | E1 | H1 | ||||||
| CMP034 | Porcine | G2 | P[27] | I5 | E9 | H1 | ||||||
| A131 | Porcine | G3 | P[7] | I5 | R1 | C2 | M1 | A1 | N1 | T1 | E1 | H1 |
| CRW-8 | Porcine | G3 | P[7] | I5 | ||||||||
| 4F | Porcine | G3 | P[19] | I5 | ||||||||
| A411 | Porcine | G3 | I5 | A1 | N1 | T1 | E1 | H1 | ||||
| Gottfried | Porcine | G4 | P[6] | I1 | R1 | C1 | M1 | A8 | N1 | T1 | E1 | H1 |
| OSU | Porcine | G5 | P[7] | I5 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| A34 | Porcine | G5 | P[23] | E1 | ||||||||
| 134/04-15 | Porcine | G5 | P[26] | I5 | E1 | |||||||
| 344/04-1 | Porcine | G5 | P[27] | E1 | ||||||||
| HP140 | Porcine | G6 | P[13] | I2 | E1 | H1 | ||||||
| HP113 | Porcine | G6 | P[13] | I2 | E1 | H1 | ||||||
| A253 | Porcine | G11 | P[7] | I5 | R1 | C2 | M1 | A1 | N1 | T1 | E1 | H1 |
| YM | Porcine | G11 | P[7] | I5 | R1 | C1 | M1 | A8 | N1 | T1 | E1 | H1 |
| RU172 | Porcine | G12 | P[7] | I5 | E1 | H1 | ||||||
| H2 | Equine | G3 | P[12] | I2 | A10 | E2 | ||||||
| FI14 | Equine | G3 | P[12] | I6 | A10 | E2 | ||||||
| H1 | Equine | G5 | P[7] | I5 | A8 | E1 | ||||||
| R-22 | Equine | G10 | P[11] | I2 | ||||||||
| L338 | Equine | G13 | P[18] | I6 | M6 | A6 | ||||||
| FI23 | Equine | G14 | P[12] | I2 | A10 | E2 | ||||||
| EDIM (EW) | Murine | G16 | P[16] | I7 | A7 | E7 | ||||||
| EC | Murine | G16 | P[16] | E7 | ||||||||
| EB | Murine | G16 | P[16] | E7 | ||||||||
| EHP | Murine | G16 | P[20] | A7 | E7 | |||||||
| SA11g4“O” (5N) | Simian | G3 | P[1] | I2 | R2 | C5 | M5 | A5 | N5 | T5 | E2 | H5 |
| SA11-5S | Simian | G3 | P[1] | I2 | R2 | C5 | M5 | A5 | N5 | T5 | E2 | H5 |
| SA11-30/19 | Simian | G3 | P[1] | I2 | R2 | C5 | M5 | A5 | N5 | T5 | E2 | H5 |
| SA11-30/1A | Simian | G3 | P[1] | I2 | R2 | C5 | M5 | A5 | N5 | T5 | E2 | H5 |
| SA11-H96 | Simian | G3 | P[2] | I2 | R2 | C5 | M5 | A5 | N5 | T5 | E2 | H5 |
| SA11-Both | Simian | G3 | P[2] | I2 | R2 | C5 | M5 | A5 | N2 | T5 | E2 | H5 |
| RRV | Simian | G3 | P[3] | I2 | R2 | C3 | M3 | A9 | T3 | E3 | H6 | |
| TUCH | Simian | G3 | P[24] | I9 | R3 | C3 | M3 | A9 | ||||
| OVR762 | Ovine | G8 | P[14] | I2 | R2 | C2 | M2 | A11 | N2 | T6 | E2 | H3 |
| Lp14 | Ovine | G10 | P[15] | I2 | E2 | H3 | ||||||
| 30/96 | Lapine | G3 | P[14] | I2 | R2 | C2 | M3 | A9 | N2 | T6 | E5 | H3 |
| C-11 | Lapine | G3 | P[14] | A9 | E5 | |||||||
| ALA | Lapine | G3 | P[14] | A9 | E5 | H3 | ||||||
| 160/01 | Lapine | G3 | P[22] | E5 | ||||||||
| FRV64 | Feline | G3 | P[3] | A9 | E3 | |||||||
| Cat2 | Feline | G3 | P[9] | A3 | ||||||||
| FRV1 | Feline | G3 | P[9] | E3 | ||||||||
| CU-1 | Canine | G3 | P[3] | E3 | ||||||||
| RV198/95 | Canine | G3 | P[3] | E8 | ||||||||
| RV52/96 | Canine | G3 | P[3] | E8 | ||||||||
| K9 | Canine | G3 | P[3] | A9 | ||||||||
Recommendations
In order to render the classification system useful and to explore its biological significance in full, a set of recommendations is herein summarized, which is already followed by many, when investigating the VP4, VP7 or NSP4 genotype(s) of a new rotavirus strain but now comprises all genes [19]. The new classification system [19] is based on the nt sequences of complete open reading frames (ORF), and therefore the nt sequence of the entire ORFs of all genes of a new strain should preferentially be obtained in order to unequivocally assign it to one of the known/established genotypes or to a new genotype. As the UTRs of group A rotavirus genes are relatively short (see above) and terminal nt sequences are partially conserved, they are, at this stage, excluded from classification attempts within group A rotaviruses. In addition, it is much less defined what drives the evolution of these gene portions.
Classification strategy
Complete ORF analysis
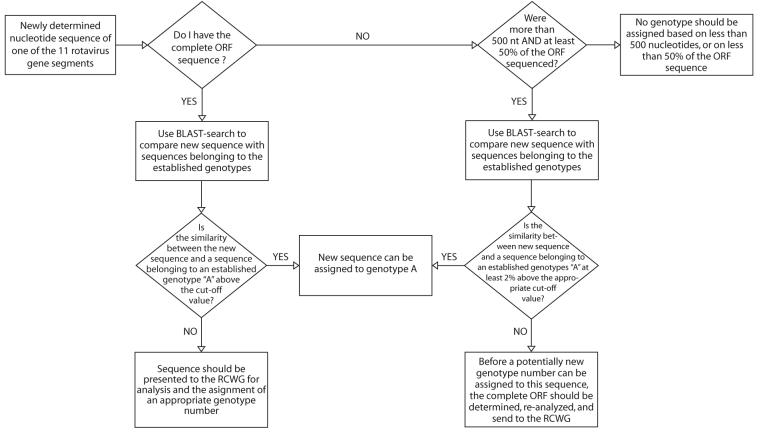
Once the complete ORF of a rotavirus gene under investigation has been determined, it will be compared to other complete ORFs of cognate genes available in the GenBank database through a BLAST (Basic Local Alignment Search Tool) –search (http://www.ncbi.nlm.nih.gov/BLAST/). If pairwise nucleotide identities between the gene of the novel strain under investigation (strain X) and strains belonging to an established genotype A are above the cut off value of that gene segment (Table 1), strain X can be assigned to genotype A. The exact relationship between the gene of strain X and cognate genes of all established genotypes, has to be obtained phylogenetically. When all the pairwise nucleotide identities between a gene of the new strain Y, and the cognate genes of all the established genotypes are below the cut-off value for that gene segment (Table 1), strain Y may be the prototype of a new genotype (Figure 1).
Figure 1.
Flowchart summarizing the guidelines for genotype classification provided in this manuscript.
In order to avoid the appearance of duplications in the literature and identical numbering of different new genes, a Rotavirus Classification Working Group (RCWG) including molecular virologists, infectious disease physicians, epidemiologists and public health specialists has been formed (Table 3), who would be prepared and responsible to help validate the new sequence and to assign an appropriate successive genotype number, in close consultation with the ReoviridaeStudy Group of the ICTV.
Table 3.
Current members of the RCWG
| Name | Telephone number | |
|---|---|---|
| Houssam Attoui | houssam.attoui@bbsrc.ac.uk | +44 1483 231 194 |
| Krisztián Bányai | bkrota@hotmail.com | +36 1 467 4082 |
| Max Ciarlet | max_ciarlet@merck.com | +1 267 305 7424 |
| Ulrich Desselberger | ud207@medschl.cam.ac.uk | +44 1223 763403 |
| Mary K. Estes | mestes@bcm.edu | + 1 713 798 3585 |
| Jon R. Gentsch | Jrg4@cdc.gov | +1 404 639 2860 |
| Miren Iturriza-Gómara | Miren.iturriza@hpa.org.uk | +44 208 327 6225 |
| Carl Kirkwood | Carl.kirkwood@mcri.edu.au | +613 8341 6439 |
| Vito Martella | v.martella@veterinaria.uniba.it | +39 80 467 9805 |
| Jelle Matthijnssens | jelle.matthijnssens@uz.kuleuven.be | +32 16 332166 |
| Peter P.C. Mertens | Peter.mertens@bbsrc.ac.uk | +44 1483 231 017 |
| Osamu Nakagomi | onakagom@nagasaki-u.ac.jp | +81 95 819 7061 |
| John T. Patton | jpatton@niaid.nih.gov | +1 301 594 1615 |
| Mustafizur Rahman | rahman.mustafizur1@gmail.com | +32 16 332166 |
| Franco M. Ruggeri | Franco.Ruggeri@iss.it | +39 06 49902980 |
| Linda J. Saif | saif.2@osu.edu | +1 330263 3742 |
| Norma Santos | nsantos@micro.ufrj.br | +55 21 2560 8344 |
| Andrej Steyer | andrej.steyer@mf.uni-lj.si | +386 1 543 74 62 |
| Koki Taniguchi | kokitani@fujita-hu.ac.jp | +81 562 93 2467 |
| Marc Van Ranst | marc.vanranst@uz.kuleuven.be | +32 16 347909 |
Partial ORF analysis
If only the partial ORF sequence of a rotavirus genome segment is available, assigning it to a certain genotype is less certain because the genotypic diversity across the ORF is not a constant value. Some regions of the ORF may be highly variable, while others may be more conserved. Since the cut-off percentage values for each of the 11 genome segments has been calculated based on entire ORFs, applying these cut off percentages to only a part of the ORF, might lead to erroneous conclusions. Only under certain circumstances when all three of the following restrictions are obeyed, a partial gene sequence might be used to assign a rotavirus gene to an established genotype:
At least 50% of the ORF sequence should be determined.
At least 500 nt of the ORF should be determined.
Identity between strain X and a strain belonging to an established genotype A should be at least 2% above the appropriate cut-off sequence (Table 1), before strain X can be assigned to genotype A (Figure 1).
Remarks
To assign a genotype to a new ORF sequence, whether complete or partial, the comparison should only be done to strains for which the genotype has been established based on the entire ORF analysis, and not with other partial ORF sequences. Due to intra-segmental recombination [30-32, 40] or to different rates of diversification throughout a genome segment, classification assignments based on partial ORFs may yield misleading or incorrect results.
Practical Recommendations
Any sequence of a rotavirus RNA segment that had been analyzed as described above, and found to clearly belong to one of the established genotypes, can be assigned to that genotype. When a potential new genotype is found, the complete ORF should be determined and can be sent (in confidence and out of competition) to a member of the RCWG (Table 3). The sequence will be analyzed and, if appropriate, a new successive genotype number will be assigned. The new number can be used for publication. Most reputable journals will request to provide the GenBank accession number of the new sequence, and this should be obtained as soon as possible and the correct new genotype be assigned to it. The whole process of the RCWG: (i) receiving the sequence, (b) appropriate phylogenetic analysis, (c) presentation to the RCWG members, and (d) approval by the RCWG, should not take more than 6 weeks. An annual report of the RCWG will be published summarizing all newly assigned genotypes.
Acknowledgments
We would like to thank all the colleagues of the Laboratory of Clinical & Epidemiological Virology, Department of Microbiology & Immunology, Rega Institute for Medical Research, University of Leuven, Belgium, for their helpful comments and discussions. J.M. was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen). JTP was supported by the Intramural Research Program of the NIH, National Institutes of Health, USA.
Reference List
- 1.Ball LA. The universal taxonomy of viruses in theory and practice. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eight Report of the International Committee on taxonomy of Viruses. Elsevier, Academic Press; Amsterdam, Holland: 2005. pp. 3–8. [Google Scholar]
- 2.Banerjee I, Iturriza-Gómara M, Rajendran P, Primrose B, Ramani S, Gray JJ, Brown DW, Kang G. Molecular characterization of G11P[25] and G3P[3] human rotavirus strains associated with asymptomatic infection in South India. J Med Virol. 2007;79:1768–1774. doi: 10.1002/jmv.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciarlet M, Liprandi F, Conner ME, Estes MK. Species specificity and interspecies relatedness of NSP4 genetic groups by comparative NSP4 sequence analyses of animal rotaviruses. Arch Virol. 2000;145:371–383. doi: 10.1007/s007050050029. [DOI] [PubMed] [Google Scholar]
- 4.Ciarlet M, Estes M. Rotaviruses: basic biology, epidemiology and methodologies. In: Britton G, editor. Encyclopedia of Environmental Microbiology. John Wiley & Sons; New York: 2002. pp. 2573–2773. [Google Scholar]
- 5.Cunliffe NA, Woods PA, Leite JP, Das BK, Ramachandran M, Bhan MK, Hart CA, Glass RI, Gentsch JR. Sequence analysis of NSP4 gene of human rotavirus allows classification into two main genetic groups. J Med Virol. 1997;53:41–50. [PubMed] [Google Scholar]
- 6.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Desselberger U, Wolleswinkel-van den Bosch J, Mrukowicz J, Rodrigo C, Giaquinto C, Vesikari T. Rotavirus types in Europe and their significance for vaccination. Pediatr Infect Dis J. 2006;25:S30–41. doi: 10.1097/01.inf.0000197707.70835.f3. [DOI] [PubMed] [Google Scholar]
- 8.Estes M, Kapikian A. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5th edition Kluwer Health/Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 1917–1974. [Google Scholar]
- 9.Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Bányai K, Ramachandran M, Jain V, Cunliffe NA, Nakagomi O, Kirkwood CD, Fischer TK, Parashar UD, Bresee JS, Jiang B, Glass RI. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192(Suppl 1):146–159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Estes MK. Proposed working serologic classification system for rotaviruses. Annales de l'Institut Pasteur de Virologie. 1985;136:5–12. [Google Scholar]
- 11.Horie Y, Masamune O, Nakagomi O. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. The J Gen Virol. 1997;78:2341–2346. doi: 10.1099/0022-1317-78-9-2341. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Sugiyama M, Masubuchi K, Mori Y, Minamoto N. Complete nucleotide sequence of a group A avian rotavirus genome and a comparison with its counterparts of mammalian rotaviruses. Virus Res. 2001;75:123–138. doi: 10.1016/s0168-1702(01)00234-9. [DOI] [PubMed] [Google Scholar]
- 13.Iturriza Gómara M, Wong C, Blome S, Desselberger U, Gray J. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence of independent segregation. J Virol. 2002;76:6596–6601. doi: 10.1128/JVI.76.13.6596-6601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khamrin P, Maneekarn N, Peerakome S, Yagyu F, Okitsu S, Ushijima H. Molecular characterization of a rare G3P[3] human rotavirus reassortant strain reveals evidence for multiple human-animal interspecies transmissions. J Med Virol. 2006;78:986–994. doi: 10.1002/jmv.20651. [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood CD, Palombo EA. Genetic characterization of the rotavirus nonstructural protein, NSP4. Virology. 1997;236:258–265. doi: 10.1006/viro.1997.8727. [DOI] [PubMed] [Google Scholar]
- 16.Li DD, Duan ZJ, Zhang Q, Liu N, Xie ZP, Jiang B, Steele D, Jiang X, Wang ZS, Fang ZY. Molecular characterization of unusual human G5P[6] rotaviruses identified in China. J Clin Virol. 2008 doi: 10.1016/j.jcv.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Matthijnssens J, Rahman M, Martella V, Xuelei Y, De Vos S, De Leener K, Ciarlet M, Buonavoglia C, Van Ranst M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J Virol. 2006;80:3801–3810. doi: 10.1128/JVI.80.8.3801-3810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthijnssens J, Rahman M, Yang X, Delbeke T, Arijs I, Kabue JP, Muyembe JJ, Van Ranst M. G8 rotavirus strains isolated in the Democratic Republic of Congo belong to the DS-1-like genogroup. J Clin Microbiol. 2006;44:1801–1809. doi: 10.1128/JCM.44.5.1801-1809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo AE, Iturriza-Gómara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol. 2008;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maunula L, Von Bonsdorff CH. Frequent reassortments may explain the genetic heterogeneity of rotaviruses: analysis of Finnish rotavirus strains. J Virol. 2002;76:11793–11800. doi: 10.1128/JVI.76.23.11793-11800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens PPC, Duncan R, Attoui H, Dermody TS. Reoviridae. In: Fauquet C, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eight Report of the International Committee on Taxonomy of Viruses. Alsevier, Academic Press; Amsterdam, Holland: 2005. pp. 447–560. [Google Scholar]
- 22.Mori Y, Borgan MA, Ito N, Sugiyama M, Minamoto N. Sequential analysis of nonstructural protein NSP4s derived from Group A avian rotaviruses. Virus Res. 2002;89:145–151. doi: 10.1016/s0168-1702(02)00112-0. [DOI] [PubMed] [Google Scholar]
- 23.Nakagomi O, Nakagomi T, Akatani K, Ikegami N. Identification of rotavirus genogroups by RNA-RNA hybridization. Molecular and cellular probes. 1989;3:251–261. doi: 10.1016/0890-8508(89)90006-6. [DOI] [PubMed] [Google Scholar]
- 24.Nakagomi O, Nakagomi T. Genetic diversity and similarity among mammalian rotaviruses in relation to interspecies transmission of rotavirus. Arch Virol. 1991;120:43–55. doi: 10.1007/BF01310948. [DOI] [PubMed] [Google Scholar]
- 25.Nakagomi T, Horie Y, Koshimura Y, Greenberg HB, Nakagomi O. Isolation of a human rotavirus strain with a super-short RNA pattern and a new P2 subtype. J Clin Microbiol. 1999;37:1213–1216. doi: 10.1128/jcm.37.4.1213-1216.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noel JS, Lee TW, Kurtz JB, Glass RI, Monroe SS. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol. 1995;33:797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Saif LJ, Jeong C, Lim GK, Park SI, Kim HH, Park SJ, Kim YJ, Jeong JH, Kang MI, Cho KO. Molecular characterization of novel G5 bovine rotavirus strains. J Clin microbiol. 2006;44:4101–4112. doi: 10.1128/JCM.01196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parra GI, Bok K, Martinez M, Gomez JA. Evidence of rotavirus intragenic recombination between two sublineages of the same genotype. J Gen Virol. 2004;85:1713–1716. doi: 10.1099/vir.0.79851-0. [DOI] [PubMed] [Google Scholar]
- 31.Phan TG, Okitsu S, Maneekarn N, Ushijima H. Genetic heterogeneity, evolution and recombination in emerging G9 rotaviruses. Infect Genet Evol. 2007;7:656–663. doi: 10.1016/j.meegid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Phan TG, Okitsu S, Maneekarn N, Ushijima H. Evidence of intragenic recombination in G1 rotavirus VP7 genes. J Virol. 2007;81:10188–10194. doi: 10.1128/JVI.00337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman M, Matthijnssens J, Nahar S, Podder G, Sack DA, Azim T, Van Ranst M. Characterization of a novel P[25],G11 human group a rotavirus. J Clin Microbiol. 2005;43:3208–3212. doi: 10.1128/JCM.43.7.3208-3212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman M, Matthijnssens J, Yang X, Delbeke T, Arijs I, Taniguchi K, Iturriza-Gómara M, Iftekharuddin N, Azim T, Van Ranst M. Evolutionary history and global spread of the emerging g12 human rotaviruses. J Virol. 2007;81:2382–2390. doi: 10.1128/JVI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramig RF, Ciarlet M, Mertens PPC, Dermody TS. Rotavirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eight Report of the International Committee on taxonomy of Viruses. Elsevier, Academic Press; Amsterdam, Holland: 2005. pp. 484–496. [Google Scholar]
- 36.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 37.Schuffenecker I, Ando T, Thouvenot D, Lina B, Aymard M. Genetic classification of “Sapporo-like viruses”. Arch Virol. 2001;146:2115–2132. doi: 10.1007/s007050170024. [DOI] [PubMed] [Google Scholar]
- 38.Small C, Barro M, Brown TL, Patton JT. Genome heterogeneity of SA11 rotavirus due to reassortment with “O” agent. Virology. 2007;359:415–424. doi: 10.1016/j.virol.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriano-Gabarro M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J. 2006;25 doi: 10.1097/01.inf.0000197622.98559.01. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Gojobori T, Nakagomi O. Intragenic recombinations in rotaviruses. FEBS letters. 1998;427:183–187. doi: 10.1016/s0014-5793(98)00415-3. [DOI] [PubMed] [Google Scholar]
- 41.Svensson L, Sheshberadaran H, Vesikari T, Norrby E, Wadell G. Immune response to rotavirus polypeptides after vaccination with heterologous rotavirus vaccines (RIT 4237, RRV-1) J Gen Virol. 1987;68:1993–1999. doi: 10.1099/0022-1317-68-7-1993. [DOI] [PubMed] [Google Scholar]
- 42.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]