Abstract
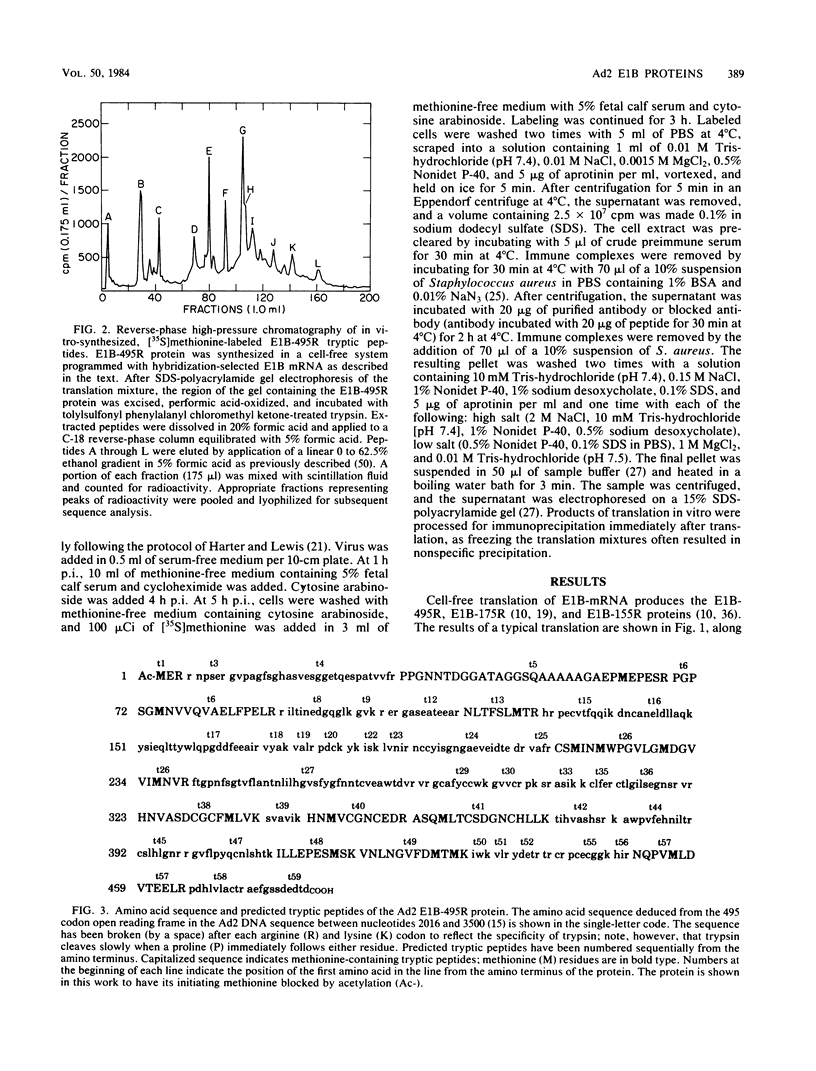
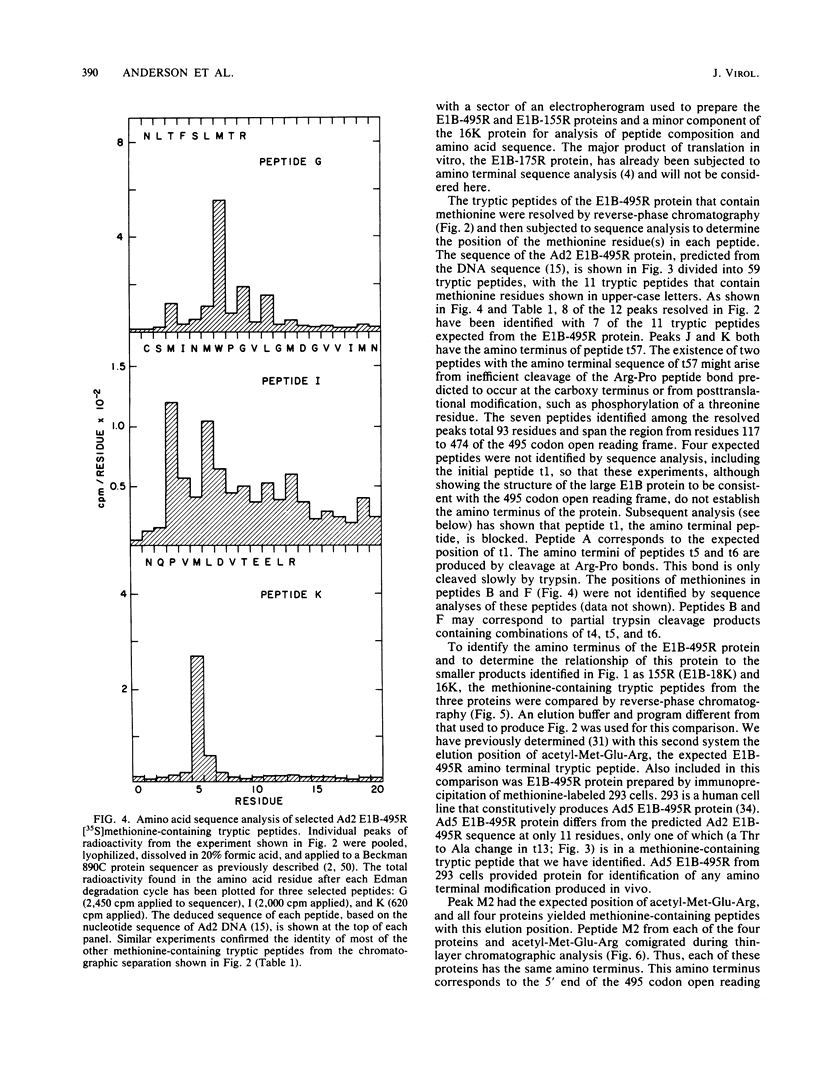
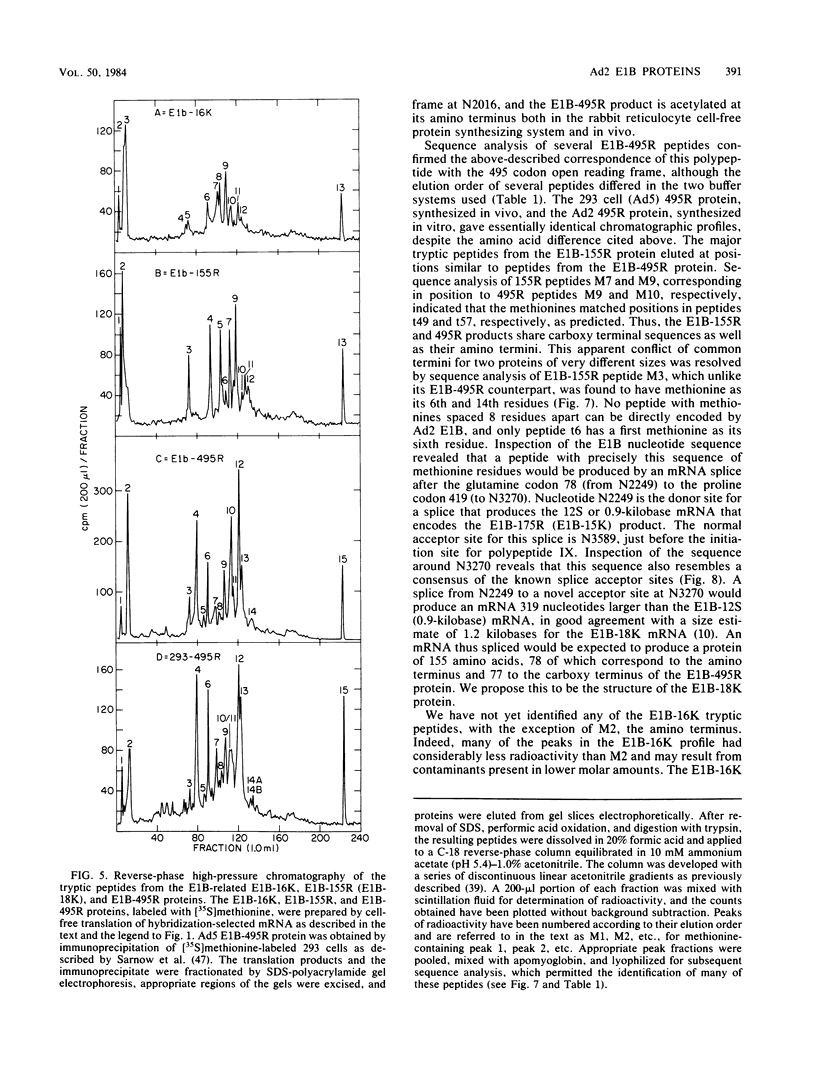
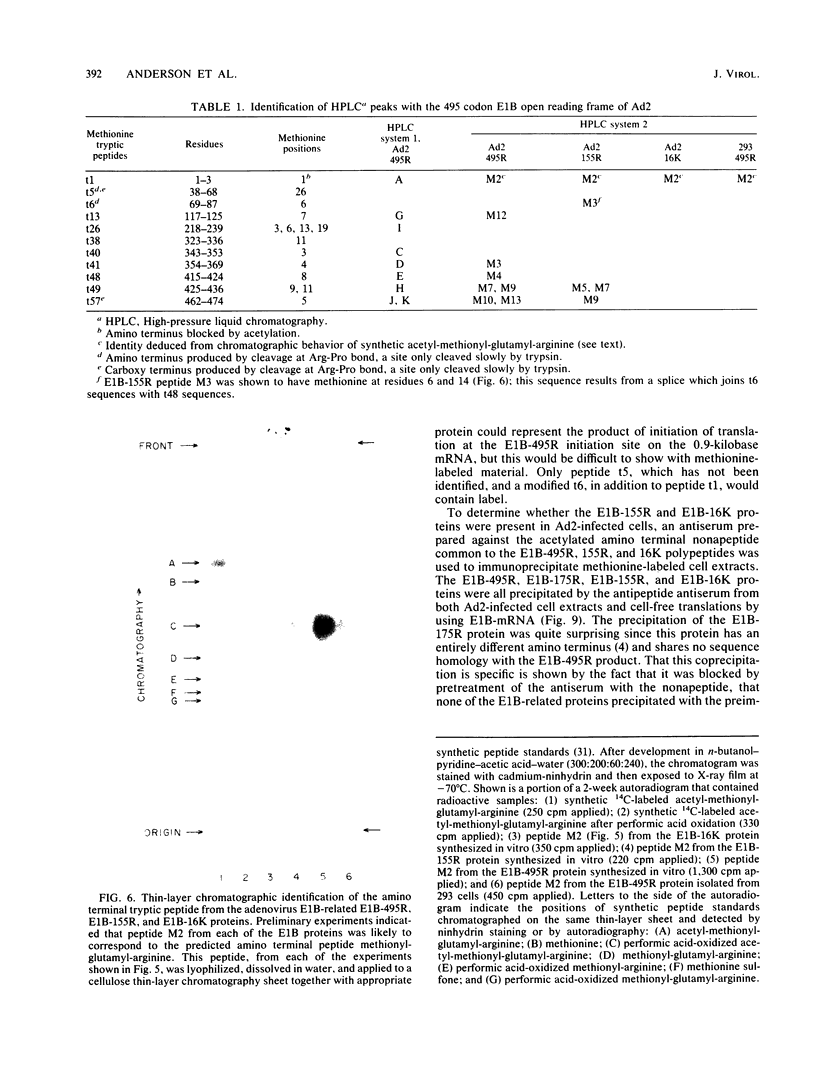
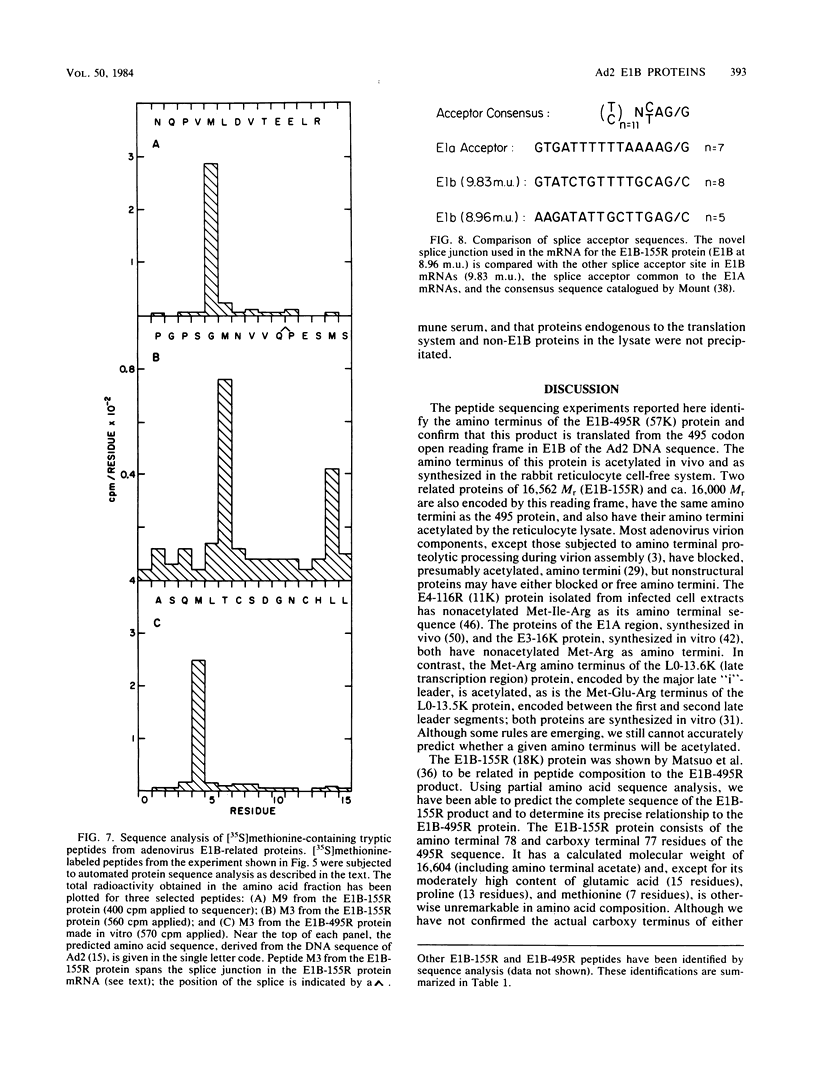
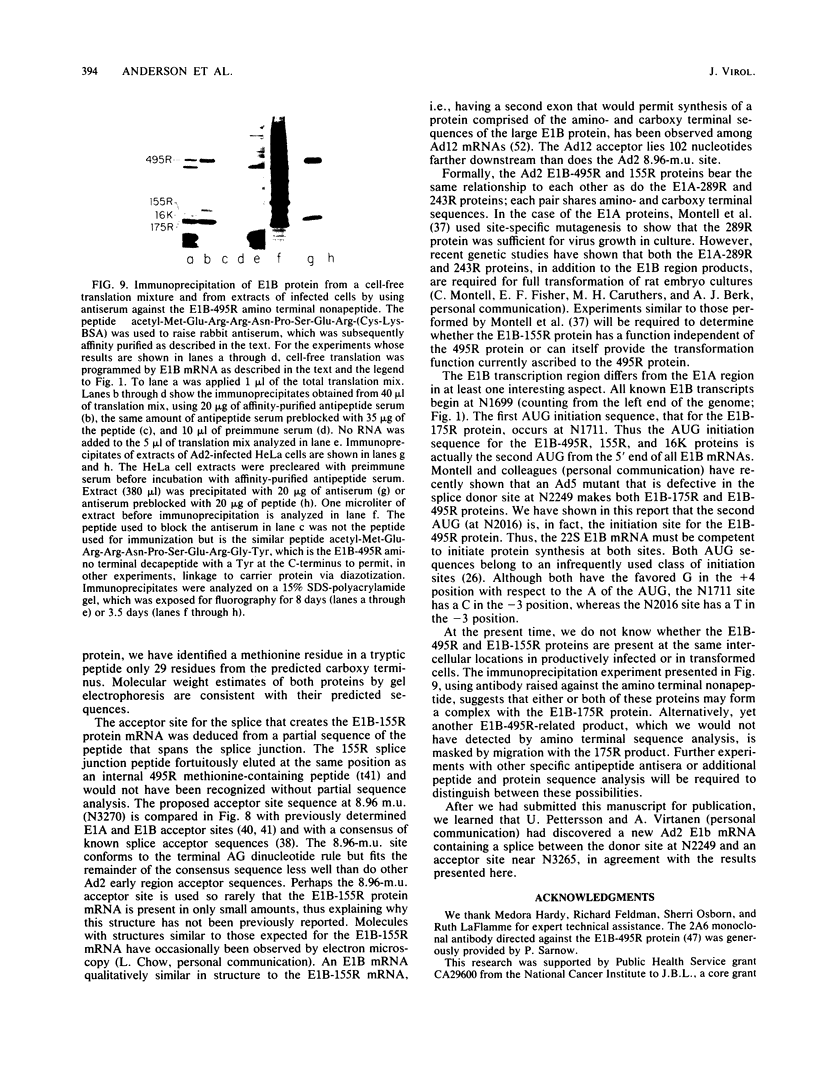
Partial sequence analysis of tryptic peptides has identified the E1B-495R (E1b-57K) (early transcription region 1B of 495 amino acid residues, with an approximate molecular weight of 57,000) protein of adenovirus 2 as encoded by the 495 amino acid open reading frame located in the adenovirus 2 DNA sequence between nucleotides 2016 and 3500. Additional proteins of 16,000 Mr and 18,000 Mr that are related to the E1B-495R protein were identified by cell-free translation of hybridization-selected mRNA. Analysis of [35S]methionine-containing amino terminal tryptic peptides by thin-layer chromatography showed that the E1B-495R, E1B-18K, and E1B-16K proteins all begin at the same initiation codon. The E1B-495R protein from 293 cells also has the same initial tryptic peptide, acetyl-methionyl-glutamyl-arginine. Sequence analysis of E1B-18K tryptic peptides indicated that this protein also has the same carboxy terminus as the E1B-495R protein and that it is derived from an mRNA that is spliced to remove sequences between nucleotides 2250 and 3269, resulting in a protein product of 155 amino acid residues. Analysis of E1B-16K tryptic peptides has not yet revealed the carboxy terminal structure of this protein. Both the E1B-495R and the E1B-155R (E1B-18K) proteins, as well as the E1B-16K protein, were precipitated from cell-free translations and from extracts of infected cells by antiserum against an amino terminal nonapeptide common to these proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B. Amino-terminal sequence of adenovirus type 2 proteins: hexon, fiber, component IX, and early protein 1B-15K. Virology. 1980 Jul 15;104(1):27–41. doi: 10.1016/0042-6822(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Bos J. L., Van der Eb A. J. Role of adenovirus types 5 and 12 early region 1b tumor antigens in oncogenic transformation. Virology. 1983 May;127(1):45–53. doi: 10.1016/0042-6822(83)90369-0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Brackmann K. H., Green M., Wold W. S., Cartas M., Matsuo T., Hashimoto S. Identification and peptide mapping of human adenovirus type 2-induced early polypeptides isolated by two-dimensional gel electrophoresis and immunoprecipitation. J Biol Chem. 1980 Jul 25;255(14):6772–6779. [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Esche H., Mathews M. B., Lewis J. B. Proteins and messenger RNAs of the transforming region of wild-type and mutant adenoviruses. J Mol Biol. 1980 Sep 25;142(3):399–417. doi: 10.1016/0022-2836(80)90279-x. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Cartas M. A., Matsuo T. Identification and purification of a protein encoded by the human adenovirus type 2 transforming region. J Virol. 1982 Apr;42(1):30–41. doi: 10.1128/jvi.42.1.30-41.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., RajBhandary U. L. Organization of tRNA and rRNA genes in N. crassa mitochondria: intervening sequence in the large rRNA gene and strand distribution of the RNA genes. Cell. 1979 Jul;17(3):583–595. doi: 10.1016/0092-8674(79)90266-6. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L. Tumor antigens of human Ad5 in transformed cells and in cells infected with transformation-defective host-range mutants. Cell. 1979 Nov;18(3):781–791. doi: 10.1016/0092-8674(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Suriano J. R., Green M. Adenovirus proteins. II. N-terminal amino acid analysis. J Virol. 1967 Aug;1(4):723–728. doi: 10.1128/jvi.1.4.723-728.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A., Levine A. J. The isolation and identification of the adenovirus group C tumor antigens. Virology. 1977 Jan;76(1):1–11. doi: 10.1016/0042-6822(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W. Proteins encoded near the adenovirus late messenger RNA leader segments. Virology. 1983 May;127(1):112–123. doi: 10.1016/0042-6822(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Viral messenger RNAs in six lines of adenovirus-transformed cells. Virology. 1981 Dec;115(2):345–360. doi: 10.1016/0042-6822(81)90116-1. [DOI] [PubMed] [Google Scholar]
- Malinowski K., Manski W. Microprocedures for quantitative immunochemical analysis of antigenic molecules and antigenic determinants. Methods Enzymol. 1981;73(Pt B):418–436. doi: 10.1016/0076-6879(81)73082-9. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Wold W. S., Hashimoto S., Rankin A., Symington J., Green M. Polypeptides encoded by transforming region E 1b of human adenovirus 2: immunoprecipitation from transformed and infected cells and cell-free translation of E 1b-specific mRNA. Virology. 1982 Apr 30;118(2):456–465. doi: 10.1016/0042-6822(82)90366-x. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterom-Dragon E. A., Anderson C. W. Polypeptide structure and encoding location of the adenovirus serotype 2 late, nonstructural 33K protein. J Virol. 1983 Jan;45(1):251–263. doi: 10.1128/jvi.45.1.251-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Le Moullec J. M., Pettersson U. Predicted structure of two adenovirus tumor antigens. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3778–3782. doi: 10.1073/pnas.77.7.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Jörnvall H., Zabielski J. Multiple mRNA species for the precursor to an adenovirus-encoded glycoprotein: identification and structure of the signal sequence. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6349–6353. doi: 10.1073/pnas.77.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. doi: 10.1128/jvi.42.3.905-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. R., Flint S. J., Levine A. J. Identification of the adenovirus early proteins and their genomic map positions. Virology. 1980 Jan 30;100(2):419–432. doi: 10.1016/0042-6822(80)90533-4. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Transformation of rodent cells by DNA extracted from transformation-defective adenovirus mutants. J Virol. 1983 Jun;46(3):1039–1044. doi: 10.1128/jvi.46.3.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Hearing P., Anderson C. W., Reich N., Levine A. J. Identification and characterization of an immunologically conserved adenovirus early region 11,000 Mr protein and its association with the nuclear matrix. J Mol Biol. 1982 Dec 15;162(3):565–583. doi: 10.1016/0022-2836(82)90389-8. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Sullivan C. A., Levine A. J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982 Jul 30;120(2):510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Van Den Elsen P. J., Hertoghs J. J., Van Der Eb A. J. Characterization of tumor antigens in cells transformed by fragments of adenovirus type 5 DNA. Virology. 1979 Dec;99(2):372–385. doi: 10.1016/0042-6822(79)90016-3. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Maruyama K., Saito I., Fukui Y., Shimojo H. Incomplete transformation of rat cells by a deletion mutant of adenovirus type 5. J Virol. 1981 Jun;38(3):1048–1054. doi: 10.1128/jvi.38.3.1048-1054.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Lewis J. B., Mathews M. B., Harter M. L., Anderson C. W. Adenovirus type 2 early proteins: assignment of the early region 1A proteins synthesized in vivo and in vitro to specific mRNAs. Virology. 1981 Jul 30;112(2):703–713. doi: 10.1016/0042-6822(81)90315-9. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Pettersson U., Le Moullec J. M., Tiollais P., Perricaudet M. Different mRNAs from the transforming region of highly oncogenic and non-oncogenic human adenoviruses. Nature. 1982 Feb 25;295(5851):705–707. doi: 10.1038/295705a0. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M. Adenovirus type 2 early polypeptides immunoprecipitated by antisera to five lines of adenovirus-transformed rat cells. J Virol. 1979 Apr;30(1):297–310. doi: 10.1128/jvi.30.1.297-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., de Pater S., Houweling A., van der Veer J., van der Eb A. The relationship between region E1a and E1b of human adenoviruses in cell transformation. Gene. 1982 May;18(2):175–185. doi: 10.1016/0378-1119(82)90115-9. [DOI] [PubMed] [Google Scholar]