Abstract
Background
Production of interferon (IFN)-γ is key to efficient anti-tumor immunity. The present study was set out to investigate effects of IFNγ on the release of the potent pro-angiogenic mediator IL-8 by human A549 lung carcinoma cells.
Methods
A549 cells were cultured and stimulated with interleukin (IL)-1β alone or in combination with IFNγ. IL-8 production by these cells was analyzed with enzyme linked immuno sorbent assay (ELISA). mRNA-expression was analyzed by real-time PCR and RNase protection assay (RPA), respectively. Expression of inhibitor-κ Bα, cellular IL-8, and cyclooxygenase-2 was analyzed by Western blot analysis.
Results
Here we demonstrate that IFNγ efficiently reduced IL-8 secretion under the influence of IL-1β. Surprisingly, real-time PCR analysis and RPA revealed that the inhibitory effect of IFNγ on IL-8 was not associated with significant changes in mRNA levels. These observations concurred with lack of a modulatory activity of IFNγ on IL-1β-induced NF-κB activation as assessed by cellular IκB levels. Moreover, analysis of intracellular IL-8 suggests that IFNγ modulated IL-8 secretion by action on the posttranslational level. In contrast to IL-8, IL-1β-induced cyclooxygenase-2 expression and release of IL-6 were not affected by IFNγ indicating that modulation of IL-1β action by this cytokine displays specificity.
Conclusion
Data presented herein agree with an angiostatic role of IFNγ as seen in rodent models of solid tumors and suggest that increasing T helper type 1 (Th1)-like functions in lung cancer patients e.g. by local delivery of IFNγ may mediate therapeutic benefit via mechanisms that potentially include modulation of pro-angiogenic IL-8.
Background
Interleukin (IL)-1β is a cytokine with a key role in the pathophysiology of local and systemic inflammation [1]. Moreover, owing to its pro-inflammatory nature, IL-1β is regarded a tumor-promoting cytokine. In fact, enhanced tumor metastasis and angiogenesis has been observed under the influence of IL-1β [2,3]. Accordingly, IL-1β is able to facilitate tumor progression in murine models of lung cancer. Upregulation of metastasis and tumor angiogenesis by IL-1β as observed in those studies was associated with increased activity of matrix metalloproteinases and expression of the pro-angiogenic molecule hepatocyte growth factor. Furthermore, blockage of the chemokine receptor CXCR2 inhibited tumor growth in vivo indicating that a functional murine IL-8 homologue contributes to IL-1β-mediated progression of disease [4,5]. Notably, the chemokine IL-8 (CXCL-8) is an efficient mediator of angiogenesis [6,7] and thus located at the crucial interface of inflammation and tumor biology. Neutralization of IL-8 reduced tumorigenesis of human non-small cell lung cancer (NSCLC) in the SCID mouse model [8]. A key role for IL-1β-inducible IL-8 in the progression of lung cancer is strongly suggested by various clinical studies demonstrating that IL-8 detected in patient biopsy specimens positively correlates with tumor angiogenesis and metastasis. Moreover, IL-8 is associated with shortened survival, particularly in NSCLC [9-12]. Cell culture data suggest that lung carcinoma cells are a highly relevant source of IL-8 in the tumor microenvironment [9,13]. Interestingly, a recent study also demonstrates that IL-8 mediates proliferation of the human NSCLC cell lines A549 and NCI-H292, respectively [14]. Those observations further underscore that IL-8 can be regarded a pivotal factor in the progression of lung cancer.
Bulk of data from preclinical research indicates that interferon (IFN)-γ mediates important tumor-suppressive functions. Those include supression of proliferation and angiogenesis, induction of apoptosis, and activation of leukocytes with anti-cancer activity such as NK cells, NKT cells and T cells [15]. Interestingly, inhaled IFNγ showed therapeutic efficacy in a murine model of lung cancer [16]. Moreover, application of IFNγ by aerosol is able to activate alveolar macrophages in human beings [17] and shows therapeutic potential in tuberculosis patients [18,19]. In order to further characterize IL-8 as an immunopharmacological target, we set out to investigate in the present study effects of the tumorsuppressive Th1-like cytokine IFN-γ on the production of IL-8 by NSCLC A549 cells under the influence of IL-1β.
Methods
Cell Culture
Human A549 lung carcinoma/epithelial cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Cells were maintained in RPMI 1640 supplemented with 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated FCS (GIBCO-BRL, Eggenstein, Germany). For the experiments, confluent cells on polystyrene plates (Greiner, Frickenhausen, Germany) were washed with PBS and incubated in the aforementioned medium. Human IFNγ was obtained from TEBU/Peprotech (Frankfurt, Germany) and IL-1β from Invitrogen/Biosource (Karlsruhe, Germany).
Detection of IL-8 and IL-6 by enzyme-linked immunosorbent assay (ELISA)
Levels of IL-8 and IL-6 in cell-free culture supernatants were determined by ELISA according to the manufacturer's instructions (BD Bioscience/Pharmingen, Heidelberg, Germany). Two splice variants of IL-8 with different C-terminal regions can be discriminated (database Swiss-Prot/TrEMBL, http://www.expasy.org/cgi-bin/get-all-varsplic.pl?P10145). According to information provided by the manufacturer both splice variants of IL-8 are being detected by the ELISA assay used in the current study. In addition, various N-terminal IL-8 variants that are being generated by proteolytic cleavage are recognized by the assay.
Analysis of IL-8 and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA expression by real-time polymerase chain reaction (PCR) analysis, standard PCR analysis and RNase protection assay (RPA)
After RNA isolation (peqGold TriFast, Peqlab, Erlangen, Germany), 1 μg of total RNA was transcribed using random hexameric primers and Moloney virus reverse transcriptase (RT) (Applied Biosystems, Darmstadt, Germany). During real-time PCR analysis changes in fluorescence are caused by the Taq-polymerase degrading the probe that contains a fluorescent dye (FAM used for IL-8, VIC used for GAPDH) and a quencher (TAMRA). For IL-8 (Hs00174103_m1) and GAPDH (4310884E) pre-developed assay reagents were obtained from Applied Biosystems. Assay-mix was purchased from Invitrogen (Karlsruhe, Germany). Real-time PCR was performed using the AbiPrism 7700 Sequence Detector (Applied Biosystems) as follows: One initial step at 50°C for 2 minutes and 95°C for 2 minutes was followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Detection of the dequenched probe, calculation of threshold cycles (Ct values), and further analysis of these data were performed by the Sequence Detector software. Relative changes in IL-8 mRNA expression compared to unstimulated control and normalized to GAPDH were quantified by the 2-ddCt method.
For detection of both IL-8 splice forms 1 μg of RNA was used for standard RT-PCR (Applied Biosystems, Darmstadt, Germany) with GoTaq DNA polymerase (Promega, Mannheim, Germany). The following sequences were performed for PCR: 94°C for 10 min (1 cycle); 95°C for 30 s, 59°C for 30 s, and 72°C for 1 min (with variable numbers of cycles); extension phase at 72°C for 7 min. Numbers of cycles: GAPDH, 24; IL-8, as indicated. Sequences of the primers and length of resulting amplicons: IL-8 (F) 5'-atgacttccaagctggcc gtggct-3'; IL-8 (R1): 5'-ttatgaattctcagccctcttcaaaaa-3' (detects the dominat form of IL-8), 299 bp; IL-8 (R2): 5'-ccctgttttcagggacctctgc-3' (detects the minor/rare form of IL-8, this form is denoted as IL-8 variant in the figures in the results, 294 bp; GAPDH (F): 5'-accacagtccatgccatcac-3', GAPDH (R): 5'-tccaccaccctgttgctgta-3', 452 bp.
10 μg of total RNA were used for RPA analysis of IL-8 mRNA expression. DNA probes were cloned into the transcription vector pBluescript II KS (+) (Stratagene, Heidelberg, Germany). After linearization, an antisense transcript was synthesized in vitro by T7 RNA polymerase and [α-32P]UTP (800 Ci/mmol). RNA samples were hybridized at 42°C overnight with 50,000 c.p.m. of the labeled antisense transcript. Hybrids were digested by RNase A and RNase T1 (Roche) for 1 hour at 30°C. Under these conditions every single mismatch was recognized by the RNases. Protected fragments were separated on 5% (w/v) polyacrylamide/8 M urea gels and analyzed using a PhosphoImager device (Fuji, Straubenhardt, Germany). Individual expression of IL-8 was evaluated on the basis of the GAPDH housekeeping gene expression and is shown as n-fold induction. The cloned cDNA probes for detection of IL-8 and GAPDH correspond to nucleotides (nt) 16–270 and nt 961–1071 of the published sequences (hIL-8, NM000584.2; hGAPDH, AC M33197).
Detection of inhibitor of κBα(IκBα), cyclooxygenase-2 (COX-2), IL-8, and β-actin by Western blot analysis
A549 cells were harvested using lysis buffer (150 mM NaCl, 1 mM CaCl2, 25 mM TrisCl, pH 7.4, 1% Triton-X-100, supplemented with protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and DTT, Na3VO4, PMSF (each 1 mM) and NaF (20 mM)). 50 μg of total protein/lane were used. Antibodies and SDS-PAGE conditions: IκBα, 12% SDS-PAGE, polyclonal antibody (Santa Cruz Biotechnology); COX-2, 10% SDS-PAGE, monoclonal antibody (Santa Cruz Biotechnology); IL-8, 18% SDS-PAGE, monoclonal antibody (R&D Systems, Wiesbaden, Germany); for detection of β-actin (monoclonal antibody, Sigma) blots were stripped and reprobed. For detection of intracellular IL-8, cells were incubated with Brefeldin A (BfA) at 10 μg/ml (Sigma, Hamburg, Germany) in order to suppress the cellular secretory machinery. In those experiments all conditions were adjusted to a final concentration of 0.05% DMSO in order to control for the BfA vehicle.
Statistics
Data are shown as mean ± SD and are presented as pg/ml, ng/ml, or as fold-induction compared to unstimulated control. Data were analyzed by unpaired Student's t test on raw data using Sigma PLOT/STAT (Jandel Scientific).
Results
IFNγ impairs release of IL-8 from IL-1β-stimulated A549 cells
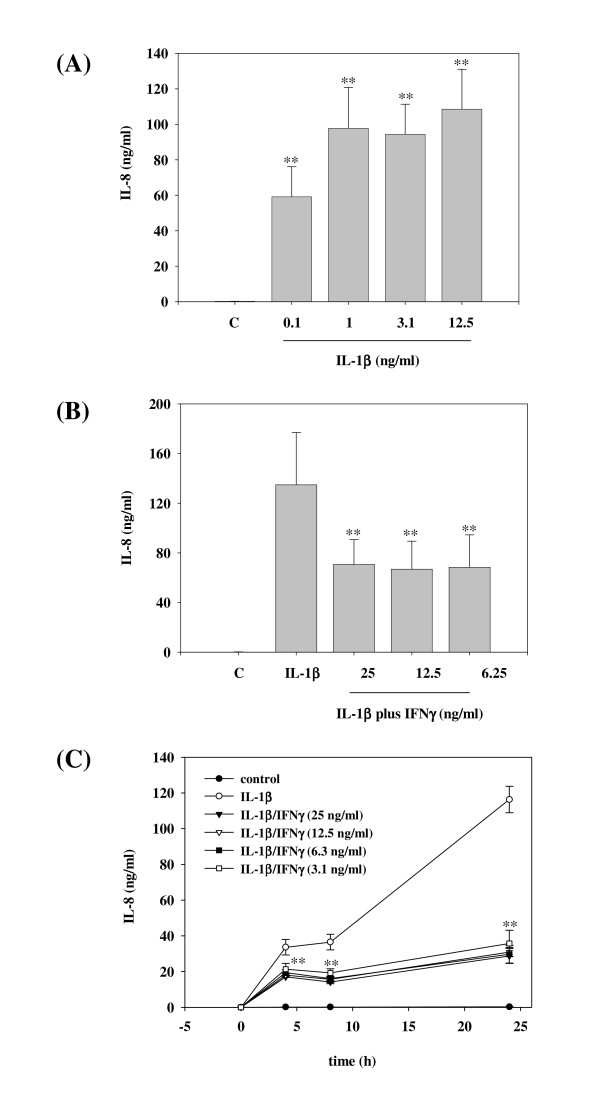
In accord with previous reports [20] we observed strong induction of IL-8 secretion by A549 cells under the influence of IL-1β. A saturation range was reached at an IL-1β concentration of 1 ng/ml (Figure 1A). IL-8 release could not be further enhanced, even by increasing the dose of IL-1β to 25 ng/ml or 50 ng/ml, respectively (data not shown). In the present study we sought to investigate a potential regulatory role of IFNγ concerning IL-1β-induced IL-8 in A549 cells. All subsequent experiments were performed by using high saturating IL-1β concentrations (≥ 1 ng/ml) in order to ensure complete pro-inflammatory activation of those cells and thus to set a high hurdle for modulation of IL-8 production by anti-inflammatory intervention. As shown in Figure 1B, coincubation with IFNγ significantly impaired release of IL-8 from IL-1β-stimulated A549 cells during a 24 h incubation period. IFNγ as a single stimulus was unable to mediate IL-8 release by A549 cells (data not shown). Figure 1C demonstrates that preincubation of A549 cells with IFNγ for 16 h even more enhanced this inhibitory action of IFNγ on IL-1β-induced IL-8 release. Notably, the modulatory function of IFNγ was already detectable after a 4 h incubation period with IL-1β (Figure 1C).
Figure 1.
IFNγ modulates release of IL-8 by IL-1β-stimulated A549 cells.(A) A549 cells were incubated as unstimulated control, or stimulated with the indicated concentrations of IL-1β. After 24 h, cell-free supernatants were assayed for IL-8 protein content by ELISA analysis. Data are expressed as mean IL-8 concentrations ± SD (n = 4). **p < 0.01 compared with untreated control. (B) A549 cells were incubated as unstimulated control, stimulated with IL-1β (25 ng/ml), or with IL-1β (25 ng/ml) in combination with the indicated concentrations of IFNγ. After 24 h, cell-free supernatants were assayed for IL-8 content by ELISA analysis. Data are expressed as mean IL-8 concentrations ± SD (n = 8). **p < 0.01 compared with IL-1β alone. (C) A549 cells were incubated as unstimulated control or were stimulated with IL-1β (25 ng/ml). Where indicated, A549 cells were preincubated for 16 h with IFNγ at different concentrations (ranging from 3.1 ng/ml up to 25 ng/ml). IL-1β was added directly thereafter to the cultures without further washing. After the indicated time periods with or without IL-1β stimulation, cell-free supernatants were assayed for IL-8 content by ELISA analysis. Data are expressed as mean IL-8 concentrations ± SD (n = 7). **p < 0.01 (for IFNγ at 3.1 ng/ml) compared with IL-1β alone.
The modulatory function of IFNγ on IL-8 secretion is not associated with changes in cellular IL-8 mRNA or protein expression
Since reduction of IL-8 mRNA expression is an appealing potential mechanism responsible for the inhibitory IFNγ action observed herein, real-time PCR analysis for IL-8 gene expression was performed (Figure 2A). Notably, despite thorough investigation we were unable to detect significant changes of IL-8 mRNA levels under the influence of IFNγ. In this same set of experiments IL-1β-induced IL-8 protein release (10 h stimulation period) was inhibited by IFNγ by 52.3% ± 5.0% compared to IL-1β alone (set as 100%). A lack of an IFNγ effect on the level of IL-8 mRNA expression was also obtained using RNase protection assay as an alternative method for quantification of mRNA populations (Figure 2B). Although both splice variants of IL-8 (Swiss-Prot/TrEMBL, http://www.expasy.org/cgi-bin/get-all-varsplic.pl?P10145) would be recognized by the ELISA used herein, we sought to investigate whether IL-8 splicing is affected by coincubation of A549 cells with IFNγ. For that purpose standard PCR analysis was performed using primers pairs that specifically discriminate between the different C-termini of both IL-8 forms. Figure 2C demonstrates that we were unable to detect the minor/rare splice form of IL-8 (denoted as IL-8 variant) in A549 cells, irrespective of the presence or absence of IFNγ.
Figure 2.
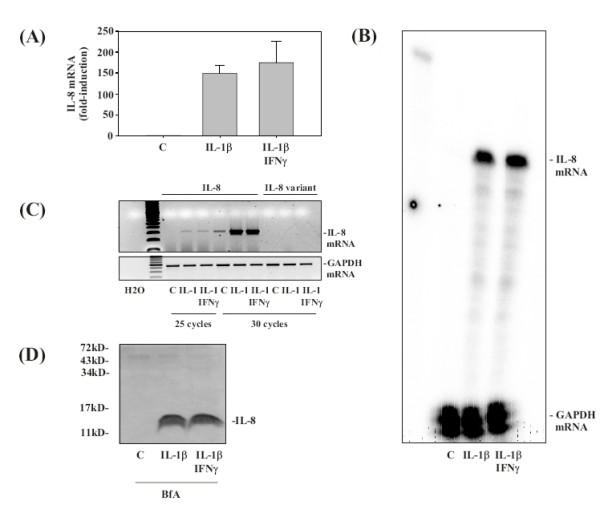
IFNγ is unable to modulate IL-8 mRNA and protein expression under the influence of IL-1β. A549 cells were incubated as unstimulated control, or stimulated with IL-1β (1 ng/ml). Where indicated, A549 cells were preincubated for 16 h with IFNγ at 20 ng/ml. IL-1β was added directly thereafter to the cultures without further washing. After 10 h, IL-1β-induced IL-8 mRNA accumulation was evaluated by realtime PCR analysis (A) and RPA (B), respectively. IL-8 mRNA expression was normalized to that of GAPDH. (A) Data are expressed as fold-induction compared to unstimulated control ± S.D. (n = 3). IL-8 protein levels in cell-free culture supernatants of those same cultures were determined by ELISA analysis (see results section). (B) One representative of three independently performed RPA analyses is shown. (C) RNA populations were analyzed by standard PCR using primers pairs that specifically detect the two IL-8 splice variants. The minor/rare splice form of IL-8 is denoted as 'IL-8 variant'. (D) A549 cells were incubated as unstimulated control, or stimulated with IL-1β (1 ng/ml). Where indicated, A549 cells were preincubated for 16 h with IFNγ at 20 ng/ml. Furthermore, 1 h before stimulation with IL-1β, BfA (10 μg/ml) was added to all cultures in order to block the cellular secretory machinery. After 8 h of incubation with IL-1β, cells were harvested and cellular IL-8 protein expression was assessed by Western blot analysis. One representative of three independently performed experiments is shown.
In order to further characterize the mechanism of IFNγ action on IL-1β-induced IL-8 production, secretion of the cytokine was blocked by coincubation with BfA. Western blot analysis under those condition clearly demonstrated that translation of IL-8 mRNA into protein is not affected by IFNγ (Figure 2D).
IFNγ is not a general inhibitor of IL-1β action on A549 cells
In order to investigate whether IFNγ should be regarded as a general inhibitor of IL-1β action on A549 cells, COX-2 (Figure 3A) and IL-6 (Figure 3B) were investigated as additional prototypic IL-1β-inducible proteins. Interestingly, Western blot and ELISA analysis revealed that expression/release of neither parameter was significantly affected by IFNγ in the context of IL-1β-activated A549 cells. Those data concur with the further observation that IFNγ was likewise unable to modulate degradation of IκBα in response to IL-1β, indicating that IFNγ left activation of nuclear factor-κB (NF-κB) under those conditions unaffected (Figure 3C). In addition to that, experiments using conditioned media from IFNγ-stimulated A549 cells also excluded the possibility that IFNγ mediates production of an 'IL-8 binding protein' that might have impaired IL-8 detection by ELISA (data not shown).
Figure 3.
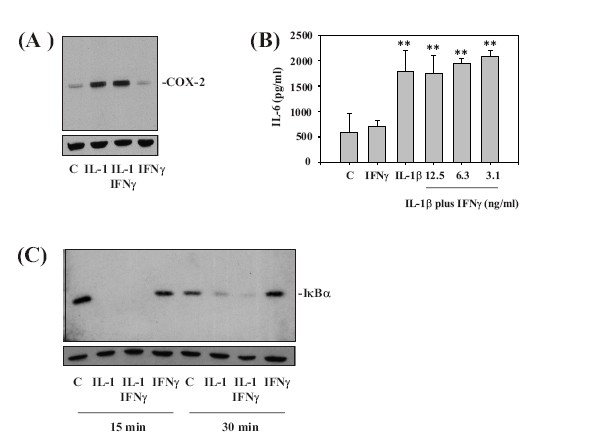
Analysis of IL-6 release, COX-2 expression, and IκB degradation reveals that IFNγ can not be regarded as a general inhibitor of IL-1β action on A549 cells.(A) A549 cells were incubated as unstimulated control, or stimulated with IL-1β (12.5 ng/ml). Where indicated, A549 cells were preincubated for 16 h with IFNγ at 20 ng/ml. IL-1β was added directly thereafter to the cultures without further washing. After 24 h, cell-lysates were assayed for COX-2 expression by Western blot analysis. One representative of three independently performed experiments evaluating COX-2 expression is shown. (B) A549 cells were incubated as unstimulated control, or stimulated with IL-1β (12.5 ng/ml). Where indicated, A549 cells were preincubated for 16 h with either IFNγ at 12.5 ng/ml (without later addition of IL-1β) or with the indicated concentrations of IFNγ in combination with IL-1β (12.5 ng/ml). IL-1β was added directly thereafter to the cultures without further washing. After 24 h, cell-free supernatants were assayed for IL-6 secretion by ELISA. Data are expressed as mean IL-6 concentrations ± SD (n = 3). **p < 0.01 compared with untreated control. (C) A549 cells were incubated as unstimulated control or stimulated with IL-1β (12.5 ng/ml). Where indicated, A549 cells were preincubated for 16 h with IFNγ at 20 ng/ml. IL-1β was added directly thereafter to the cultures without further washing. After 15 min or 30 min, cells were harvested and homogenates were evaluated for IκBα-protein by Western blot analysis. One representative for three independently performed experiments is shown.
Discussion
Impaired production of Th1-like cytokines and/or enhanced expression of Th2-like cytokines and IL-10 has been associated with tumor progression in a variety of malignancies, including lung cancer [21-23]. Modulation of angiogenesis appears to be a prime mechanism by which anti-cancer immunity restrains growth of solid tumors [24]. Specifically, production of the IFNγ-inducible angiostatic non-ELR+chemokines CXCL-9 (MIG), CXCL-10 (IP-10), and CXCL-11 (I-TAC) is of key relevance in this context [6]. Accordingly, production of CXCL-9 and CXCL-10 has been associated with tumorsuppression in animal models of NSCLC [25,26]. In the present study we demonstrate for the first time that IFNγ has the capability to significantly inhibit secretion of the pro-angiogenic chemokine IL-8 by A549 NSCLC cells. It is important to bear in mind that IL-8 not only is a mediator of angiogenesis but is obviously a crucial chemoattractant for neutrophils [27]. In this context it is of interest that lung carcinogenesis driven by inflammation has been associated with influx of neutrophils into the airway compartment [28]. The current observation concurs with distinct anti-inflammatory properties of immunoregulatory IFNγ [29] that include modulation of IL-8 secretion as previously noted for other human cell types including monocytes/macrophages [30], synoviocytes [31], and melanoma cells [32]. Effects of IFNγ on IL-8 were not of unspecific nature but displayed discrete specificity since IL-1β-induced expression/secretion of COX-2 and IL-6 were left unaffected. Analysis of cellular IL-8 mRNA and protein expression furthermore revealed that effects of IFNγ on IL-8 release by A549 cells do not affect IL-8 production but are obviously mediated by an unforeseen mechanism that targets the process of secretion and warrants further investigation.
Generally speaking, clinical trials investigating the therapeutic potential of IFNγ in cancer had a disappointing outcome [33]. However, one phase II clinical study suggests that a subgroup of NSCLC patients may benefit from therapeutic intravenous application of IFNγ in combination with chemotherapy. This trend observed did not reach statistical significance, possibly due to the fact that numbers of patients included in that trial were limited (26 for chemotherapy alone versus 27 for chemotherapy plus IFNγ). Moreover, an increased incidence of hematological toxicity was evident in the study arm undergoing intraveneous IFNγ treatment [34]. In the context of lung pathology application by aerosol might be of considerable advantage with regard to the therapeutic potential of IFNγ. Delivery of IFNγ by inhalation represents a route of administration that achieves high local concentrations at the lung along with reduced systemic toxicity in human beings [35]. In fact, this strategy proved promising in a murine model of lung cancer [16]. Moreover, inhalation of IFNγ has already been suggested for the treatment of tuberculosis and appears to show clinical efficacy [18,19]. In light of the current data and regarding previously published information delineating the tumorsuppressive potential of IFNγ it is tempting to speculate that inhaled IFNγ may pharmacologically differ from systemically applied and may indeed act as an immunostimulatory/-regulatory adjuvant with the potential to provide therapeutic benefit.
Conclusion
Taken together, data presented herein suggest that the Th1 signature cytokine IFNγ may not only be able to modulate angiogenesis in the NSCLC microenvironment by increasing the production of anti-angiogenic non-ELR+ chemokines. Concurrently, IFNγ clearly has the potential to suppress the production of pro-angiogenic IL-8 by A549 NSCLC carcinoma cells. This observation might be of translational relevance as clinical studies identified IL-8 as being a factor that is strongly associated with reduced survival of NSCLC patients [9,10].
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KAB and CDS performed the cell culture, ELISA analysis, RPAs, and Western blot analyses. CDS and MB performed PCR-analyses. HM, JP, BZ and KAB designed the study and drafted out the manuscript. All authors have read and approved the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The study was supported by departmental funding.
Contributor Information
Kim A Boost, Email: kim.boost@med.uni-muenchen.de.
Christian D Sadik, Email: c.sadik@med.uni-frankfurt.de.
Malte Bachmann, Email: m.bachmann@med.uni-frankfurt.de.
Bernhard Zwissler, Email: bernhard.zwissler@med.uni-muenchen.de.
Josef Pfeilschifter, Email: j.pfeilschifter@mde.uni-frankfurt.de.
Heiko Mühl, Email: h.muehl@em.uni-frankfurt.de.
References
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- Dinarello CA. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 2006;25:307–13. doi: 10.1007/s10555-006-9000-8. [DOI] [PubMed] [Google Scholar]
- Bar D, Apte RN, Voronov E, Dinarello CA, Cohen S. A continuous delivery system of IL-1 receptor antagonist reduces angiogenesis and inhibits tumor development. FASEB J. 2004;18:161–3. doi: 10.1096/fj.03-0483fje. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, Hong X, Tazawa R, Kikuchi T, Matsushima K, Nukiwa T. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–75. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- Yano S, Nokihara H, Yamamoto A, Goto H, Ogawa H, Kanematsu T, Miki T, Uehara H, Sajio Y, Nukiwa T, Sone S. Multifunctional interleukin-1beta promotes metastasis of human lung cancer cells in SCID mice via enhanced expression of adhesion-, invasion- and angiogenesis-related molecules. Cancer Sci. 2003;94:244–52. doi: 10.1111/j.1349-7006.2003.tb01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–78. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–37. [PubMed] [Google Scholar]
- Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY, Kuo SH, Luh KT. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am J Respir Crit Care Med. 2000;162:1957–63. doi: 10.1164/ajrccm.162.5.2002108. [DOI] [PubMed] [Google Scholar]
- Boldrini L, Gisfredi S, Ursino S, Lucchi M, Mussi A, Basolo F, Pingitore R, Fontanini G. Interleukin-8 in non-small cell lung carcinoma: relation with angiogenic pattern and p53 alterations. Lung Cancer. 2005;50:309–17. doi: 10.1016/j.lungcan.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wielke CA, Strieter RM. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179:1409–15. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Wang CH, Huang YC, Liao WY, Wang SS, Chen JJ, Yang PC. Autocrine and paracrine regulation of interleukin-8 expression in lung cancer cells. Am J Respir Cell Mol Biol. 2005;32:540–7. doi: 10.1165/rcmb.2004-0223OC. [DOI] [PubMed] [Google Scholar]
- Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Kessler R, Dumont S, Bartholeyns J, Weitzenblum E, Poindron P. Antitumoral potential of aerosolized interferon-gamma in mice bearing lung metastases. Am J Respir Cell Mol Biol. 1994;10:202–6. doi: 10.1165/ajrcmb.10.2.8110475. [DOI] [PubMed] [Google Scholar]
- Halme M, Maasilta P, Repo H, Ristola M, Taskinen E, Mattson K, Cantell K. Inhaled recombinant interferon gamma in patients with lung cancer: pharmacokinetics and effects on chemiluminescence responses of alveolar macrophages and peripheral blood neutrophils and monocytes. Int J Radiat Oncol Biol Phys. 1995;31:93–101. doi: 10.1016/0360-3016(94)00365-R. [DOI] [PubMed] [Google Scholar]
- Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–5. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- Milanés-Virelles MT, García-García I, Santos-Herrera Y, Valdés-Quintana M, Valenzuela-Silva CM, Jiménez-Madrigal G, Ramos-Gómez TI, Bello-Rivero I, Fernández-Olivera N, Sánchez-de la Osa RB, Rodríguez-Acosta C, González-Méndez L, Martínez-Sánchez G, López-Saura PA, MACGAM Study Group Adjuvant interferon gamma in patients with pulmonary atypical Mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect Dis. 2008;8:17. doi: 10.1186/1471-2334-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter KR, Wewers MD, Lowe MP, Knoell DL. Extracellular regulation of interleukin (IL)-1β through lung epithelial cells and defective IL-1 type II receptor expression. Am J Respir Cell Mol Biol. 1999;20:964–75. doi: 10.1165/ajrcmb.20.5.3458. [DOI] [PubMed] [Google Scholar]
- Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol Immunother. 2002;51:521–31. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YM, Yang WK, Whang-Peng J, Tsai CM, Perng RP. An analysis of cytokine status in the serum and effusions of patients with tuberculous and lung cancer. Lung Cancer. 2001;31:25–30. doi: 10.1016/S0169-5002(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, Chouaib S, Mami-Chouaib F. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer. 1998;77:7–12. doi: 10.1002/(SICI)1097-0215(19980703)77:1<7::AID-IJC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Benelli R, Lorusso G, Albini A, Noonan DM. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr Pharm Des. 2006;12:3101–15. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–92. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, Iannettoni MD, Strieter RM. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther. 2000;11:247–61. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Gungor N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–36. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3:1247–55. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S, Strieter RM, Kunkel SL, Walz A. Interferon-alpha and interferon-gamma down-regulate the production of interleukin-8 and ENA-78 in human monocytes. J Leukoc Biol. 1995;57:929–35. doi: 10.1002/jlb.57.6.929. [DOI] [PubMed] [Google Scholar]
- Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–9. [PubMed] [Google Scholar]
- Möhler T, Scheibenbogen C, Häfele J, Willhauck M, Keilholz U. Regulation of interleukin-8 mRNA expression and protein secretion in a melanoma cell line by tumour necrosis factor-alpha and interferon-gamma. Melanoma Res. 1996;6:307–11. doi: 10.1097/00008390-199608000-00005. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Murphy WJ, Chertox O, Schirrmacher V, Wang JM. Prospects for cytokine and chemokine biotherapy. Clin Cancer Res. 1997;3:2682–6. [PubMed] [Google Scholar]
- Halme M, Maasilta PK, Pyrhonen SO, Mattson KV. Interferons combined with chemotherapy in the treatment of stage III-IV non-small cell lung cancer – a randomised study. Eur J Cancer. 1994;30A:11–5. doi: 10.1016/S0959-8049(05)80009-7. [DOI] [PubMed] [Google Scholar]
- Virgolini I, Kurtaran A, Leimer M, Smith-Jones P, Agis H, Angelberger P, Kletter K, Valent P, Linkesch W, Eichler HG. Inhalation scintigraphy with iodine-123-labeled interferon gamma-1b: pulmonary deposition and dose escalation study in healthy volunteers. J Nucl Med. 1997;38:1475–81. [PubMed] [Google Scholar]