Abstract
Even in a simple Pavlovian memory task an animal may form several associations that can be independently assessed by the appropriate tests. Studying conditioned odor discrimination of the fruit fly Drosophila melanogaster we found that animals store quality and intensity of an odor as separate memory traces. The trace of odor intensity is short-lived, decaying in <3 h. Only the last intensity value is stored. In contrast to odor-quality memory, odor-intensity memory does not require the rutabaga-dependent cAMP signaling pathway. Flies rely on their memory of intensity in a narrow concentration range in which they can generalize intensity. Larger concentration differences they treat like different qualities. This study shows that the perceptual identity of an odor is based on at least three lines of processing in the brain: (i) a memory of odor quality, (ii) a memory of odor intensity, and (iii) a range of intensities (and qualities), in which the odor is generalized.
Keywords: concentration invariance, generalization, odor learning, olfaction, rutabaga
A scent is characterized by its quality and whether it is intense or faint. Quality signals chemical properties of the source; intensity serves, among others, in orientation. Yet, for some odorants quality and quantity are not fully separated: At sufficiently different concentrations the same odorant may appear as distinct qualities (1).
Flies (Drosophila melanogaster) are attracted by some odors and repelled by others. They can be trained to discriminate between different odorants (ref. 2 and for review see ref. 3) and also between concentrations of a single odorant (4, 5). These findings alone do not prove that flies perceive and store the quality of an odor independently of its intensity. They might distinguish between “good” and “bad” and otherwise treat qualities and intensities as one and the same (6). The strongest evidence against this latter idea stems from a generalization experiment by Borst (5). Using two-component mixtures of odorants (A and B) Borst showed that flies treated mixtures differing in the ratio of A/B as more different from mixtures in which the ratio A/B had been kept constant but concentrations were changed. Apparently, flies like humans perceive both the quality of an odor and its intensity.
Surprisingly, this confounding problem has been largely neglected. Evidently, if quality and intensity are perceived separately, they are likely to also be stored as distinct memory traces with different properties. In this case, much of what we know about olfactory learning in Drosophila needs to be specified as to whether it applies to odor quality, intensity, or both.
Here, we characterize (short-term) olfactory memory (7) with respect to odor intensity (OIM) and odor quality (OQM). It is experimentally difficult to present two odorants at the same (fly-subjective) intensity, whereas it is easy to present the same odorant at different intensities. Therefore, we focus on odor-intensity learning and look at odor-quality learning only indirectly by comparing odor-intensity learning to two-odor learning. As flies can exploit odor gradients (8) and distinguish small concentration differences (5), they also need to generalize them to keep track of their identity. We show that in a narrow concentration range this is indeed the case.
Results
Properties of Conditioned Odor Discrimination.
During training under standard conditions (8–13) one conditional stimulus (CS+) is paired with the reinforcer (electric shock), the other (CS−) is presented without. In the simplest case, only the paired odor should be remembered (5, 16). Indeed, the CS− was dispensable for associative learning (see Fig. 3) as long as adaptation (14) did not bias the memory score, e.g., at low concentrations. Using low concentrations offered another advantage: While under standard conditions, half-scores (ref. 15 and Methods) needed to be averaged to compensate for uneven odor avoidance and adaptation to the two odors. Without these effects, we could use the half-scores as equivalents of the full learning scores.
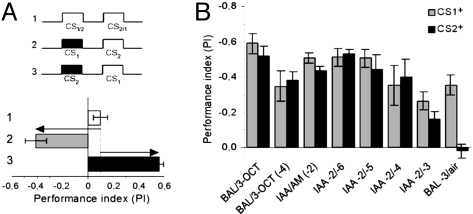
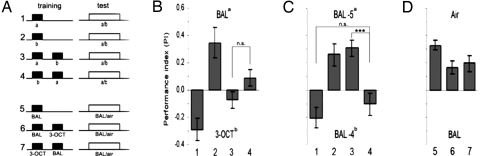
Fig. 3.
Half-scores. (A) Protocols (Upper) and schematic calculation of half scores (Lower). Distribution of naive flies between CS1 and CS2 in the test is set to be around PI ≈ 0 (protocol 1). Flies shocked in the presence of CS1 (protocol 2; gray bars in B) avoided it in the test. Shock paired with CS2 (protocol 3; black bars in C) led to the avoidance of CS2. For half-scores responses of naive flies are subtracted from PIs (arrows). (B) Both half-scores (e.g., after punishing CS1 or CS2) are plotted upward and negative here (in contrast to A) to make them easily comparable. Their contribution to the final learning score is similar, both for two odorants and two concentrations of one odor. The only exception is air, because no association is formed with air.
Characterizing Odor Intensity Learning.
Studying OIM we found similar results. We tested a small and a large concentration ratio. In both cases flies showed similar learning scores with and without the CS− [Fig. 1 A and B; performance index (PI) about −0.20 and −0.50, respectively]. The CS− had no influence on the test score. As for OQM, OIM half-scores had similar values (Fig. 1C; see also Fig. 3).
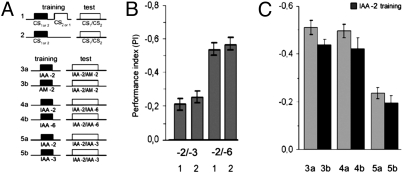
Fig. 1.
Learning score is not influenced by the presence or absence of CS−, but is influenced by the difficulty of the test. (A) Experimental design. To obtain learning scores, protocols 1 and 2 are performed twice (a/b), exchanging the two concentrations for CS+ and if applicable CS− (see Methods). (B) Flies were trained and tested with high (dilution −2; integers are log6 units) and low concentrations of IAA (dilution −3 and −6, respectively) with and without CS−. Naive responses would be close to zero. PIs are learning scores. (C) In experiments 3a, 4a, and 5a flies were trained with IAA dilution −2. Memories after the identical training must be identical. PIs (half-scores) depend on the particular pair of odors in the test. Low PI is found with two close concentrations (−2 and −3; ratio 1:6), and high PIs are found with two different odorants (IAA vs. AM) or large concentration difference (−2 and −6; ratio 1:1,296). Gray and black bars represent half-scores for the complementary training protocol (a/b). In all three experiments (nos. 3–5) PIs of naïve flies in choices between the two respective odors are about zero (see Methods). As in all subsequent figures error bars are SEMs.
An absolute value of odor concentration is stored.
If presentation of the CS− during training has no effect on the memory score, the fly must memorize the absolute concentration of the CS+. We designed an experiment (Fig. 2) that directly reveals whether the fly's behavior in the test is guided by the absolute concentration of the CS+ or by its relation to that of the CS−.
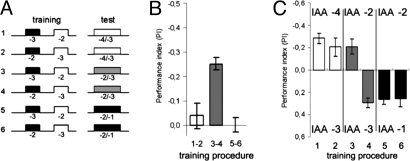
Fig. 2.
Flies learn absolute odor intensity. (A) Experimental design. All six protocols show training with two dilutions of IAA (−3 and −2), with either −3 (protocols 1, 3, and 5) or −2 (groups 2, 4, and 6) dilution being paired with electric shock. Flies were tested at three different conditions regarding the two IAA concentrations (indicated by the white, gray, and black bars). In protocols 3 and 4 (gray bars) flies are tested at the training conditions (−3 vs. − 2). In protocols 1 and 2 flies were tested at dilution −3 vs. − 4 (white bars) and in protocols 5 and 6 they were tested at dilutions −1 vs. − 2 (black bars). (B) Learning scores. Protocols 3 and 4 give learning score for same concentrations during training and test (gray column). Flies that underwent shift-up (protocols 5 and 6) or shift-down (protocols 1 and 2) showed no learning, if learning scores were calculated according to the status of the relative concentrations as high and low in training and test. (C) PIs are half-scores. Protocols 1–6 in A correspond to columns 1–6 in C. Above and below the columns the dilutions are indicated that were used in the test. Columns 3 and 4 show the results of the standard experiment. In protocols 1 and 6 the concentrations avoided in the test matched the concentrations combined with electric shock during training but as relative concentrations have the opposite status with respect to that during training (switch from high to low and vice versa). In other words, if flies had relied on relative concentrations they should have avoided the other odor. In protocols 2 and 5 none of the test concentrations had been paired with electric shock during training. The concentrations avoided in the test are the ones closest to the shock-associated concentrations during training (generalization).
In the training, we successively presented two concentrations, one paired with shock. In the test afterward the two concentrations were presented simultaneously (protocol 3, Fig. 2A). The process was repeated with the other concentration being paired with shock followed by the same test (protocol 4, Fig. 2A) to provide a full learning score. In the critical part of the experiment, training was the same as just described, but for the test we changed both concentrations by one dilution step, in one experiment down (protocols 1 and 2), in the other up (protocols 5 and 6). Another way to look at this change between training and test is that one odor stayed at the same concentration but changed its status as the lower or higher one with respect to the other, whereas the other odor changed both its status in relation to the first and its concentration [36-fold (2 log6 units; dilutions are given in log6 units throughout)] either from low to high (protocols 5 and 6) or from high to low (protocols 1 and 2).
We calculated learning scores assuming that flies remembered relative concentrations, i.e., we replaced the high concentration in the training by the high concentrations in the test and the same for the low concentrations. No learning was observed (Fig. 2B). As the change of concentrations between training and test was within the generalization range (1:10), we conclude that flies did not evaluate relative concentrations in this experiment.
If flies had learned absolute concentrations, however, they should have avoided in protocol 1 the odor dilution −3 and in protocol 6 the dilution −2. In protocols 2 and 5 flies experienced two odors in the tests after the shift, for which they had no memory trace. Calculating learning scores from protocols 1 and 2 (shifts down) and protocols 5 and 6 (shifts up) one would have expected values of 50% of that of the standard protocol.
To follow up this discrepancy, we plotted half-scores (Fig. 2C). They showed that flies always avoided the concentration closest to the one paired with the shock (−3 in protocol 2; −2 in protocol 5). As will be shown below this result is to be expected because flies can generalize concentration within a range of 1:10. The above finding implies that flies avoided the concentration that was presented during training as the “safe,” unpunished odor. The experiment unambiguously shows that the concentration was learned as an absolute value and not in relation to the CS−.
Influence of the test on memory read-out.
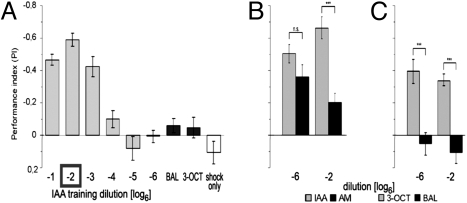
Our data suggest that the memory score in the paradigm depends on the training and the test. Given that the CS− does not need to be presented during training (Fig. 1B) and that no nonassociative components influence the test, one can easily demonstrate it by training flies with one concentration of one odor only (leaving out the CS−) and varying the difficulty of the test. To define “difficulty,” we assumed that to discriminate different odors was easier than to tell apart two concentrations of one odor and to discriminate two vastly different concentrations simpler than two similar ones. We chose a moderately high concentration of isoamylacetate (IAA) (dilution −2) as CS+ and had the flies in the test choose between the CS+ and a different concentration of that odorant or between the CS+ and a different odorant. Flies were tested under three conditions (Fig. 1C): (i) against a much lower concentration of IAA (column 5a; dilution −6; concentration ratio 1:1,296); (ii) against a slightly lower concentration of IAA (column 4a; dilution −3; concentration ratio 1:6); and (iii) against a different odorant (column 3a; AM, dilution −2). Flies tested at the small concentration ratio performed poorly (PI = −0.24), whereas with the large concentration ratio or different odorants in the test flies reached scores two times higher (PI = −0.50 and −0.51, respectively). As all flies in the three groups had exactly the same training the difference in the PIs could be explained only by the different difficulty of the test.
Same memory strength for different odors and concentrations.
In the two-odor learning paradigm, the concentrations of odorants are usually adjusted such that naïve flies distribute about equally between them (PI ≈ 0; Fig. 3A). During training, one odor is paired with shock, and as a consequence, in the test, it is avoided more than the other. Our measurements show that for the two odors the amplitudes of the avoidance increases are equal but of opposite polarity (compare the half-scores in Fig. 3B). Because the memory test for the two half-scores was the same, any difference between the two half-scores could be attributed to the different strengths of the memories of the two odors. Indeed, if air was the CS+ the fly had no cues for the formation of a useful association and the PI was zero (Fig. 3B, compare the last two columns). However, memories of concentrations of IAA as different as 1:1,296 (IAA dilutions −2/−6) still had the same strength. In none of the examples, except in the experiment with air as CS+, did we find a difference between the two half-scores, indicating that odor memories under our conditions had a standard strength.
Odor intensity memory is independent of cAMP signaling.
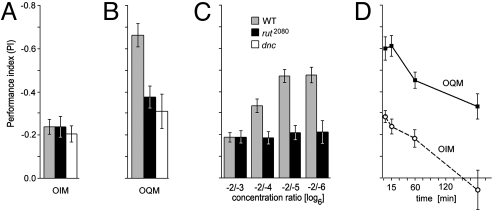
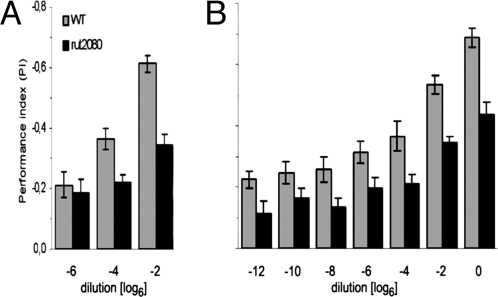
We tested odor intensity learning in the mutants rutabaga2080 (rut2080) and dunce1 that disturb cAMP signaling. As reported previously, in two-odor learning [3-octanol (3-OCT) and benzaldehyde (BAL)] these strains have a 3-min memory score of ≈50% of WT (Fig. 4B) (10, 17, 18). With two concentrations of IAA at the ratio 1:6 the two mutants performed as well as WT (Fig. 4A). If the concentration ratio was increased the memory score increased for WT but not for rut2080(Fig. 4C). This finding might indicate that at higher concentration differences scents are perceived as different odor qualities, as will be argued more conclusively below.
Fig. 4.
OIM is independent of the rutabaga (rut)/cAMP signaling pathway. (A and B) Mutant rut and dunce (dnc) flies show only ≈50% of the WT score in two-odor learning (A) but perform equal to WT flies in intensity learning (B). (C) Increase of difference between test concentrations in intensity learning increases PI in WT flies. Mutant rut flies perform at WT level with a dilution ratio of 1:6 but do not improve their PI with higher dilution ratios. (D) Memory kinetics. OIM (○) completely decays in <3 h, whereas two-odor memory is ≈50% of 3-min memory. If two-odor memory is the sum of OIM and OQM, the latter does not significantly decay in the first 3 h.
Decay of odor intensity memory.
OIM turned out to be rather short lasting. At 3 min the PI was −0.28. It decreased ≈18% in 15 min (PI = −0.24) and ≈36% in 1 h (PI = −0.18) and was zero at 3 h (PI = 0.06, not significant) (Fig. 4D). In comparison, for two-odor memory at 3 min, PI = −0.60. It decreased by 25% in 1 h and still retained >50% at 3 h (PIs = −0.45 and −0.33, respectively). A similarly fast memory decay has been reported for olfactory memory in mutant rut flies (18).
Multiple reinforced odors.
Can flies memorize more than one odor simultaneously? Flies were trained with the normal succession of two odors or two concentrations of one odor but this time both odors were combined with electric shock. With two odors the outcome was as expected: In the test, flies were equally deterred by both odors providing a PI close to (and statistically indistinguishable from) zero (Fig. 5B). If only the last odor had been remembered, the response in the third column in Fig. 5B should have been positive, the one in the forth column in Fig. 5B should have been negative.
Fig. 5.
Multiple olfactory memories. (A) Experimental design. Odors used as were BAL dilution −4 or undiluted BAL (a) and as BAL dilution −5 or undiluted 3-OCT (b), in odor-intensity learning or two-odor learning, respectively. (B) Half-scores are shown; i.e., all values are standardized to a zero that is the naïve response to the odors without any preexposure. Training with two different odors in the standard protocol (undiluted BAL and 3-OCT) leads to avoidance of the previously shocked odorant. When both odorants are shocked in the same training session (A3, A4), the results for the two reciprocal experiments are close to zero and not significantly different from each other (P > 0.05), because independent memories are formed for each odorant and cancel each other out. (C) Intensity learning. If one concentration of BAL (dilution −4 or −5) is paired with shock and flies are tested with two concentrations (dilution −4 and −5), they perform comparably as with two odorants. When both concentrations are paired with shock in the same training session, flies avoid the concentration that was shocked last in the sequence. The difference between the two experiments (C3, C4) shows that the order (A3, A4) matters (P < 0.001). (D) To show that with two odorants (B) indeed both memories are formed, training is as in B but in the test the choice is between one odor (BAL) and air. When shocked in the presence of BAL, flies avoid BAL. When BAL and 3-OCT are both punished, flies also avoid BAL. Position of BAL in the sequence (A6, A7) is irrelevant. n.s., not significant; ***, P < 0.001.
Surprisingly, when two concentrations (ratio 1:6) of one odor were paired with electric shock during training, the effects of punishment did not cancel out. Rather, flies avoided the concentration paired with the last punishment. With the high concentration following the low one, the avoidance was as pronounced as if only the high concentration had been paired with electric shock. With the reversed sequence (high/low) avoidance was not significantly different from the avoidance after pairing only the low concentration with electric shock (Fig. 5C). The asymmetry in the data toward a low concentration of BAL (dilution −5) indicates an additional nonassociative shock-induced avoidance of the high-concentration BAL (dilution −4). The results show that if several intensities are successively reinforced flies store only the last value.
To confirm that in the experiment with two odorants (Fig. 5B) the two memories were indeed stored (rather than being both erased), we repeated the training procedure but tested only one odor (BAL) against air (Fig. 5D). Flies were either exposed only to BAL paired with the shock, BAL followed by 3-OCT (both paired with shock), or 3-OCT followed by BAL (both paired with shocks). All sequences led to a significant avoidance of BAL in the test. Results of the sequences BAL/3-OCT and 3-OCT/BAL did not differ from each other. We conclude that repeating the punishment did not abolish both memories. Together, theses experiments indicate that distinct memories are formed for quality and intensity.
Odor Generalization.
Generalization of odor intensity.
We wondered to which extent flies would generalize odor concentrations. We tested always the same concentration (−2) against air and compared the responses (half-scores) of naïve and conditioned flies (Fig. 6A). We varied the concentration of the odor during training. Full generalization would have resulted in the same increase of avoidance after training at any concentration. Absence of generalization would have led to an increase of avoidance only at the same concentration as used in the test.
Fig. 6.
Odor generalization. (A) Generalization of concentrations. Flies were shocked in the presence of a range of IAA concentrations, diluted BAL, or 3-OCT, or they were given shock alone. After training, all flies were tested with IAA dilution −2 against air. Training performed with the test dilution of IAA led to the highest score. Trained concentrations six times higher or lower also resulted in significant scores. No other stimuli changed spontaneous responses to the tested dilution of IAA (marked by black frame). (B) Generalization between two odors: IAA at high (−2) or low (−6) concentration was tested against air. Training was with IAA (gray bars) or AM (black bars) at the same dilution as in the test. At low concentrations IAA and AM were generalized; at high concentrations only partial generalization was observed. (C) No generalization between 3-OCT and BAL. As in B, one odorant was tested after training with either the same or a different odorant. 3-OCT at high (−2) or low (−6) concentration was tested against air. Training was with 3-OCT (gray bars) or BAL (black bars) at the same dilution as in the test. n.s., not significant; ***, P < 0.001.
We applied six different concentrations of IAA during the training. Training with the test concentration (−2) gave the highest avoidance score (PI = −0.59; conditioned–naïve). Training with concentrations six times more or less diluted than the test concentration (−1 and −3, respectively) were similar enough to the test concentration to make flies show significantly higher avoidance than naïve animals [for responses of naive animals to IAA see supporting information (SI) Fig. S1]. Any concentration diluted 36 times more or less did not lead to such increased avoidance. No change in avoidance was observed when other odorants (BAL, 3-OCT) were used during training instead of IAA or when shock alone was given (Fig. 6A). This effect was indeed generalization rather than an inability to discriminate, because flies have been shown to discriminate concentration differences as small as 30% (5, 19).
Generalization of odor quality.
From the previous experiments it is apparent that generalization does not occur between two different odorants. We extensively tested this possibility. We trained with BAL as CS+ varying the concentration from undiluted to clean air and always tested one dilution of 3-OCT. No significant difference between conditioned and naïve flies was observed (data not shown). This experiment implies that generalization between different odorants does not occur regardless of the concentration.
To test whether this result was generally applicable, we performed similar experiments with chemically very similar odorants, IAA and amylacetate (AM). In the test, flies had always a choice between IAA and air. During training, IAA or AM were paired with shock. We performed the experiment at high (dilution −2) and low (dilution −6) concentrations of the two odorants. After training and testing at the high concentration of the same odor (IAA), a high conditioned avoidance score was obtained (PI = −0.66). After training with the other odor (AM), the conditioned score was much lower (PI = −0.20) but still different from zero. Apparently, some generalization across odorants had occurred. In the parallel experiment at the low concentrations, training and testing of the same odor (IAA) also led to a high conditioned avoidance score (PI = −0.50). But now, conditioned avoidance of flies trained with AM (PI = −0.36) was not significantly different from that of flies trained with IAA (Fig. 6B). The same experiment was repeated with two other odorants (BAL and 3-OCT). Between those, flies failed to generalize regardless of concentration (Fig. 6C), which would imply that generalization between some (presumably chemically similar) odorants such as IAA and AM is possible and depends on odor intensity.
Interestingly, at concentrations at which IAA and AM are generalized, rut is dispensable for learning (Fig. 7A). Mutant rut flies seem to be lacking with respect to WT only, if the two odors are not generalized (Fig. 7). It would be interesting to see whether in mutant rut flies for any odor pair relative concentrations could be found that would be generalized.
Fig. 7.
In mutant rut2080 flies two-odor learning was normal for odors that are generalized. Lower concentrations of odors in two-odor learning led to lower PIs. (A) At high odor concentration of similar odorants (IAA and AM) mutant rut flies performed at ≈60% of WT. At a concentration at which these odorants are generalized (Fig. 6B), their learning scores for WT and mutant were the same. (B) Dissimilar odorants (BAL and 3-OCT), which are not generalized at any concentration (Fig. 6C), showed rut-dependent memory throughout the tested range of dilutions, with mutant rut flies performing always at ≈50–60% of WT.
Discussion
Concentration Invariance.
In insects, odors are assumed to be represented in the antennal lobes (ALs) by a specific combination of activated glomeruli, and in the mushroom bodies (MBs) by a specific set of Kenyon cells (KCs) (review in ref. 20). The MB model of olfactory short-term memory in Drosophila (17) proposes that output synapses of the KCs representing the CS+ increase their gain in the course of conditioning to drive a MB output neuron (conditioned response). Two recent reports (21, 22) describe extrinsic MB neurons in the MB output region displaying the properties expected of such output neurons. For this model to work outside of the laboratory, the representation of the CS+ in the MBs has to be, at least to some extent, concentration invariant. Otherwise, any deviation of the test concentration from that in the training would fail to trigger the output neuron, unless each slightly different concentration of a conditioned odor would have its own memory trace. To store many dozens of memory templates for the same odorant would seem not very efficient.
Our generalization experiments have shown that odor conditioning, indeed, is concentration invariant. Within a concentration ratio of 1:10 (or 10:1) the CS+ is still recognized and treated as the “same” between training and test. Beyond this range concentration differences seem to be treated like distinct scents. These findings are in line with imaging data: In recordings of odor responses in the projection neurons at the level of the AL or calyx, the difference in the signal corresponding to a 10-fold concentration difference is small (23). On the other hand, with increasing concentration in a larger range, new glomeruli in the AL are recruited in addition to the already activated ones (23, 24).
If in the AL the glomerular patterns of lower concentrations would be nested within those of higher concentrations (23, 24) the MB model outlined above would predict that the half-scores in experiments with two concentrations (ratio >1:10), should be very different. The memory trace for intensity should be matched by the activation of all sets of KCs representing any higher concentrations than that of the CS+, implying that the half-score should be zero if the CS+ is the lower of the two concentrations (16). Our results (Fig. 3) are clearly at odds with this prediction. Perhaps the glomerular patterns of the lower concentrations are not completely nested in those of higher concentrations but differ as those of distinct odorants, may be caused by an inhibition between glomeruli (25). Interestingly, honey bees are able to solve this learning task only under some test conditions (26–28).
What Is Learned in Conditioned Odor Discrimination?
Under a variety of conditions, we did not find any influence of the CS− during the formation of associative odor memory (Figs. 1B and 2). All data support the conclusion that olfactory learning is one-odor learning and only during the test flies discriminate between two stimuli. They cannot predict what will be important to learn for the test. Thus, one cannot experimentally separate intensity and quality learning during training. Obviously, flies exposed to the CS+ learn both intensity and quality at the same time.
The memory scores obtained in the test represent the product of two parameters: odor discrimination and memory strength. If either of the two is zero, then the memory score is zero. To compare the strengths of two memories, one has to measure their half-scores in the same test. In most cases they are equal as if they had a unit strength (Fig. 3).
Separation of Quality and Intensity Memories.
The memories for quality and intensity can be distinguished as two independent processes by three criteria. First, the memory store for intensity keeps only the value of the last conditioned odor in successive conditioning trials regardless of whether it is of the high or low concentration (Fig. 5B). In contrast, with two odors, independent memories are stored for each odor (Fig. 5 C and D). In the test, these memories compete against each other, resulting in no measurable memory score (Fig. 5C). Tully and Quinn (8) had demonstrated that flies are able to simultaneously store two memories of odors by using a protocol for reversal learning. After the second training, they found a memory score of PI = 0.26 for avoidance of the second CS+ (compared with PI = 0.90 after a single conditioning phase). They concluded that despite what flies had learned during the first cycle, they avoided the odor more recently paired with the shock. The 70% reduction in learning score, however, is in line with our findings that independent odor memories of the first and second conditioning event are stored.
We assume that multiple memories of odor quality are stored in parallel and do not interfere with each other, unless at the level of the behavioral output. Intensity memories, on the other hand, interact already at the level of memory storage. Multiple memories of odor quality would fit into the MB model of olfactory learning (16), whereas the observed interference between intensity memories argues for a different storage site and/or mechanism.
The second discriminating criterion is stability (Fig. 4D). We observed complete decay of intensity memory within <3 h, whereas the memory in two-odor learning persisted to >50% at that time. If the memories of intensity and quality were assumed to add up in the score of two-odor memory, one would have to conclude that the memory for odor quality is stable during the first 3 h.
Third, intensity and quality memory are distinguished by the role of cAMP signaling. It has been known for a long time that ≈50% of short-term memory is retained in rut and dnc mutants (18, 29, 30). This remaining memory has been assumed to be rut- and dnc-independent rather than being caused by the residual functions of these genes. No effect of calcium on adenylate cyclase activity could be detected in the rut1 allele (31, 32), suggesting that it may be an amorph with complete loss of function (7) regarding learning and memory. The P-insertion mutant rut2080 was reported to be a loss-of-function allele as well (33). Finally, dncM14 appeared to be amorphic (34, 35). The same alleles have been used in the present study. We therefore propose that intensity memory is rut- and dnc-independent and that the rut-dependent short-term component is odor-quality memory. Yet, not all components of odor-quality memory are rut-dependent. Anesthesia-resistant memory, which is rut-independent (43), must be a memory for odor quality, as no intensity memory remains at 3 h.
Other components of cAMP signaling were shown to be necessary for two-odor learning as well (36). Connolly et al. (37) expressed a constitutively active form of a Gαs subunit specifically in MBs, which completely abolished two-odor memory. Because there was no remaining memory as in the case of rutabaga we assumed that OIM would be completely disrupted by this approach, which was indeed the case, as was shown recently (38).
We have shown that flies treat an odor as the same even at ≈10-fold higher or lower concentration. In this range of concentrations odor-intensity learning results in low memory scores. Apparently, concentration ratios within this range are difficult to discriminate in the T-maze. We suggest that in this concentration range the system of OQM in the MBs is concentration-invariant. Notably, almost the same range for concentration generalization has been found in Musca (39).
Generalization between two odorants was observed only between low concentrations of IAA and AM. Although these odors do not smell the same to us, they are similar in their chemical structure (amylacetates). Possibly, with decreasing concentrations chemically similar odors get more similar (see ref. 25). Flies might perceive low concentrations of IAA and AM as two intensities of the same scent and not as different qualities. At least, flies treat the two odorants as they would be two close concentrations of the same odorant: they rely on their rut-independent intensity memory (Fig. 7A) for discrimination in odor conditioning. This reliance is not observed with BAL and 3-OCT, probably because they are chemically very different (Fig. 7B).
This conjecture is supported by physiological data. IAA and AM activate similar sets of glomeruli (23). These activity patterns, which contain a common prominent glomerulus, may get identical if with decreasing concentration, eventually, only this glomerulus remains active (23). In contrast, BAL and 3-OCT activate different olfactory sensillae (40) and very different glomerular patterns in the AL. Moreover, BAL has been reported to be processed separately from all other tested odorants (41).
Conclusion
For a particular scent (i) a memory trace of its chemical identity (OQM) and (ii) a separate memory trace of its intensity (OIM) are deposited in the fly brain. OQMs are concentration invariant within a limited range (10:1; 1:10), implying that OQM is retrieved by the scent at any of these concentrations but not by concentrations outside of this range. We call this the identity range of an OQM (regarding concentration), which is the range in which generalization of concentration is observed. An OQM contains no information about odor concentration within its identity range but represents a scent at a certain range of concentrations (identity range). The information about concentration within this range is stored separately in the OIM. An OIM is meaningful only for a certain OQM and should therefore be functionally linked to it.
Methods
Fly Stocks and Care.
Flies were raised on corn-meal food medium (42) at a 14:10 h light/dark cycle at 25°C and 60% relative humidity. Experimental flies were fed on fresh food vials for up to 48 h before being tested. For behavioral experiments, we used 3- to 6-day-old males and females in mixed groups, either taken from homozygote lines or progeny of crosses between homozygote parental lines. All behavioral experiments were done at 26°C and 80% relative humidity, under red light (invisible to the flies) during the training phase and in complete darkness during the test.
Canton-S (Wuerzburg) served as WT. For rutabaga experiments, rut2080 or rut2080; +; UAS-rut+ was used (10). For dunce experiments we used dunceM14 (34).
Behavioral Paradigm.
Flies were trained and tested in a modified version (18) of the T-maze paradigm (7). About 50–100 flies were placed into a training tube, the inside of which was covered with an electrifiable copper grid. Flies were then exposed sequentially to two odors, which were applied in an air current of constant speed sucked through the machine at 750 ml/min. During 60-s exposure to the first odor (CS+) flies were given 12 times electric shock of 90 V and 1.3-s duration. After 45 s of fresh air, flies were exposed for 1 min to the second odor (CS−) without electric shock, followed by 45 s of air. This completed one training cycle. After training, flies were gently tapped into a compartment, from where they could be transported to the choice point of the T-maze. There they were exposed to two air streams, one scented with the formerly shocked, the other with the formerly nonshocked odor. Flies were allowed to choose between them for 120, 60, or 15 s, depending on the experiment. After the test, flies were trapped in one of the two tubes, collected, anesthetized, and counted. Unless stated otherwise, two groups of flies were reciprocally trained and tested. The punished odor (CS+) from the first experiment became the nonpunished one (CS−) in the second experiment. The performance index for one of those groups (PI1, PI2) is referred to as a half-score (15). For each experiment, performance indices PI1, PI2 = (N nonpunished − N punished)/(N punished + N nonpunished) were calculated and averaged to a final learning score as PI = (PI1 + PI2)/2.
Odorants were placed at the end of the tubes in small cups of various diameters to balance their mutual intensity. They were presented as pure substances or diluted in paraffin oil (Fluka). We used two different pairs of odorants: BAL (Fluka; presented in 4.3-mm diameter cups) with 3-OCT (Fluka; 15-mm cups) and IAA (Aldrich; 15-mm cups) with AM (Merck; 15-mm cups).
For concentration learning, two different dilutions of one odorant were used. One concentration served as odor A and the other as odor B. Different dilutions of IAA or BAL in paraffin oil were used. Odorants were diluted in steps of 6:1 [log6 units]. These steps are denoted as nx, i.e., a dilution in three steps of 6:1 (63:1 = 216:1) would be written as −3. The absolute odor concentrations in the air stream (molecules per volume) were not known.
Supplementary Material
Acknowledgments.
We thank members of M.H.'s laboratory for advice and discussions, T. Zars (University of Missouri–Columbia, Columbia, MO) for rut 2080;+;UAS-rut+ flies, and J. Rister for comments on the manuscript. This work was supported by German Science Foundation Grant SFB554.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804086105/DCSupplemental.
References
- 1.Buck L. Olfactory receptors and odor coding in mammals. Nutrition Rev. 2004;62:184–188. doi: 10.1111/j.1753-4887.2004.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 2.Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y. Properties of learning and memory in Drosophila melanogaster. J Comp Physiol A. 1977;114:69–89. [Google Scholar]
- 5.Borst A. Computation of olfactory signal in Drosophila melanogaster. J Comp Physiol A. 1983;152:373–383. [Google Scholar]
- 6.Kramer E. The orientation of walking honeybee in odor fields with small concentration gradients. Physiol Entomol. 1976;1:27–37. [Google Scholar]
- 7.Borst A, Heisenberg M. J Comp Physiol A. 1982;147:479–484. [Google Scholar]
- 8.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 9.Preat T. Decreased odor avoidance after electric shock in Drosophila mutants biases learning and memory tests. J Neurosci. 1998;18:8534–8538. doi: 10.1523/JNEUROSCI.18-20-08534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 11.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 13.Waddell S. Courtship learning: Scent of a woman. Curr Biol. 2005;15:R88–R90. doi: 10.1016/j.cub.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Stoerkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 17.Dubnau J, Tully T. Gene discovery in Drosophila: New insights for learning and memory. Annu Rev Neurosci. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- 18.Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 19.Heisenberg M. Mutants of brain structure and function: What is the significance of the mushroom bodies for behavior? In: Siddiqi O, Babu, Hall LM, Hall JC, editors. Development and Behavior of Drosophila. New York: Plenum; 1980. pp. 373–390. [DOI] [PubMed] [Google Scholar]
- 20.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Riemensperger T, Voeller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Silbering AF, Sachse S, Eisermann B, Galizia CG. Odor-induced activity patterns in the antennal lobe of Drosophila melanogaster. In: Elsner N, Zimmermann H, editors. Proceedings of the 29th Goettingen Neurobiology Conference 2003; Neurowissenschaftliche Gesellschaft; 2003. pp. 481–482. [Google Scholar]
- 24.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 25.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–674. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 26.Bhagavan S, Smith BH. Olfactory conditioning in the honey bee, Apis mellifera: Effects of odor intensity. Physiol Behav. 1997;61:107–117. doi: 10.1016/s0031-9384(96)00357-5. [DOI] [PubMed] [Google Scholar]
- 27.Pelz C, Gerber B, Menzel R. Odorant intensity as a determinant for olfactory conditioning in honeybees: Roles in discrimination, overshadowing, and memory consolidation. J Exp Biol. 1997;200:837–847. doi: 10.1242/jeb.200.4.837. [DOI] [PubMed] [Google Scholar]
- 28.Ditzen M, Evers JF, Galizia CG. Odor similarity does not influence the time needed for odor processing. Chem Senses. 2003;28:781–789. doi: 10.1093/chemse/bjg070. [DOI] [PubMed] [Google Scholar]
- 29.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci USA. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tully T, Preat T, Boynton SC, DelVecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 31.Dudai Y, Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984;47:119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- 32.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 33.Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci USA. 2003;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis RL, Kiger JA., Jr Dunce mutants of Drosophila melanogaster: Mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981;90:101–107. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salz HK, Kiger JA. Genetic analysis of chromomere 3D4 in Drosophila melanogaster. Genetics. 1984;108:377–392. doi: 10.1093/genetics/108.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis RL. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 37.Connolly JB, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 38.Xia S, Tully T. Segregation of odor identity and intensity during odor discrimination in Drosophila mushroom body. PLoS Biol. 2007 doi: 10.1371/journal.pbio.0050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukushi T. Olfactory conditioning in the housefly, Musca domestica. Annot Zool Jap. 1973;46:135–143. [Google Scholar]
- 40.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 41.Keene AC, Waddell S. Drosophila memory: Dopamine signals punishment? Curr Biol. 2005;15:R932–R934. doi: 10.1016/j.cub.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 42.Guo A, et al. Conditioned visual flight orientation in Drosophila: Dependence on age, practice, and diet. Learn Mem. 1996;3:49–59. doi: 10.1101/lm.3.1.49. [DOI] [PubMed] [Google Scholar]
- 43.Isabel G, Pacual A, Preat P. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.