Abstract
The auditory conditioned stimulus (CS) pathway that is necessary for delay eyeblink conditioning was investigated with induced lesions of the medial auditory thalamus contralateral to the trained eye in rats. Rats were given unilateral lesions of the medial auditory thalamus or a control surgery followed by twenty 100-trial sessions of delay eyeblink conditioning with a tone CS and then five sessions of delay conditioning with a light CS. Rats that had complete lesions of the contralateral medial auditory thalamic nuclei, including the medial division of the medial geniculate, suprageniculate, and posterior intralaminar nucleus, showed a severe deficit in conditioning with the tone CS. Rats with complete lesions also showed no cross-modal facilitation (savings) when switched to the light CS. The medial auditory thalamic nuclei may modulate activity in a short-latency auditory CS pathway or serve as part of a longer latency auditory CS pathway that is necessary for eyeblink conditioning.
Keywords: eyelid, learning, medial geniculate, posterior intralaminar nucleus, suprageniculate
Pavlovian conditioning paradigms are optimal for establishing the neural structures that are necessary and sufficient for learning (Thompson, 1976). The amygdala and cerebellum have been implicated as sites of learning-induced plasticity that form associations among stimuli during fear and eyeblink conditioning, respectively (Fanselow & Poulos, 2005; Thompson, 2005). These learning centers receive converging information from both conditioned stimuli (CSs) and unconditioned stimuli (USs) to form the association that is necessary for producing a conditioned response (CR). Stimulus information is thought to be relayed to learning centers by neural pathways that are distinct for different types of CRs.
Neural pathways for an auditory CS in fear-motivated conditioning include the medial division of the medial geniculate nucleus (MGm), posterior intralaminar nucleus (PIN), suprageniculate nucleus (SG), and their projections to the lateral and basolateral amygdala (Bordi & LeDoux, 1994; Campeau & Davis, 1995; Edeline & Weinberger, 1992; Gabriel, Saltwick, & Miller, 1975; LeDoux, Farb, & Ruggiero, 1990; LeDoux, Sakaguchi, & Reis, 1983; McCabe, McEchron, Green, & Schneiderman, 1993; McEchron, McCabe, Green, Llabre, & Schneiderman, 1995; Supple & Kapp, 1989). The MGm is also involved in appetitive conditioning (Birt & Olds, 1981; Disterhoft & Olds, 1972; Olds, Disterhoft, Segal, Kornblith, & Hirsh, 1972). The medial auditory thalamic nuclei (MGm, PIN, and SG) provide CS input to the amygdala but are also activated by aversive USs (Bordi & LeDoux, 1994; Edeline & Weinberger, 1992; Lennartz & Weinberger, 1992). Auditory conditioning produces learning-related facilitation of neuronal activity in the MGm, which might be due to convergence of CS and US inputs in the MGm (Birt & Olds, 1981; Bordi & LeDoux, 1994; Edeline & Weinberger, 1992; Gabriel et al., 1975; Lennartz & Weinberger, 1992; Love & Scott, 1969; McEchron et al., 1995; Ryugo & Weinberger, 1978; Supple & Kapp, 1989; Wepsic, 1966) or to CS–US convergence in the amygdala with feedback to the MGm (Maren, Yap, & Goosens, 2001; Poremba & Gabriel, 2001). Although the medial auditory thalamic nuclei have been shown to play a role in many of the most commonly used auditory conditioning paradigms, very little is known about their contributions to eyeblink conditioning.
Auditory CS information in eyeblink conditioning is conveyed to the pontine nuclei via a monosynaptic projection from the cochlear nuclei (Steinmetz et al., 1987; Steinmetz & Sengelaub, 1992). The pontine nuclei then provide the cerebellum with auditory CS information through their mossy fiber inputs via the middle cerebellar peduncle, primarily to the contralateral interpositus nucleus and granule cell layer of the cerebellar cortex (Brodal, 1981; Mihailoff, 1993; Shinoda, Sugiuchi, Futami, & Izawa, 1992; Steinmetz & Sengelaub, 1992). The projection from the cochlear nuclei to the pons constitutes a unilateral short-latency auditory CS pathway. Abundant evidence indicates that the pontine mossy fiber projection is the proximal part of the necessary and sufficient CS pathway in eyeblink conditioning (Bao, Chen, & Thompson, 2000; Freeman & Rabinak, 2004; Freeman, Rabinak, & Campolattaro, 2005; Hesslow, Svensson, & Ivarsson, 1999; Steinmetz, 1990; Steinmetz et al., 1987; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986; Tracy, Thompson, Krupa, & Thompson, 1998). Less conclusive data have been reported regarding the various auditory nuclei that could contribute to processing of an auditory CS in eyeblink conditioning.
The short-latency pathway clearly provides auditory CS information to the pontine nuclei, but the monosynaptic pathway may not be sufficient for conditioning. Neurons in the pons that receive input from the cochlear nuclei show a response latency of 3−5 ms after the onset of a brief acoustic stimulus (Gould, Sears, & Steinmetz, 1993; Steinmetz et al., 1987). However, an interstimulus interval (ISI) greater than 50 ms is required for acquisition of eyeblink conditioning (Smith, Coleman, & Gormezano, 1969). Other auditory structures may be critically involved in relaying tone CS information after 5 ms to the pontine nuclei to support eyeblink conditioning. Evidence for the involvement of auditory areas other than the cochlear nuclei in eyeblink conditioning comes from studies that used stimulation of the ventral division of the medial geniculate, inferior colliculus, superior olive, auditory cortex, and cochlear nuclei as an effective CS to support conditioning (Knowlton & Thompson, 1992; Knowlton, Thompson, & Thompson, 1993; Nowak, Kehoe, Macrae, & Gormezano, 1999; Patterson, 1970). Single-unit activity in the medial geniculate recorded during differential auditory trace conditioning in rabbits showed CS- and CR-related facilitation in the dorsal and medial nuclei; the strongest facilitation occurred in the medial nucleus (O'Connor, Allison, Rosenfield, & Moore, 1997). The stimulation and unit recording data suggest that auditory areas that are efferent to the cochlear nuclei might play a role in eyeblink conditioning.
Lesion studies have provided further evidence for which of the auditory areas mentioned above might be relaying auditory CS information to the cerebellum. Decerebrations rostral to the red nucleus do not prevent retention of auditory eyeblink conditioning, and decortication does not prevent acquisition or retention, indicating that auditory cortex is not essential (Mauk & Thompson, 1987; Oakley & Russell, 1972, 1977). Mauk and Thompson decerebrated rabbits following initial conditioning with a tone CS by first aspirating the cerebral cortex and hippocampus and then sectioning the brain stem rostral to the red nucleus. This decerebration procedure might have spared the caudal end of the medial auditory thalamus. It is possible, therefore, that the medial auditory thalamic nuclei play a role in auditory eyeblink conditioning through direct or indirect projections to the pons. The current experiment examined the possibility that the contralateral medial auditory thalamic nuclei are involved in the acquisition of delay eyeblink conditioning with a tone CS.
Method
Subjects
The subjects were 39 male Long-Evans rats (250 − 400 g). The rats were housed in the animal colony in Spence Laboratories at the University of Iowa (Iowa City, IA). All rats were maintained on a 12-hr light–dark cycle, with light onset at 7 a.m., and were given ad libitum access to food and water.
Surgery
Two weeks before training, rats were removed from their home cages and anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg). Rats were given injections of atropine sulfate (0.45 mg/kg) to reduce respiratory tract secretions during anesthesia. After the onset of anesthesia, the rats were fitted with differential electromyograph (EMG) electrodes that were implanted in the left orbicularis oculi muscle in the upper eyelid. The reference electrode was attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins in a plastic connector. A bipolar stimulating electrode (Plastics One, Roanoke, VA) for delivering the shock US was implanted subdermally, caudal to the left eye. A 30-gauge stainless steel infusion cannula was lowered into the right MG in three sites. The infusion cannula was connected to polyethylene tubing (PE 10; 100 cm), which was connected to a 10-μL gas-tight syringe (Hamilton, Reno, NV). The syringe was placed in an infusion pump (Hamilton Apparatus, Holliston, MA), and either 0.25 μL (MG) or 0.10 μL (MGm) of ibotenic acid (10 mg/ml, pH 7.4) or sterile saline (pH 7.4) was infused at each coordinate. The infusion rate was 2.0 ml/hr for MG lesions and 1.0 ml/hr for smaller lesions targeting the MGm; the infusion cannula was allowed to sit for 7 min before being raised to prevent upflow. The stereotaxic coordinates for the MG lesion taken from bregma were 5.0, 5.5, and 6.1 mm posterior; 3.3, 3.4, and 3.6 mm lateral; and 5.9 mm ventral to the skull surface. The coordinates for the MGm lesion were 5.1, 5.5, and 5.9 mm posterior; 2.9, 2.8, and 3.1 mm lateral; and 6.0 and 6.1 mm ventral to the skull surface. The plastic connectors housing the EMG electrode leads, the bipolar stimulating electrode, and two skull screws were secured to the skull with dental acrylic (Dentsply International, York, PA). Animals were maintained on 0.006% Sulfatrim (Alpharma, Baltimore, MD) in water for 4 days after surgery.
Apparatus
The conditioning apparatus consisted of seven small-animal sound-attenuating chambers (BRS/LVE, Laurel, MD). Within each sound-attenuating chamber was a small-animal operant chamber (BRS/LVE), in which the rats were kept during conditioning. One wall of the operant chamber was fitted with two speakers that independently produce tones of up to 120 dB (sound pressure level) with a frequency range of approximately 1,000 to 9,000 Hz. The middle of the back wall of the sound-attenuating chamber was equipped with a small 4-W house light, and the corner was equipped with a 6-W light CS. An exhaust fan on one of the walls provided a 65-dB masking noise. The CSs used in training were a 2,000- or 8,000-Hz pure tone (88 dB; range in conditioning chamber = 83−88 dB) and a 6-W white light flash. The electrode leads from the rat's head stage were connected to peripheral equipment by lightweight cables that allowed the rat to move freely during conditioning. A desktop computer was connected to the peripheral equipment. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC). One circuit controlled the delivery of a shock stimulus through a stimulus isolator (Model 365A; World Precision Instruments, Sarasota, FL). Another circuit differentially amplified (gain = 2000; sampling rate = 250 Hz), filtered (500−5,000 Hz), and integrated (time constant = 20 ms) eyelid EMG activity. The intensity of the shock US was set at two times the threshold for eliciting a discrete eyeblink (range of final current intensity = 2−5 mA).
Conditioning Procedure
The rats were allowed to adapt to the training environment for 5 min before each training session. They were then given delay eyeblink conditioning using a tone CS (2 or 8 kHz) for 20 days followed by 5 days of delay conditioning with a light CS. Daily training sessions consisted of 10 blocks of 9 paired CS–US presentations and 1 CS-alone trial. The stimuli were presented with a pseudorandom distribution of intertrial intervals between 18 and 42 s that averaged 30 s. The 500-ms tone or light CS coterminated with a 25-ms shock US, yielding an interstimulus interval of 475 ms. The values relayed to the computer software from the EMG integrator were units of voltage of integrated EMG activity. The CR threshold was set at 0.4 V above the amplified and integrated EMG activity during baseline. The EMG baseline was usually zero (except for the direct current offset), because the orbicularis oculi muscle does not produce spontaneous or tonic activity (Hesslow, 1994; Pellegrini & Evinger, 1997). Integrated EMG responses exceeding the threshold value during the first 80 ms of the CS period were startle responses; responses that exceeded the threshold value during the last 395 ms of the CS but before the US were CRs; and responses that exceeded the threshold after the onset of the US were URs.
Histology
After training, the rats were euthanized with an injection of sodium pentobarbital (150 mg/kg) and transcardially perfused with ∼100 ml of physiological saline followed by ∼300 ml of 10% buffered formalin. After perfusion, the brains were postfixed for a minimum of 24 hr, cryoprotected in 10% and 30% sucrose in phosphate-buffered saline, and subsequently sectioned at 50 μm with a sliding microtome. Sections were then stained with cresyl violet or thionin. The location and size of the lesions were then examined with a light microscope (Leica DMLS, Wetzlar, Germany) and a stereotaxic brain atlas (Paxinos & Watson, 1998).
Groups
The rats were initially assigned to one of three groups: lesion (n = 25), saline-infused control (saline, n = 8), or behavioral control in which training took place only with the light CS (light acquisition, n = 6). The lesion group was subsequently subdivided on the basis of histological assessment of the lesions (see the Results section) into two groups: rats that had complete lesions of the MGm, PIN, and SG (complete, n = 14) and rats that exhibited sparing of at least one of those nuclei (n = 11).
Results
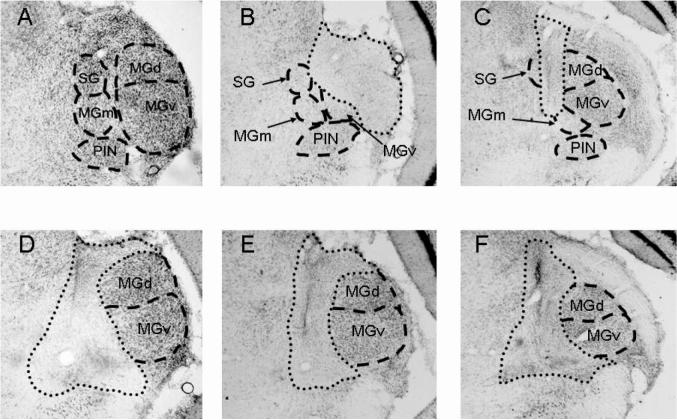
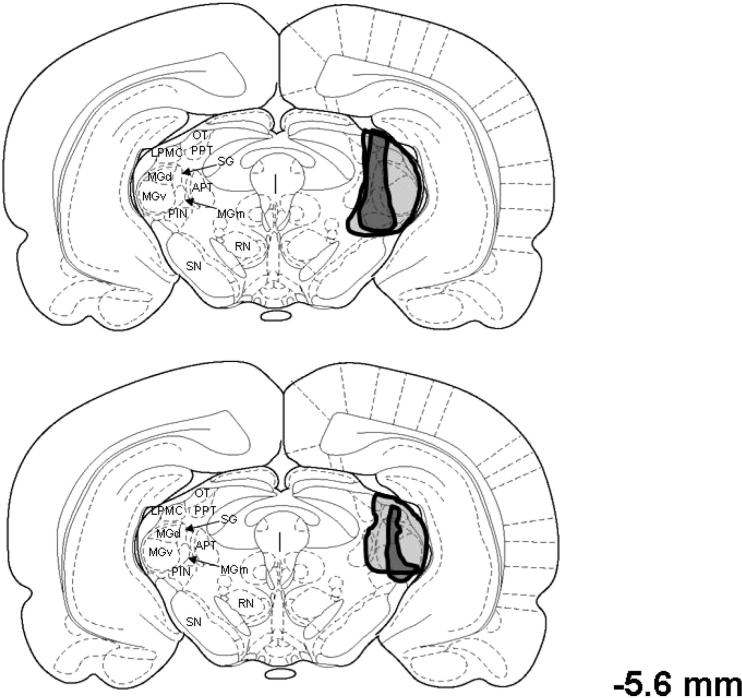
Figure 1 shows examples of complete and incomplete ibotenic acid lesions of the medial auditory thalamic nuclei. Lesions were designated as complete if they included total cell loss in the MGm, PIN, and SG (n = 14). Lesions that spared the MGm, PIN, or SG were considered to be incomplete (n = 11). Other areas were damaged in some of the rats including the medial CA3 and ventral CA1 areas of the hippocampus; dorsal and ventral divisions of the medial geniculate, peripeduncular nucleus, pretectal nucleus, and the dorsolateral substantia nigra (Figure 2). These areas were not damaged consistently in rats with complete lesions, but more rats in the complete group (n = 5) sustained hippocampal damage than in the incomplete group (n = 2). Therefore, the behavioral data from rats with hippocampal lesions were not used in the statistical analyses.
Figure 1.
Images of nissl stained sections of the contralateral medial auditory thalamus, which includes the medial division of the medial geniculate (MGm), the posterior intralaminar nucleus (PIN), and the suprageniculate nucleus (SG) in a control rat (A), two rats with incomplete lesions (B, C), and three rats with complete lesions (D, E, and F). The dorsal (MGd) and ventral (MGv) divisions of the medial geniculate are also labeled. Dotted lines indicate the borders of the lesions, and dashed lines indicate the borders of the nuclei.
Figure 2.
Drawing of coronal sections of the rat brain depicting the smallest (black regions) and largest (gray regions) lesions in rats with complete (upper) or incomplete (lower) lesions of the medial auditory thalamus, which includes the medial division of the medial geniculate (MGm), the posterior intralaminar nucleus (PIN), and the suprageniculate (SG). APT = anterior pretectal nucleus; LPMC = lateral posterior thalamic nucleus, mediocaudal division; MGd = dorsal division of the medial geniculate; MGv = ventral division of the medial geniculate; OT = optic tract nucleus; PPT = posterior pretectal nucleus; RN = red nucleus; SN = substantia nigra. The number to the right of the drawings indicates the anterior–posterior stereotaxic coordinate relative to bregma. From The Rat Brain in Stereotaxic Coordinates (Figure 42) by G. Paxinos and C. Watson, 1998, New York: Academic Press. Copyright 1998 by Academic Press. Adapted with permission.
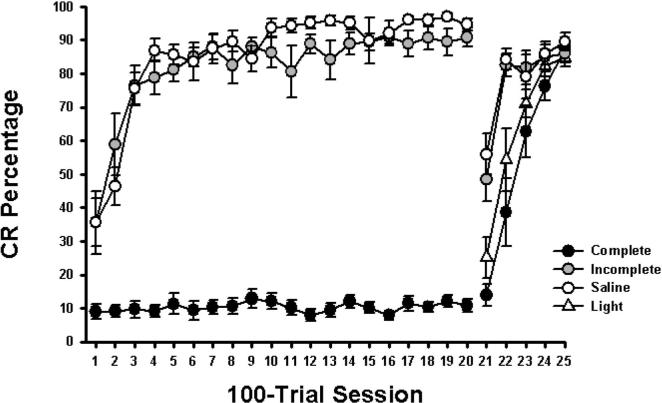
Rats with complete lesions of the medial auditory thalamus were severely impaired on conditioning to a tone CS compared with rats in the incomplete and saline groups (Figure 3; Sessions 1−20). When rats in the complete group were switched to conditioning with a light CS, their rate of acquisition was the same as the light acquisition group (Figure 3; Sessions 21−25). In contrast, the incomplete and saline groups showed cross-modal facilitation (savings) relative to the light acquisition group when switched from the tone to a light CS.
Figure 3.
Mean (±) standard error of the mean conditioned response (CR) percentage over twenty 100-trial sessions of delay eyeblink conditioning with a tone-conditioned stimulus (CS) and five sessions of conditioning with a light CS in rats with compete lesions of the medial auditory thalamic nuclei (complete), incomplete lesions (incomplete), saline-infused controls (saline), and controls that were given training only with a light stimulus (light).
Unpaired control groups to assess nonassociative changes in eyeblink responses were not used in the current study. It is unlikely, however, that nonassociative effects contributed to the pattern of results. The percentage of CRs in the group with complete lesions was similar to the percentage of CRs typically seen in rats given unpaired training in our laboratory (e.g., Kleim et al., 2002). It is possible that some of the eyeblink responses in the group given incomplete lesions were due to nonassociative responding, but the rate of acquisition and CR timing suggest that the conditioning observed in this group was associative.
We performed a repeated measures analysis of variance (ANOVA) on the percentage of CRs during acquisition of tone conditioning for the complete, incomplete, and saline groups (Sessions 1−20), which revealed a significant interaction of the group and session variables, F(38, 437) = 8.51, p < .0001. Post hoc tests (Tukey–Kramer) indicated that the incomplete and saline groups produced more CRs during all tone sessions (Sessions 1−20) than did the complete group (all comparisons, p < .05). The incomplete group showed fewer CRs than did the saline group on Sessions 2, 11, and 13 of tone conditioning (all comparisons, p < .05).
A repeated measures ANOVA was also performed on the percentage of CRs during subsequent light conditioning for the complete, incomplete, saline, and light acquisition groups (Sessions 21−25), which revealed a significant interaction of the group and session variables, F(12, 112) = 6.80, p < .0001. Post hoc tests indicated that the complete and light acquisition groups showed fewer CRs on Sessions 21 and 22, as compared with the incomplete and saline groups (both comparisons, p < .05). The complete and light acquisition groups did not differ significantly during any of the light conditioning sessions. The incomplete and saline groups also did not differ significantly during any of the light conditioning sessions. The absence of cross-modal facilitation in the complete group indicates that they acquired no conditioning during tone training.
Separate ANOVAs were performed on UR amplitude and UR peak latency during the first day of training for the saline, complete, and incomplete groups. The UR measures were taken on the first day of training because there were fewer CRs that would contaminate measurement of the UR than during the subsequent sessions. There was no group effect for either UR amplitude, F(2, 23) = 0.25; p < .78, or UR peak latency, F(2, 23) = 0.40, p < .68, indicating that motor performance was intact in the rats that failed to show auditory eyeblink conditioning.
Discussion
Complete lesions of the medial division of the medial geniculate, suprageniculate, and posterior intralaminar nuclei (the medial auditory thalamic nuclei) contralateral to the trained eye severely impaired acquisition of eyeblink conditioning with a tone CS. Lesions that did not completely destroy the medial auditory thalamic nuclei had little effect on acquisition of eyeblink conditioning. Rats with complete lesions of the medial auditory thalamic nuclei acquired eyeblink conditioning with a light CS but showed no evidence of cross-modal facilitation relative to a control group that was only trained with the light CS. Rats with incomplete lesions and rats in the control group showed facilitated acquisition of conditioning when switched to the light CS. Rats with complete lesions did not exhibit deficits in the amplitude or latency of the unconditioned response, suggesting that the lesions did not affect motor performance.
Cross-modal facilitation like that observed in the control and incomplete lesion groups has been investigated in detail in rabbits (Kehoe, 1988). The facilitation of conditioning seen when the CS modality is changed is not due to primary stimulus generalization; it is most accurately characterized as more rapid acquisition due to previously established plasticity within the neural circuitry underlying conditioning (Kehoe, 1988). The absence of cross-modal facilitation in the rats with complete lesions indicates that the impairment in conditioning seen during tone training is due to a learning deficit and cannot be attributed to a deficit in performance of the CR. The rats with complete lesions, therefore, failed to acquire associative plasticity during the 2,000 trials of tone conditioning.
A somewhat unexpected finding of the current experiment is that bilateral lesions of the medial auditory thalamic nuclei were not necessary to produce the conditioning impairment. It may be that the contralateral medial auditory thalamic nuclei are selectively engaged when a unilateral US is presented. Evidence for unilateral neural processing with a unilateral US has been demonstrated recently for measures of fear conditioning in which a periorbital shock was used as the US (Blair et al., 2005). Fear conditioning with a bilaterally applied US such as foot shock requires the amygdala in both hemispheres, but fear conditioning with a unilateral US such as periorbital shock requires only the contralateral amygdala (Blair et al., 2005). Alternatively, unilateral thalamic contributions to auditory associative learning may be unique to eyeblink conditioning. A follow-up study to the current experiment will examine the effects of unilateral medial auditory thalamic lesions on fear and eyeblink conditioning with a within-subjects design.
The precise role of the medial auditory thalamic nuclei in relaying acoustic CS information to the cerebellum in eyeblink conditioning is not known. It is possible that two pathways are involved in relaying the necessary and sufficient auditory CS information to the pontine nuclei: the well-documented short-latency projection from the cochlear nuclei to the pons and a longer latency parallel pathway through the medial auditory thalamus and directly or indirectly to the pons (Figure 4). The medial auditory thalamic nuclei project directly to the pontine nuclei (Campolattaro & Freeman, raw data) and indirectly through a feedback projection to the inferior colliculus, which then projects to the pons (Kawamura, 1975). Another indirect pathway from the auditory cortex to the pontine nuclei is probably not an essential component of the auditory CS pathway because decortication does not abolish conditioning using an auditory CS (Oakley & Russell, 1972, 1977). The longer latency pathway could modulate activity in the short-latency pathway or be part of the essential auditory CS pathway. In the absence of physiological data, it is difficult to form a plausible hypothesis for how the longer latency pathway could modulate the short-latency pathway, but a reasonable possibility is that the longer latency pathway increases the gain of the short-latency pathway. Accordingly, lesions of the longer latency pathway would diminish gain in the short-latency pathway and thereby decrease its input to the cerebellum. It is also possible that lesions of the medial auditory thalamus impair conditioning by producing abnormal activity in the eyeblink conditioning circuitry, which has been shown following lesions of the septum (Solomon, Solomon, Vander Schaaf, & Perry, 1983). Alternatively, the medial auditory thalamic nuclei could be part of the essential auditory CS pathway. The present data do not distinguish between these possible roles for the medial auditory thalamus in eyeblink conditioning.
Figure 4.
(A) The short-latency auditory CS pathway for eyeblink conditioning, which includes the cochlear nuclei (CN), the pontine nuclei (PN), and their mossy fiber inputs to the cerebellar cortex (CTX) and interpositus nucleus (IPN). (B) A possible long-latency auditory CS pathway for eyeblink conditioning, which includes the inferior colliculus (IC), medial auditory thalamic nuclei (MATN), and their projections to the PN.
Although the current findings suggest that the auditory CS pathway is more complex than previously thought, it appears to be simpler than the visual CS pathway. Bilateral lesions of the lateral geniculate or pretectal nuclei or superficial layers of the superior colliculus did not prevent acquisition of nictitating membrane conditioning with a light CS (whole-field illumination) in rabbits (Koutalidis, Foster, & Weisz, 1988). Combined bilateral lesions of these nuclei, however, completely blocked acquisition of conditioning to a light CS but did not impair acquisition to a tone CS. The effects of unilateral combined lesions of the visual nuclei have not been reported. The findings of the Koutalidis et al. (1988) study suggest that there are parallel pathways by which visual stimulation can reach the cerebellum. The auditory CS pathway might also involve parallel pathways to the cerebellum, with separate projections from the MGm, PIN, and SG providing sufficient input for conditioning. Most of the incomplete/ineffective lesions in the current experiment spared only the PIN or the SG, suggesting that each spared nucleus was capable of supporting conditioning to a tone CS. The main difference between the auditory and visual CS pathways seems to be that the latter has parallel projections from collicular and thalamic nuclei to the pons, whereas the former probably includes a serial circuit from the inferior colliculus to the medial auditory thalamic nuclei and then parallel thalamic inputs to the pons. Neuroanatomical tract tracing and neurophysiological studies are needed to conclusively determine whether the auditory CS pathway involves parallel inputs to the pons from the MGm, PIN, and SG that are independently sufficient for auditory eyeblink conditioning.
Auditory CS pathways for eyeblink conditioning and fear-motivated conditioning may be more similar than previously thought. The auditory CS pathway for fear and eyeblink conditioning includes the medial auditory thalamic nuclei. The inferior colliculus is an essential source of auditory input to the medial auditory thalamic nuclei in fear-motivated conditioning (Heldt & Falls, 2003; LeDoux et al., 1983; LeDoux et al., 1990). It is likely, therefore, that the inferior colliculus is also necessary for eyeblink conditioning. Future studies will examine the neuranatomical connections among the medial auditory thalamic nuclei, inferior colliculus, and the eyeblink conditioning circuitry. A better understanding of the neural substrates that these different forms of associative learning have in common is important for elucidating neural mechanisms that underlie behavioral adaptations to aversive stimuli.
Acknowledgments
This work was supported by National Institute of Mental Health Grant MH065483 and National Institute of Neurological Disorders and Stroke Grant NS038890.
References
- Bao S, Chen L, Thompson RF. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behavioral Neuroscience. 2000;114:254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Birt D, Olds M. Associative response changes in lateral midbrain tegmentum and medial geniculate during differential appetitive conditioning. Journal of Neurophysiology. 1981;46:1039–1055. doi: 10.1152/jn.1981.46.5.1039. [DOI] [PubMed] [Google Scholar]
- Blair HT, Huynh VK, Vaz VT, Van J, Patel RR, Hiteshi AK, Lee JE, Tarpley JW. Unilateral storage of fear memories by the amygdala. Journal of Neuroscience. 2005;25:4198–4205. doi: 10.1523/JNEUROSCI.0674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. Experimental Brain Research. 1994;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Brodal A. Neurological anatomy. Oxford University Press; New York: 1981. [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. (raw data). [Medial auditory thalamic projections to the basal pons].
- Disterhoft JF, Olds J. Differential development of conditioned unit changes in thalamus and cortex of rat. Journal of Neurophysiology. 1972;35:665–679. doi: 10.1152/jn.1972.35.5.665. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: Receptive field plasticity in the medial division of the medial geniculate body. Behavioral Neuroscience. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Rabinak CA. Eyeblink conditioning in rats using pontine stimulation as a conditioned stimulus. Integrative Physiological and Behavioral Science. 2004;39:180–191. doi: 10.1007/bf02734438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learning and Memory. 2005;12:255–259. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M, Saltwick SE, Miller JD. Conditioning and reversal of short-latency multiple-unit responses in the rabbit medial geniculate nucleus. Science. 1975;189:1108–1109. doi: 10.1126/science.1162365. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Sears LL, Steinmetz JE. Possible CS and US pathways for rabbit eyelid conditioning: Electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral and Neural Biology. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Falls WA. Destruction of the inferior colliculus disrupts the production and inhibition of fear conditioned to an acoustic stimulus. Behavioural Brain Research. 2003;144:175–185. doi: 10.1016/s0166-4328(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. The Journal of Physiology. 1994;476:77–87. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Kawamura K. The pontine projection from the inferior colliculus in the cat: An experimental anatomical study. Brain Research. 1975;95:309–322. doi: 10.1016/0006-8993(75)90109-2. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ. A layered network model of associative learning: Learning to learn and configuration. Psychological Review. 1988;95:411–433. doi: 10.1037/0033-295x.95.4.411. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Freeman JH, Jr., Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D. Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of Sciences. 2002;99:13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson JK, Thompson RF. Projections from the auditory cortex to the pontine nuclei in the rabbit. Behavioural Brain Research. 1993;56:23–30. doi: 10.1016/0166-4328(93)90019-m. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson RF. Conditioning using a cerebral cortical conditioned stimulus is dependent on the cerebellum and brain stem circuitry. Behavoral Neuroscience. 1992;106:509–517. doi: 10.1037//0735-7044.106.3.509. [DOI] [PubMed] [Google Scholar]
- Koutalidis O, Foster A, Weisz DJ. Parallel pathways can conduct visual CS information during classical conditioning of the NM response. Journal of Neuroscience. 1988;8:417–427. doi: 10.1523/JNEUROSCI.08-02-00417.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. Journal of Neuroscience. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. Journal of Neuroscience. 1983;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency-specific receptive field plasticity in the medial geniculate body induced by Pavlovian fear conditioning is expressed in the anesthetized brain. Behavioral Neuroscience. 1992;106:484–497. doi: 10.1037//0735-7044.106.3.484. [DOI] [PubMed] [Google Scholar]
- Love JA, Scott JW. Some response characteristics of cells of the magnocellular division of the medial geniculate body of the cat. Canadian Journal of Physiology and Pharmacology. 1969;47:881–888. doi: 10.1139/y69-145. [DOI] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. The Journal of Neuroscience. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain Research. 1987;403:89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- McCabe PM, McEchron MD, Green EJ, Schneiderman N. Electrolytic and ibotenic acid lesions of the medial subnucleus of the medial geniculate prevent the acquisition of classically conditioned heart rate to a single acoustic stimulus in rabbits. Brain Research. 1993;619:291–298. doi: 10.1016/0006-8993(93)91623-z. [DOI] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Simultaneous single unit recording in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit's classically conditioned heart rate. Brain Research. 1995;682:157–166. doi: 10.1016/0006-8993(95)00331-j. [DOI] [PubMed] [Google Scholar]
- Mihailoff GA. Cerebellar nuclear projections from the basilar pontine nuclei and nucleus reticularis tegmenti pontis as demonstrated with PHA-L tracing in the rat. Journal of Comparative Neurology. 1993;330:130–146. doi: 10.1002/cne.903300111. [DOI] [PubMed] [Google Scholar]
- Nowak AJ, Kehoe EJ, Macrae M, Gormezano I. Conditioning and reflex modification of the rabbit nictitating membrane response using electrical stimulation in auditory nuclei. Behavioural Brain Research. 1999;105:189–198. doi: 10.1016/s0166-4328(99)00073-x. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Neocortical lesions and Pavlovian conditioning. Physiology & Behavior. 1972;8:915–926. doi: 10.1016/0031-9384(72)90305-8. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Subcortical storage of Pavlovian conditioning in the rabbit. Physiology & Behavior. 1977;18:931–937. doi: 10.1016/0031-9384(77)90203-7. [DOI] [PubMed] [Google Scholar]
- O'Connor KN, Allison TL, Rosenfield ME, Moore JW. Neural activity in the medial geniculate nucleus during auditory trace conditioning. Experimental Brain Research. 1997;113:534–556. doi: 10.1007/pl00005605. [DOI] [PubMed] [Google Scholar]
- Olds J, Disterhoft JF, Segal M, Kornblith CL, Hirsh R. Learning centers of rat brain mapped by measuring latencies of conditioned unit responses. Journal of Neurophysiology. 1972;35:202–219. doi: 10.1152/jn.1972.35.2.202. [DOI] [PubMed] [Google Scholar]
- Patterson MM. Classical conditioning of the rabbit's (Oryctolagus cuniculus) nictitating membrane response with fluctuating ISI and intracranial CS. Journal of Comparative and Physiological Psychology. 1970;72:193–202. doi: 10.1037/h0029463. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Evinger C. Role of cerebellum in adaptive modification of reflexive blinks. Learning and Memory. 1997;4:77–87. doi: 10.1101/lm.4.1.77. [DOI] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. Journal of Neuroscience. 2001;21:270–278. doi: 10.1523/JNEUROSCI.21-01-00270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Weinberger NM. Differential plasticity of morphologically distinct neuron populations in the medial geniculate body of the cat during classical conditioning. Behavioral Biology. 1978;22:275–301. doi: 10.1016/s0091-6773(78)92351-9. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Futami T, Izawa R. Axon collaterals of mossy fibers from the pontine nucleus in the cerebellar dentate nucleus. Journal of Neurophysiology. 1992;67:547–560. doi: 10.1152/jn.1992.67.3.547. [DOI] [PubMed] [Google Scholar]
- Smith MC, Coleman SR, Gormezano I. Classical conditioning of the rabbit's nictitating membrane response at backward, simultaneous, and forward CS–US intervals. Journal of Comparative and Physiological Psychology. 1969;2:226–231. doi: 10.1037/h0028212. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Solomon SD, Vander Schaaf E, Perry HE. Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science. 1983;220:329–331. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE. Classical nictitating membrane conditioning in rabbits with varying interstimulus intervals and direct activation of cerebellar mossy fibers as the CS. Behavioural Brain Research. 1990;38:97–108. doi: 10.1016/0166-4328(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Sengelaub DR. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits: I. Anatomical evidence for direct projections from the pontine nuclei to the cerebellar interpositus nucleus. Behavioral and Neural Biology. 1992;57:103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Supple WF, Jr., Kapp BS. Response characteristics of neurons in the medial component of the medial geniculate nucleus during Pavolvian differential fear conditioning in rabbits. Behavioral Neuroscience. 1989;6:1276–1286. doi: 10.1037//0735-7044.103.6.1276. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The search for the engram. American Psychologist. 1976;31:209–227. doi: 10.1037//0003-066x.31.3.209. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annual Review of Psychology. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Wepsic JG. Multimodal sensory activation of cells in the magnocellular medial geniculate nucleus. Experimental Neurology. 1966;15:299–319. doi: 10.1016/0014-4886(66)90053-7. [DOI] [PubMed] [Google Scholar]