Abstract
The role of the perirhinal cortex in inhibitory eyeblink conditioning was examined. In Experiment 1, rats were given lesions of the perirhinal cortex or control surgery and subsequently trained with a feature-negative discrimination procedure followed by summation and retardation tests for conditioned inhibition. Perirhinal cortex lesions impaired, but did not prevent acquisition of feature-negative discrimination. Results from the summation test showed that rats with perirhinal cortex lesions could not generalize feature-negative discrimination to a new stimulus. There were no group differences during the retardation test. Experiment 2 showed that lesions of the perirhinal cortex did not impair simple excitatory conditioning. Experiment 3 showed that perirhinal cortex lesions had no effect on acquisition of a simple tone-light discrimination. The results suggest that the perirhinal cortex plays a role in eyeblink conditioning when using discrimination procedures involving overlapping stimuli.
Keywords: Eyeblink conditioning, Eyelid conditioning, Learning, Cerebellum, Conditioned inhibition
1. Introduction
Eyeblink classical conditioning has been useful for studying the behavioral and neurobiological mechanisms of associative learning (Christian & Thompson, 2003; Gormezano, Kehoe, & Marshall, 1983). Evidence accumulated from lesion, stimulation, inactivation, and unit recording experiments has localized the underlying neural substrates for delay eyeblink conditioning to the cerebellum and interconnected brainstem nuclei (Christian & Thompson, 2003). However, little is currently known about the neural mechanisms of inhibitory eyeblink conditioning.
Conditioned inhibition can be established by presenting intermixed trials in which a conditioned stimulus (CS) is paired with an unconditioned stimulus (US) on half of the trials (A+) and an additional CS (i.e., the putative inhibitory stimulus; X) is presented within a stimulus compound without the US (XA−) on the other trials. Because the US only occurs in the presence of A and not during the compound stimulus, discriminative responding between A+ and XA− trials depends on whether X is present during a trial (Rescorla, 1969).
Recent work has examined the role of the cerebellum in the acquisition and retention of inhibitory eyeblink conditioning in rats. Muscimol inactivation of the cerebellar nuclei and overlying cortex ipsilateral to the conditioned eye blocks acquisition of excitatory conditioning, but has no effect on acquisition of inhibitory conditioning (Freeman, Halverson, & Poremba, 2005). Additionally, Nolan and Freeman (2005) showed that depleting Purkinje cells throughout the cerebellar cortex using the immunotoxin OX7-saporin retards, but does not prevent acquisition of inhibitory eyeblink conditioning in rats. The findings of the Nolan and Freeman (2005) study suggest that Purkinje cells that were not inactivated in the Freeman et al. (2005) study play a role in conditioned inhibition. However, the failure to completely block acquisition of conditioned inhibition with widespread cerebellar cortical damage indicates that brain areas other than the cerebellum may be necessary for acquiring inhibitory eyeblink conditioning.
Previous studies found that large aspirations of the neo-cortex do not prevent acquisition or retention of inhibitory eyeblink conditioning in rabbits (Moore, Yeo, Oakely, & Steele, 1980; Yeo, Hardiman, Moore, & Steele-Russell, 1983). However, all of the rabbits in these studies exhibited sparing of the ventral temporal lobe, including the perirhinal and entorhinal cortices. The perirhinal cortex receives information from numerous sensory areas important for mnemonic encoding and retrieval (Burwell & Amaral, 1998a, 1998b; Eacott, 1998; Wiig & Burwell, 1998). Even though lesions of the perirhinal cortex do not affect serial A+/X → A− discrimination in fear-potentiated startle in rats (Falls, Bakken, & Heldt, 1997), they have been shown to disrupt performance in both rats and non-human primates on tasks requiring discrimination when there is perceptual overlap between stimulus items that are to be discriminated or mnemonic interference (Buckley, Booth, Rolls, & Gaffan, 2001; Buckley & Gaffan, 1997; Buckley & Gaffan, 1998; Bussey, Saksida, & Murray, 2002, 2003; Gilbert & Kesner, 2003). Perirhinal cortex may be involved in inhibitory eyeblink conditioning because the standard feature-negative discrimination (FND) procedure (A+ vs. XA−) uses a common stimulus (A) during both types of trials, resulting in a high degree of stimulus overlap between the two types of trials.
The present experiments examined the role of the perirhinal cortex in excitatory and inhibitory eyeblink conditioning. Experiment 1 assessed the effects of perirhinal cortex lesions on feature-negative discrimination (A+/XA−) and tests of conditioned inhibition. Experiment 2 assessed the effects of perirhinal cortex lesions on excitatory eyeblink conditioning. Experiment 3 assessed the effects of perirhinal cortex lesions on simple discrimination learning (A+/X−).
2. Experiment 1
Perirhinal cortex is necessary for discrimination learning when there is perceptual overlap or mnemonic interference between the items to be discriminated (Buckley et al., 2001; Buckley & Gaffan, 1997, 1998; Bussey et al., 2002, Bussey, Saksida, & Murray, 2003; Gilbert & Kesner, 2003). Lesions of the perirhinal cortices may, therefore, impair acquisition of feature-negative discrimination because a common stimulus (A) is used in both types of trials (A+ and XA−). In Experiment 1, rats received electrolytic lesions of the perirhinal cortex or control surgery and were given 10 days of A+/XA− training followed by summation and retardation tests to assess inhibition acquired to X.
2.1. Materials and methods
2.1.1. Subjects
Subjects were 13 male Long Evans rats (200−250 g), approximately 150 days old at the beginning of the experiment. The rats were housed in Spence Laboratories of Psychology at the University of Iowa with a 12-h light–dark cycle, with light onset at 07:00 am.
2.1.2. Surgery
One week prior to training, rats were removed from their home cage and anesthetized by an i.p. injection of sodium pentobarbital (80 mg/kg). An i.p. injection of atropine sulfate (0.45 mg/kg) was administered to reduce respiratory tract secretions. After the onset of anesthesia, rats received either perirhinal cortex or control lesions. Perirhinal cortex lesions were made by passing 2 mA of DC current for 10 s at three sites in each hemisphere. The stereotaxic coordinates for drilling the skull holes were 3.3, 4.8, and 6.3 mm posterior to bregma and ±5.0 mm lateral to midline. An insect pin insulated with expoy, except for 1 mm at the tip, was lowered at each skull hole 8.5−9.0 mm below the skull surface angled at 12° from vertical. The same procedure was used for making control lesions, except no current was applied during surgery. Skull holes were sealed with bone-wax. The rats were then fitted with differential electromyograph (EMG) electrodes that were implanted in the left upper eyelid muscle (orbicularis oculi) and a ground electrode was attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins held in a plastic connector, which was secured to the skull with dental acrylic. A bipolar stimulating electrode (for delivering the shock US) was implanted subdermally, immediately caudal to the left eye. The bipolar electrode terminated in a plastic connector that was secured to the skull with dental acrylic.
2.1.3. Conditioning apparatus
The conditioning apparatus consisted of four small-animal sound attenuation chambers (BRS/LVE, Laurel, MD). Within each sound attenuation chamber was a small-animal operant chamber (BRS/LVE, Laurel, MD) where the rats were kept during conditioning. One wall of the operant chamber was fitted with two speakers. The back wall of the sound attenuating chamber was equipped with a small house light and an exhaust fan. A light bulb (for delivering the light CS) was located on the back wall of the sound attenuating chamber, positioned directly behind the operant chamber. The electrode leads from the rat's headstage were connected to peripheral equipment and a desktop computer. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC). One circuit delivered a shock stimulus (1−2 mA, DC constant current) through a stimulus isolator (Model number 365A, World Precision Instruments, Sarasota FL). EMG activity was recorded differentially, filtered (500−5000 Hz) and integrated by equipment (JSA Designs, Raleigh, NC) described in other reports (Freeman et al., 2005; Nicholson & Freeman, 2002; Nicholson, Sweet, & Freeman, 2003).
2.1.4. Conditioning procedure
The experimental design is illustrated in Table 1. Rats were assigned to either the perirhinal cortex lesion (n = 7) or control group (n = 6). All rats in this experiment received two phase of training (phases 1 and 2) and two phases of testing (phases 3 and 4). This four-phase procedure was designed to establish a light CS as a conditioned inhibitor. For all rats in this study, Tone 1 (T1) was a 2-kHz tone; Tone 2 (T2) was a 8-kHz tone; the light CS was a 6 W light; and the US was a 1−2.0 mA periorbital shock. T1 and T2 were each presented at 85 dB. A plus sign (+) after a CS indicates that it was paired with the US; a minus sign (−) indicates that it was not paired with the US. The duration of each CS was 400 ms, where the onset of the US coincided with the offset of a CS.
Table 1.
Design summary for Experiment 1
| Phase 1 (FND) | Phase 2 (Acq) | Phase 3 (Summ) | Phase 4 (Ret) |
|---|---|---|---|
| A+/XA- | B+ | B-/XB- | X+ |
Note. FND, feature-negative discrimination; Acq, acquisition; Summ, summation; Ret, retardation; B, 8 kHz tone conditioned stimulus (CS), A, 2 kHz tone CS; X, 6 W light CS; + indicates that the shock unconditioned stimulus (US) was presented; — indicates no shock US.
On each of the ten days of phase 1 training, all rats received a 100-trial session of feature-negative discrimination training, which consisted of 50 T1+ and 50 LT1− trials. This procedure has been used in previous studies to establish conditioned inhibition (Freeman & Nicholson, 1999; Nicholson & Freeman, 2002; Nolan, Nicholson, & Freeman, 2002; Freeman et al., 2005). The compound stimulus was presented simultaneously, where onsets and offsets for T1 and L occurred together. Trials were separated by a variable intertrial interval that averaged 30 s (range 18−42 s). Phase 2 training was conducted the next day. On this day, all rats received one 100-trial session of T2+ training. Phase 3 (summation test) consisted of 30 T2− and 30 LT2− trials. Phase 4 training (retardation test) consisted of one 100-trial session of L+. The summation and retardation tests are standard procedures used to show that a feature-negative stimulus acts as a conditioned inhibitor (Rescorla, 1969). If the feature-negative stimulus is a conditioned inhibitor, it will be capable of suppressing and responding to an excitatory stimulus not used during the initial discrimination training (the summation test) as well as showing relative difficulty during conditioning when subsequently used as an excitatory stimulus (the retardation test).
Conditioned responses (CRs) were defined as EMG activity that exceeded a threshold of 0.4 U (amplified and integrated units in volts) the baseline mean during the CS period, after 80 ms. CRs during CS–US trials were defined as responses obtained after the baseline period but before the onset of the US, while conditioned responses during compound stimulus trials were defined as responses that occurred between CS onset and the end of the stimulus compound.
2.1.5. Data analysis
The percentage of CRs during feature-negative discrimination training (phase 1), the summation test (phase 3), and the retardation test (phase 4) was analyzed using percentage of CRs by repeated measures ANOVA. Significant differences were evaluated by Tukey's honestly significant difference (HSD) test (all p's < .05).
2.1.6. Histology
Rats with perirhinal cortex lesions were deeply anesthetized with an overdose of sodium pentobarbital (90 mg/kg) and transcardially perfused with 0.9% saline followed by 3% formalin. The brains were removed from the skull and post-fixed in 0.1 M phosphate buffered sugared saline, and subsequently sectioned at 50 μm on a sliding microtome (American Optical, Buffalo, NY). Every fifth section in the series was mounted on a slide, stained with cresyl violet or thionin, and examined for lesion placement.
2.2. Results
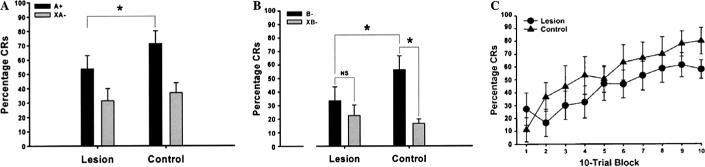
Lesions of the perirhinal cortex impaired acquisition of feature-negative discrimination in during phase 1 (Fig. 1). An ANOVA of the CR percentage data with trial-type, session, and surgery group factors revealed a significant main effect of surgery group, F(1, 11) = 5.00, p < .05 (Fig. 2A). This effect was due to higher responding during A+ trials in the control group. An ANOVA of the CR percentage data for the lesion group revealed a significant interaction of the trial-type and session factors, F(9, 108) = 5.29, p < .001. Post hoc tests showed that the interaction was due to a higher CR percentage on A+ trials during sessions 4−10 (Fig. 1). An ANOVA of the CR percentage data for the control group also revealed a significant interaction of the trial-type and session factors, F(9, 90) = 2.75, p < .01. Post hoc tests showed that the interaction was due to a higher CR percentage on A+ trials during sessions 2−10, showing that rats in control group acquired discrimination more quickly than rats in the lesion group (Fig. 1). There was no systematic relationship between the size of the lesion and performance during phase 1.
Fig. 1.
Mean (±SEM) percentage of conditioned responses (CRs) for rats that received lesions (circles) or control surgery (triangles) during 100-trial sessions of feature-negative discrimination training. Feature negative discrimination training included trials with a conditioned stimulus (CS) paired with an unconditioned stimulus (US) (A+, black symbols) and trials with a simultaneous compound stimulus that was not paired with the US (XA−, white symbols).
Fig. 2.
Mean (±SEM) percentage of conditioned responses (CRs) during feature-negative discrimination training (A) and summation testing (B) for rats that received lesion or control surgery. Mean (±SEM) percentage of CRs during retardation testing (C) for rats that received lesion (black dots) or control surgery (black triangles). The asterisks indicate statistically significant differences (p < .05). NS indicates non-significant differences in responding.
Perirhinal cortex lesions had no statistical effect on acquisition of eyeblink conditioning in phase 2 of training (data not shown). The mean CR percentage during phase 2 training for rats that received lesion or control surgery were 79.3% and 69.5%, respectively.
The results for the summation test are shown in Fig. 2B. Rats with perirhinal cortex lesions did not show evidence of a summation effect. A significant interaction of trial-type and surgery group factors, F(1, 11) = 11.70, p < .01, was due to a higher percentage of responding to B− trials in the control group, relative to the XB− trials in the control group and B− trials in the lesion group. No significant difference was found in responding to B− and XB− trial types in the lesion group.
Fig. 2C shows the results from retardation testing for both surgery groups. The largest retardation effect is typically seen early in training; therefore, the 100-trial session of retardation training was divided into ten 10-trial blocks for analysis. There were no differences during retardation training between lesion and control groups. An ANOVA of block and surgery group factors revealed a main effect of the block factor, F(9, 99), p < .001, which was due to the increase of CRs across the 10-trial blocks for both groups.
2.2.1. Lesions
Bilateral damage in the perirhinal cortex was observed in all rats given lesions. A representative digital photograph of perirhinal cortex damage for one rat is shown in Fig. 3. Lesions were transcribed onto stereotaxic figures from Paxinos and Watson (1998) and the largest and smallest lesions are shown in Fig. 4. In most of the lesioned rats (4/7), damage also extended bilaterally to the most lateral portions of the lateral entorhinal cortex. The entorhinal cortical damage was minor in all but one rat (see largest lesion in Fig. 4) and the extent of entorhinal damage was not related to the magnitude of impairment during acquisition of feature-negative discrimination or during the summation test.
Fig. 3.
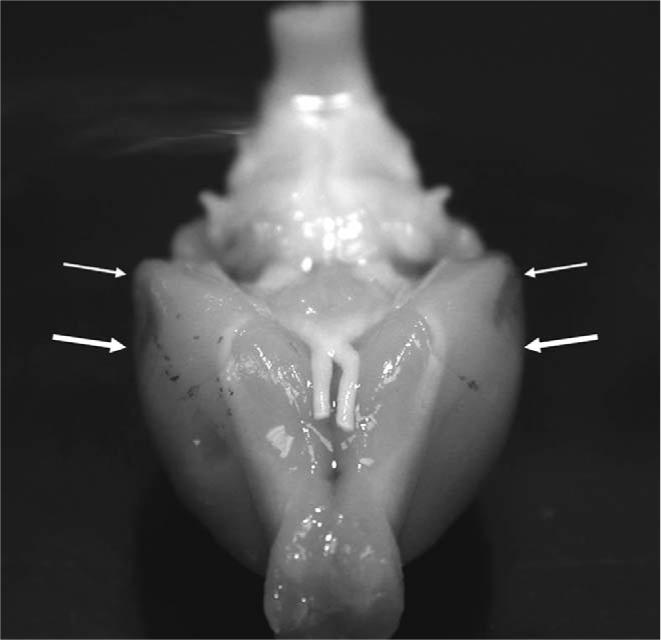
A digital photograph of the anteroventral surface of a representative rat brain with bilateral damage to the perirhinal cortex. Arrows indicate the location of the damage.
Fig. 4.

Drawing of three coronal sections showing the smallest (black regions) and largest lesions (gray regions). The values to the right of each section indicate the stereotaxic coordinates relative to bregma (Paxinos & Watson, 1998). Arrows indicate the boundaries of the perirhinal cortex, composed of areas 35 and 36 (adapted from Burwell and Amaral, 1998a, 1998b).
2.3. Discussion
Experiment 1 showed that perirhinal cortex lesions impair the rate and magnitude of eyeblink conditioning during acquisition of feature-negative discrimination. However, rats in the lesion and control groups achieved that same magnitude of discrimination by the end of feature-negative discrimination training (A+/XA−). The lesion-induced deficit during acquisition in feature-negative discrimination may be due to use of an overlapping stimulus during both types of trials (i.e., A). This finding is consistent with previous studies that showed that the perirhinal cortex is involved in making discriminations when the elements to be discriminated have a high degree of overlapping features (Buckley et al., 2001; Buckley & Gaffan, 1997, 1998; Bussey et al., 2002, 2003; Gilbert & Kesner, 2003).
The differences observed during the summation test suggest that rats in the lesion group may use a different mechanism for acquiring feature-negative discrimination (A+/XA−) than rats in the control group. The perirhinal and lateral entorhinal cortices may be necessary to generalize feature-negative discrimination to another excitatory stimulus. The failure to find a group difference during retardation testing suggests that the perirhinal and lateral entorhinal cortices are not necessary for acquiring inhibitory learning to X. Although not significant, the trend was for rats in the lesion surgery group to show a larger retardation effect than rats in the control surgery group. However, this finding may be due to weaker excitatory conditioning in the lesion group relative to the control group, as observed during feature-negative discrimination training and the summation test.
3. Experiment 2
Experiment 2 assessed whether the deficit in acquisition of feature-negative discrimination found in Experiment 1 was due to a general impairment in excitatory conditioning. Even though rats with perirhinal cortex lesions in Experiment 1 demonstrated high levels of excitatory conditioning, it is necessary to show the lesion-induced deficit in acquisition of feature-negative discrimination was not due to a general impairment in acquisition of excitatory conditioning. Impairment in excitatory conditioning could occur because the anatomical connections between the perirhinal cortex and amygdala are disrupted in rats with perirhinal cortex lesions. It has been shown that lesions of the amygdala slow, but do not prevent acquisition of excitatory eyeblink conditioning in rabbits and rats (Weisz, Harden, & Wiang, 1992; Neufeld & Mintz, 2001; Lee & Kim, 2004). An absence of impairment in excitatory conditioning might suggest that the perirhinal cortex is specifically involved in discrimination learning.
3.1. Materials and methods
The conditioning apparatus, surgical procedure, and histological methods used in Experiment 2 were the same as described in Experiment 1.
3.1.1. Groups
Rats were given either perirhinal cortex lesions (n = 18) or a control surgery (n = 17) and trained with six daily 100-trial sessions of T2+ conditioning.
3.2. Results
There were no group differences in CR percentage during acquisition of excitatory conditioning to T2+. The only significant effect found was a main effect of the session factor, which was due to an increase in the percentage of conditioned response for the lesion and control groups across the daily sessions of T2+ conditioning, F(5, 165) = 153.04, p < .001 (Fig. 5).
Fig. 5.
Mean (±SEM) percentage of conditioned responses (CRs) during excitatory conditioning for rats given control (black dots) or lesion (white dots) surgery.
3.3. Discussion
Experiment 2 found that perirhinal cortex lesions do not affect acquisition of excitatory conditioning. This finding suggests that the impairment in acquisition of feature-negative discrimination learning in Experiment 1 was not due to a general impairment in excitatory conditioning. The deficit in Experiment 1 may, therefore, be due to an impairment in learning discriminations with overlapping stimuli. However, it is possible that perirhinal cortex lesions produce a general deficit in discrimination learning in eyeblink conditioning regardless of whether there is stimulus overlap.
4. Experiment 3
Experiment 3 addressed whether the lesion-induced impairment in feature-negative discrimination found in Experiment 1 was caused by a general deficit in discrimination learning. Rats with perirhinal cortex lesions might have been impaired on feature-negative discrimination learning in Experiment 1 because they had a general impairment in discriminating between briefly (400 ms) presented stimuli. Rats that were given electrolytic lesions of the perirhinal cortex or control surgery were given simple discrimination training between light and tone CSs. A retardation test was then given in which the CS− was paired with the US.
4.1. Materials and methods
The methods regarding subjects, surgery, conditioning apparatus, data analysis, and histology were the same as in Experiments 1 and 2.
4.1.1. Groups and conditioning procedure
Rats with perirhinal cortex lesions (n = 5) or controls (n = 7) were given 10 sessions of simple tone/light discrimination training (CS+/CS−). The stimulus modalities were counterbalanced. Conditioning parameters were the same as in Experiments 1 and 2. Following discrimination training, these rats received a 100-trial session of presentations of the CS− paired with the US.
4.2. Results
Lesions of the perirhinal cortex did not impair simple discrimination or prevent CS− from becoming an inhibitory stimulus (Fig. 6). There were no statistical effects of stimulus type (i.e., tone or light) for animals that received simple CS+/CS− discrimination. The data were, therefore, collapsed across stimulus type for further analysis. Fig. 6 shows that discrimination developed across sessions in both groups and there were no significant differences between lesion and control groups. This observation was confirmed by an ANOVA of the CR percentage data, which revealed a significant interaction of the trial-type and session factors, F(9, 90) = 34.72, p < .001. The trial-type and session interaction was due to the development of a significantly greater percentage of CRs on CS+ trials compared to CS− trials in both groups. Fig. 6 also shows that acquisition of excitatory conditioning to the previous CS− did not differ between lesion and control groups.
Fig. 6.
Left, mean (±SEM) percentage conditioned responses (CRs) for rats that received perirhinal lesions (circles) or control surgery (triangles) during simple CS+/CS− discrimination training. Right, mean (±SEM) percentage CRs for rats that received perirhinal lesions (white dots) or control surgery (black dots) during retardation testing.
4.3. Discussion
Experiment 3 found that perirhinal cortex lesions do not produce a general impairment in discrimination or inhibition in eyeblink conditioning. Unpaired presentations of the CS− during simple discrimination were sufficient to produce inhibition during the retardation test in the lesion and control groups. The findings also indicate that lesions of the perirhinal cortex do not impair auditory or visual sensory functions that are necessary for processing CS related information. Additionally, the low rate of responding to the CS− in Experiment 3, when compared to XA− responding in Experiment 1, indicates that the salience of X did not completely overshadow A when they were presented together in a stimulus compound. The elevated rate in responding to the XA− compound found in Experiment 1 suggests that the rats generalized excitatory conditioning to the compound. This finding argues against the idea that the deficit in feature-negative discrimination found in Experiment 1 involved an overshadowing effect.
5. General discussion
Experiment 1 showed that lesions of the perirhinal cortex impair feature-negative discrimination. Lesion-induced impairments were found during acquisition of feature-negative discrimination and the summation test. Rats that received the lesions were slower to acquire discrimination than rats that received control surgery, but did acquire the same magnitude of discrimination by the end of feature-negative discrimination training. However, the rats that received the lesions were not able to generalize the discrimination to a different excitatory stimulus during the summation test. Most of the rats given lesions of the perirhinal cortex in Experiment 1 also sustained minor damage to the lateral entorhinal cortex, but the extent of entorhinal damage did not correlate with the magnitude of impairment during feature-negative discrimination training or during the summation test. Experiment 2 demonstrated that lesions of the perirhinal cortex do not impair acquisition of excitatory conditioning. As shown in Experiment 3, these lesions did not produce a general impairment in simple discrimination. Experiment 3 also showed that rats in both the lesion and control groups given simple discrimination did not differ in the magnitude of inhibition during training to establish the CS− as an excitatory stimulus.
The perirhinal cortex may play a role in feature-negative discrimination because it is necessary for resolving ambiguity in discriminations with perceptually overlapping features (Buckley & Gaffan, 1997, 1998; Bussey et al., 2002, 2003). Additionally, perirhinal cortex lesions impair performance in situations with a high degree of mnemonic interference among stimuli (Gilbert & Kesner, 2003). The lesion induced impairment found in the present study may, therefore, be attributed to difficultly in processing the inhibitory stimulus within the compound (XA−) as perceptually distinct from the excitatory stimulus (A+). Even though rats given the lesion surgery were able to acquire the same magnitude of discrimination by the end of feature-negative discrimination training, any inhibition that might have been acquired did not generalize to an excitatory stimulus during the summation test. This finding suggests that the mechanisms that were responsible for the acquisition of feature-negative discrimination in the lesion group in Experiment 1 may be different from those used by the control group. One possibility is that the rats with lesions were able to learn the feature-negative discrimination by processing the stimulus compound as a configural stimulus (Bussey et al., 2000; Kehoe & Gormezano, 1980). The feature-negative discrimination would, therefore, be more like a simple discrimination between A+ and a configural stimulus C− (i.e., the compound XA−) for the rats with lesions. If this is the case, they would have had difficulty showing discrimination during the summation test because the negative feature of the compound stimulus was presented with a new excitatory stimulus, which would be processed as a novel configural stimulus with no associative value. A prediction of this configural learning hypothesis is that rats with perirhinal cortex lesions should perform well on negative and positive patterning paradigms (Bussey et al., 2000).
The finding that rats with perirhinal cortex lesions were not impaired on simple discrimination learning suggests that the lesion-induced impairment on feature-negative discrimination was not due to a general deficit in the ability to perform stimulus discriminations. This finding is consistent with previous studies that showed that perirhinal cortex lesions do not impair simple discrimination learning and or associative conditioning in non-human primates and rats (Buckley & Gaffan, 1997; Buckley, Gaffan, & Murray, 1997; Lindquist, Jarrard, & Brown, 2004). Also, rats with perirhinal cortex lesions showed evidence of inhibition to the CS− when it was paired with the US during retardation testing in Experiment 3, suggesting that the lesion-induced impairment in feature-negative discrimination learning was not due the inability to acquire inhibitory learning.
A critical issue for understanding the neural mechanisms underlying inhibitory learning is to determine the pathways by which perirhinal and lateral entorhinal cortices can influence the eyeblink conditioning circuitry. Perirhinal cortical neurons probably influence the pathway that provides CS input to the cerebellum. The CS pathway in eyeblink conditioning includes the basal pontine nuclei and their mossy fiber projection to the cerebellar nuclei and cortex (Lewis, LoTurco, & Solomon, 1987; Steinmetz, Lavond, & Thompson, 1989; Steinmetz et al., 1987; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986; Hesslow, Svensson, & Ivarsson, 1999). The perirhinal cortex might influence CS input to the cerebellum through a monosynaptic projection to the basal pontine nuclei (Legg, Mercier, & Glickstein, 1989; R.D. Burwell, personal communication). The perirhinal and entorinal corticies also have strong anatomical connections with both the hippocampus and amygdala (Burwell, Witter, & Amaral, 1995; Suzuki, 1996). Amygdala lesions retard the rate of acquisition (Lee & Kim, 2004) and could play a role in feature-negative discrimination learning, although the effects of amygdala lesions on feature-negative discrimination in eyeblink conditioning have not been examined. In contrast, it has been shown that aspiration lesions of the entire hippocampus do not impair feature-negative discrimination in eyeblink conditioning with rabbits (Solomon, 1977). The perirhinal cortex may, therefore, influence the eyeblink circuitry indirectly through projections to the amygdala but probably not through projections to the hippocampus. The amygdala projects to the facial motor nucleus via the lateral tegmental area and might affect the eyeblink reflex directly during feature-negative discrimination learning (Choi, Lindquist, & Brown, 2001; Whalen & Kapp, 1991). Future studies will identify the neural pathway that is necessary for perirhinal interactions with the eyeblink conditioning circuitry.
It should be noted that because electrolytic lesions were used in the present study, any axonal projections that travel through the perirhinal and lateral entorhinal areas would have also been damaged. The lesion-induced deficits found in Experiment 1 could, therefore, be attributed to damage to the fibers of passage traveling through the perirhinal cortical region. Cortical projections to the amygdala and hippocampus, for example, could have been disrupted by the electrolytic lesions. Fibers of passage projecting to the hippocampus are probably not involved in conditioned inhibition because hippocampal lesions do not impair feature-negative discrimination (Solomon, 1977). It is possible, however, that fibers of passage projecting to the amygdala could play a role in feature-negative discrimination.
In conclusion, perirhinal cortex lesions impair feature-negative discrimination learning, but do not produce a general impairment in discrimination or inhibitory learning. The perirhinal cortex may play a role in feature-negative discrimination because it is necessary for resolving ambiguity and minimizing interference during discriminations with overlapping stimuli. A prediction of this hypothesis is that perirhinal cortex should impair feature-positive discrimination learning (Campolattaro & Freeman, 2005).
Footnotes
This research was supported by NIMH Grant MH065483 to J.H.F.
References
- Buckley MJ, Booth MCA, Rolls ET, Gaffan D. Selective perceptual impairments after perirhinal cortex ablation. The Journal of Neuroscience. 2001;21:9824–9836. doi: 10.1523/JNEUROSCI.21-24-09824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Impairment of visual object-discrimination learning after perirhinal cortex ablation. Behavioral Neuroscience. 1997;111:467–475. doi: 10.1037//0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Learning and transfer of object-reward associations and the role of the perirhinal cortex. Behavioral Neuroscience. 1998;112:15–23. doi: 10.1037//0735-7044.112.1.15. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D, Murray EA. Functional double dissociation between two inferior temporal cortical areas: perirhinal cortex versus middle temporal gyrus. Journal of Neurophysiology. 1997;77:587–598. doi: 10.1152/jn.1997.77.2.587. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rats: interconnectivity and connections with the entorhinal cortex. The Journal of Comparative Neurology. 1998a;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. The Journal of Comparative Neurology. 1998b;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. The perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings in the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Dias R, Redhead ES, Pearce JM, Muir JL, Aggleton JP. Intact negative patterning in rats with fornix or combined perirhinal and postrhinal cortex lesions. Experimental Brain Research. 2000;134:506–519. doi: 10.1007/s002210000481. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. European Journal of Neuroscience. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JF., Jr. Lesions of the perirhinal cortex impair simultaneous, but not serial feature positive discrimination. 2005. Society for Neuroscience Abstracts, 997.13
- Choi J, Lindquist DH, Brown TH. Amygdala lesions block conditioned enhancement of the early component of the rat eyeblink reflex. Behavioral Neuroscience. 2001;115:764–775. [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learning & Memory. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Eacott MJ. Acquisition and retention of visual discrimination learning after ablation of perirhinal cortex in the rat. Psychobiology. 1998;26:36–41. [Google Scholar]
- Falls WA, Bakken KT, Heldt SA. Lesions of the perirhinal cortex interfere with conditioned excitation but not with conditioned inhibition of fear. Behavioral Neuroscience. 1997;111:476–486. doi: 10.1037//0735-7044.111.3.476. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr., Halverson HE, Poremba A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. Journal of Neuroscience. 2005;25:889–895. doi: 10.1523/JNEUROSCI.4534-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr., Nicholson DA. Neuronal activity in the cerebellar interpositus and lateral pontine nuclei during inhibitory classical conditioning of the eyeblink response. Brain Research. 1999;833:225–233. doi: 10.1016/s0006-8993(99)01547-4. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learning & Memory. 2003;10:525–530. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning in the rabbit. In: Sprague JM, Epstein AN, editors. Progress in psychobiology and physiological psychology. Vol. 10. Academic Press; New York: 1983. pp. 197–275. [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Gormezano I. Configuration and combination laws in conditioning with compound stimuli. Psychological Bulletin. 1980;87:351–378. [PubMed] [Google Scholar]
- Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. Journal of Neuroscience. 2004;24:3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg CR, Mercier B, Glickstein M. Corticopontine projection in the rat: the distribution of labeled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. The Journal of Comparative Neurology. 1989;286:427–441. doi: 10.1002/cne.902860403. [DOI] [PubMed] [Google Scholar]
- Lewis JL, LoTurco JJ, Solomon PR. Lesions of the middle cerebellar peducle disrupt acquisition and retention of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1987;101:151–157. doi: 10.1037//0735-7044.101.2.151. [DOI] [PubMed] [Google Scholar]
- Lindquist DH, Jarrard LE, Brown TH. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. Journal of Neuroscience. 2004;24:3610–3617. doi: 10.1523/JNEUROSCI.4839-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Yeo CH, Oakely DA, Steele R. Conditioned inhibition of the nictitating membrane response in decorticate rabbits. Behavioural Brain Research. 1980;1:397–409. doi: 10.1016/0166-4328(80)90037-6. [DOI] [PubMed] [Google Scholar]
- Neufeld M, Mintz M. Involvement of the amygdale in classical conditioning of eyeblink response in the rat. Brain Research. 2001;889:112–117. doi: 10.1016/s0006-8993(00)03123-1. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr. Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116:22–36. [PubMed] [Google Scholar]
- Nicholson DA, Sweet JA, Freeman JH., Jr. Long-term retention of the classically conditioned eyeblink response in rats. Behavioral Neuroscience. 2003;117:871–875. doi: 10.1037/0735-7044.117.4.871. [DOI] [PubMed] [Google Scholar]
- Nolan BC, Freeman JH., Jr. Purkinje cell loss by OX7-Saporin impairs excitatory and inhibitory eyeblink conditioning. Behavioral Neuroscience. 2005;119:190–201. doi: 10.1037/0735-7044.119.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan BC, Nicholson DA, Freeman JH., Jr. Blockade of GABAA receptors in the interpositus nucleus modulates expression of conditioned excitation but not conditioned inhibition of the eye-blink response. Integrative Physiological & Behavioral Science. 2002;37:293–310. doi: 10.1007/bf02734250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat in stereotaxic coordinates. Academic Press; New York: 1998. [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Solomon PR. Role of the hippocampus in blocking and conditioned inhibition of the rabbit's nictitating membrane response. Journal of Comparative and Physiological Psychology. 1977;91:407–417. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy fiber stimulation CS. I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. The anatomy, physiology and functions of the perirhinal cortex. Current Opinion in Neurobiology. 1996;6:179–186. doi: 10.1016/s0959-4388(96)80071-7. [DOI] [PubMed] [Google Scholar]
- Weisz DJ, Harden DG, Wiang Z. Effects of amygdala lesions on reflex facilitation and conditioned response acquisition during nictitating membrane response conditioning in rabbit. Behavioral Neuroscience. 1992;106:262–273. doi: 10.1037//0735-7044.106.2.262. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS. Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behavioral Neuroscience. 1991;105:141–153. doi: 10.1037//0735-7044.105.1.141. [DOI] [PubMed] [Google Scholar]
- Wiig KA, Burwell RD. Memory Impairment on a delayed non-matching- to-position task after lesions of the perirhinal cortex in the rat. Behavioral Neuroscience. 1998;112:827–838. doi: 10.1037//0735-7044.112.4.827. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Moore JW, Steele-Russell I. Retention of conditioned inhibition of the nictitating membrane response in decorticate rabbits. Behavioral Brain Research. 1983;10:383–392. doi: 10.1016/0166-4328(83)90042-6. [DOI] [PubMed] [Google Scholar]