Abstract
Background
Hypoxia-inducible factor 1 (HIF)-1α is a transcription factor that functions as master regulator of mammalian oxygen homeostasis. In addition, recent studies identified a role for HIF-1α as transcriptional regulator during inflammation or infection. Based on studies showing that respiratory syncytial virus (RSV) is among the most potent biological stimuli to induce an inflammatory milieu, we hypothesized a role of HIF-1α as transcriptional regulator during infections with RSV.
Methodology, Principal Findings
We gained first insight from immunohistocemical studies of RSV-infected human pulmonary epithelia that were stained for HIF-1α. These studies revealed that RSV-positive cells also stained for HIF-1α, suggesting concomitant HIF-activation during RSV infection. Similarly, Western blot analysis confirmed an approximately 8-fold increase in HIF-1α protein 24 h after RSV infection. In contrast, HIF-1α activation was abolished utilizing UV-treated RSV. Moreover, HIF-α-regulated genes (VEGF, CD73, FN-1, COX-2) were induced with RSV infection of wild-type cells. In contrast, HIF-1α dependent gene induction was abolished in pulmonary epithelia following siRNA mediated repression of HIF-1α. Measurements of the partial pressure of oxygen in the supernatants of RSV infected epithelia or controls revealed no differences in oxygen content, suggesting that HIF-1α activation is not caused by RSV associated hypoxia. Finally, studies of RSV pneumonitis in mice confirmed HIF-α-activation in a murine in vivo model.
Conclusions/Significance
Taking together, these studies suggest hypoxia-independent activation of HIF-1α during infection with RSV in vitro and in vivo.
Introduction
A number of elegant studies, exemplified by those defining induction of the erythropoietin (EPO) gene [1], [2], have utilized multidisciplinary approaches to elucidate basic hypoxia-adaptive responses. Today, convincing evidence confirms a central role of hypoxia-inducible factor (HIF)-1 in mammalian oxygen homeostasis [3]–[6]. Such studies demonstrated that HIF-1 is composed of two subunits: constitutively expressed HIF-1β and oxygen-regulated HIF-1α. Under normoxic conditions, HIF-1α is subjected to hydroxylation on proline residues [7]. The modification is required for the binding of the von Hippel-Lindau (VHL) tumor suppressor protein, the recognition component of an E3 ubiquitin-protein ligase that targets HIF-1α for proteasomal degradation. Under hypoxic conditions, hydroxylation is inhibited and the VHL protein does not bind to HIF-1, eventually leading to stabilization of the alpha-subunit, heterodymerization, nuclear translocation and transcription of HIF-dependent genes.
For example, binding of HIF-1 to consensus domains in the erythropoietin promoter results in the transcriptional induction of HIF-1-bearing gene promoters [8]. A series of experiments by Wang and Semenza [9]–[11] and Maxwell et al. [12] demonstrated that reporter genes containing the erythropoetin enhancer were induced by hypoxia in a variety of cell types that did not normally produce erythropoitin. Subsequently, it was determined that HIF-1 is widely expressed and that consensus HIF-1 binding sequences exist in a number of genes other than that of erythropoitin, and were termed hypoxia responsive elements (HRE [8]). In particular, HIF-1 has been found to regulate multiple genes that include HRE in their promoter region, including vascular endothelial growth factor (VEGF), insulin-like growth factors (IGFs), their binding proteins [insuline-like growth factor binding proteins (IGFBPs)] and iron supply regulating genes [e.g. transferrin [13]]. Thus, the discovery of HIF-1 represented a major advance in the understanding of gene regulation by hypoxia. Such studies have led to an understanding that induction of HIF-1 responsive genes drives altered cellular metabolism, increased vascular mass and diameter and increased oxygen carrying capacity of the blood; all events which are conducive to an adaptive response to diminished oxygen supply [1], [14]–[16].
However, studies that are more recent have identified an additional role of HIF-1α as transcriptional regulator of inflammation and infection. For example, HIF-1α is essential for myeloid cell-mediated inflammation, bactericidal capacity of phagocytes [17] and mice with conditional knockouts of HIF-1α show profound impairment of myeloid cell aggregation, motility, invasiveness, and bacterial killing [18]. Moreover, studies of HIF-1α during infection with enterobacteriaceae revealed hypoxia-independent activation by bacterial siderophores [19]. Similarly, HIF-1α has been identified as key regulator of the inflammatory transcription factor NF-κB [20]. Other studies confirmed several parallels between the transcriptional regulation of hypoxia and inflammation/infection [21]-[27]. For example, a recent study suggests that NF-κB is a critical transcriptional activator of HIF-1α and that basal NF-κB activity is required for HIF-1 protein accumulation under hypoxia [28]. Similarly, studies of human pathogens have revealed that exposition of host cells to bacteria (e.g. Bartonella henselae) results in HIF-1α activation and VEGF secretion in vivo and in vitro [29]. Similar findings were reported when macrophages were infected with group B streptococci [17], [18]. Therefore, it is obvious that HIF-1 plays a central role in infections with human bacterial pathogens. This could have important medical implications in terms of the treatment of sepsis, as it has been shown that serum VEGF levels (known to be regulated via HIF-1) are dramatically increased in patients suffering from septicemia [30] or meningitis [31].
In addition, previous studies have found activation of HIF-1α during viral infections [32]. For example, previous studies have suggested a functional role of the hepatitis C virus in HIF stabilization [33]. Other studies found HIF-1α stabilization during hepatitis B infections [34] or during infections with the Eppstein-Barr virus [35]. Respiratory syncytial virus (RSV) is the major cause of serious lower respiratory disease in infancy and early childhood [36]. Bronchiolitis, the more severe clinical manifestation of RSV infection, is characterized by necrosis and sloughing of the respiratory epithelium and plugging of the small bronchioles with fibrin and mucus. As such, RSV is characterized by a particularly prominent inflammation of the pulmonary mucosa-both in natural and experimental infections [36]. In fact, RSV is among the most potent biological stimuli that induce the expression of inflammatory genes, including those encoding chemokines, and studies on mechanism(s) that control virus-mediated airway inflammation are currently areas of intense investigation [37]–[41]. In fact, a previous studies suggested that RSV-elicited release of nitric oxide could be associated with HIF-1α stabilization during RSV infection [42]. Therefore, we combined in vitro and in vivo approaches to study HIF-1α activation and gene-transcription during RSV infection.
Methods
Culture of epithelial cells
A549 cells (American Type Culture Collection, Wesel, Germany) were cultured as described previously [38]. In short, A549 were grown in F-12 HAM medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (Invitrogen, Karlsruhe, Germany) and 1% Antibiotic-Antimycotic-Solution (Sigma-Aldrich, Steinheim, Germany). L-Glutamin was adjusted to a total quantity of 2 mM. The cells were maintained under standard-conditions of 37°C in 20% O2 and 5% CO2.
Infection with RSV
Human RSV was purified by polyethylene glycol precipitation, followed by centrifugation on 35 to 65% discontinuous sucrose gradients as described previously [43], [44]. The virus was stored in aliquots at −80°C until use. Virus titers were determined by a methylcellulose plaque assay [45]. For experiments with inactivated RSV, the virus was exposed to an UV light source for 20 minutes as described previously [43]. A549 cells were infected when they reached 70–80% confluence, using different multiplicities of infection (MOI). The virus was added immediately after removal of the culture medium in a small amount of serum-free medium for 1 h. Additional media was added and the infection was continued for indicated time periods [38].
Protein Extraction
Supernatants were discarded and 200 µl Lysis-Buffer (1 mM Tris-HCl, 250 mM NaCl, 1 mM EDTA, Triton X 100 1%, NP40 1× Igepal Electrophoresis Reagent, Aprotinin 1 µg/ml, Leupeptin 1 µg/ml, Pepstatin 1 µg/ml, PMSF 1 mM and OV 1 mM) was added. After scraping and collecting into tubes, the cell-lysate was incubated at 4°C for 20 minutes on a rotator. Cell debris was removed by centrifugation at 13000 g for 15 minutes and discharged. Protein concentrations were determined using the BCATM Protein Assay Kit (Pierce, Bonn, Germany) according to the manufacturer's instructions.
Nuclear Protein Extraction
Nuclear Proteins were isolated from A549 cells using a modification of methods previously described [46]. In short, cells were lysed in 500 µl cold buffer A (10 mM Hepes-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM Dithiothreitol (DTT), 0.2 mM phenylmethylsulfonyl fluoride (PMSF)), scraped and collected into tubes and incubated for 15 min on ice. After adding 7,5 µl solution containing 10% NP40, vortexing for 3 seconds and incubation for three minutes on ice, the tubes were centrifuged for 2 minutes at 6000 g at 4°C. The cytoplasmic proteins in the supernatant were collected and flash-frozen. Next, the pellet was resuspended in 100 µl of cold buffer B (20 mM Hepes-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF). After centrifugation (12000 g at 4°C for 30 minutes), the supernatant was discarded and the pellet was resuspended in 50 µl of buffer C (25% glycerol, 20 mM Hepes-KOH, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, 2 mM benzamidine, 5 mg/ml leupeptin) and incubated for 45 minutes at 4°C. Cellular debris was removed by 5 minutes of centrifugation (6000 g at 4°C) and the supernatant was flash-frozen at −80°C. Protein concentrations were determined using the BCATM protein assay kit (Pierce, Bonn, Germany) as instructed by the manufacturer.
Western blotting
Proteins were diluted in radio-immuno precipitation assay (RIPA) buffer to equivalent protein concentrations. After adding 4× Laemmli sample buffer they were immediately heated for 10 minutes at 70°C, separated on a 12% polyacrylamide gel and transferred to a nitrocellulose membrane (Polyvinylidene Difluoride, Bio-Rad Laboratories, Inc., München, Germany). Rainbow (Amersham, Buckinghamshire, UK) and MagicMark (Invitrogen, Karlsruhe, Germany) were used for size analysis and blotting control. The membranes were blocked overnight at 4°C in TBS containing 0,05% Tween and supplemented with 3% BSA and 3% skimmed milk. The membranes were then incubated in 1∶500 COX2 goat polyclonal IgG (Santa Cruz, Heidelberg, Germany) or 1∶500 anti RSV mouse IgG (Acris, Hiddenhausen, Germany) or 1∶500 FN goat polyclonal IgG (Santa Cruz, Heidelberg, Germany) or 1∶1000 beta-Actin antibody (Cell Signaling, Danvers, MA) in blocking buffer. After three washes, membranes were incubated with horse-radish-peroxidase-labelled secondary antibodies (goat anti-rabbit or donkey anti-goat or goat anti-mouse 1∶1000; Santa Cruz, Heidelberg, Germany) for 45 minutes at room temperature. The wash was repeated and proteins were detected by enhanced chemiluminescence, using the Chemiluminescent Substrate Kit (Pierce, Bonn, Germany). Western immunoblotting for HIF1-alpha was performed using 1∶500 Anti-HIF-1α rabbit polyclonal IgG (upstate, Lake Placid, NY) following the manufacturers protocol without the use of Tween.
Enzyme-linked immunosorbent assay (ELISA) for determination of chemokines
Total protein samples of infected or non-infected A549 cells were tested for VEGF by use of a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA) as instructed by the manufacturer. In short, samples were added to a 96 well microtiter plate, which was coated with murine monoclonal antibody to VEGF. The unbound protein was removed by washing and an enzyme linked polyclonal antibody specific to VEGF was added. After additional washing, substrate solution was added and incubated for 20 min. The color-reaction was stopped with stop solution and the amount of VEGF was determined by optical density of the samples by comparing the standards at 450 nm using an ELISA reader.
Immunohistochemistry
A549-cells were cultured on glass slides (NalgeNuc International, Naperville, IL) and infected with RSV at a MOI of 3. After 24 hours, they were fixed and permeabilized for immunofluorescent staining using Cytofix/Cytoperm (PharMingen, BD-Bioscience, Heidelberg, Germany). After two washes with Perm/Wash-solution (PharMingen, BD-Bioscience, Heidelberg, Germany) the slides were blocked for 30 minutes with 5% skimmed milk in Perm/Wash-solution. Purified mouse anti-HIF1α Mab (BD Transduction Laboratories, BD-Bioscience, Heidelberg, Germany) and anti-RSV mouse IgG (Acris, Hiddenhausen, Germany) were diluted 1∶100 in Perm/Wash-solution and the slides were incubated for 30 minutes. Normal mouse and normal rabbit control IgG in a dilution of 1∶200 were used. After two washes with Perm/Wash-solution, the slides were incubated for 30 minutes with the secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 594 goat anti-mouse IgG, Invitrogen, Karlsruhe, Germany) in Perm/Wash. The slides were embedded with a reagent containing DAPI (Invitrogen, Karlsruhe, Germany) for staining of the nuclei. Fluorescence was visualized with a confocal laser scanning microscope (Leica, Bensheim, Germany).
Reverse Transcription Polymerase Chain Reaction Analysis
Realtime RT-PCR (iCycler; Bio-Rad Laboratories Inc., Hercules, California, USA), was used to verify COX2, FN-1, VEGF and CD73 transcript levels of RSV-infected A549 cells. After infection with RSV with an infection dose of MOI3 for 24 h, total RNA was isolated using the RNA II Kit (Macherey & Nagel, Düren, Germany) and real-time RT-PCR was performed as described previously [47]–[52]. The PCR reaction contained 10 pM each of the sense primer 5′-AAA CCT CAG CTC AGG ACT GC-3′ and the antisense primer 5′-GGC ACT AGC CTC TTT GCA TC-3′ for COX2, sense primer 5′-AAG GAA GGG GAA GAA CAG GA-3′ and the antisense primer 5′-GGC AGA GCT GAT GGA ATC TC-3′ for CD73, sense primer 5′-TTG CCT TGC TGC TCT ACC TC-3′ and the antisense primer 5′-AGC TGC GCT GAT AGA CAT CC-3′ for VEGF, sense primer 5′-AGG CTC AGC AAA TGG TTC AG-3′ and the antisense primer 5′-TCG GCT TCC TCC ATA ACA A-3′ for FN1. The primer set for COX2, FN1 and VEGF was amplified using increasing numbers of cycles of 95°C for 15 sec, 58°C for 30 sec, 72°C for 10 sec, and a final extension of 72°C for 1 minute. The primer set for CD73 was amplified using increasing numbers of cycles of 95°C for 15 sec, 60°C for 30 sec, 72°C for 10sec, and a final extension of 72°C for 1 minute. Human beta-actin (sense primer, 5′-GGT GGC TTT TAG GAT GGC AAG-3′; and antisense primer, 5′-ACT GGA ACG GTG AAG GTG ACA G-3′) was used as control.
Stable repression of HIF-1α by siRNA
Repression of HIF-1α by siRNA was achieved based on a modification of methods previously described [26], [53]–[56]. In short, a hairpin primer with the sequence 5′-ACCTCGCTGACCAGTTATGATTGT-GATCAAGAGTCACAATCATAACTGGTCAGCTT-3′ and 5′-CAAAAAGCTGACCAG-TTATGATTGTGACTCTTGATCACAATCATAACTGGTCAGCG-3′ corresponding to position 2666-2685 of the HIF1α gene was selected. A549-cells were transfected using electroporation, followed by selection with G418 (1 mg/ml). The control cell line was transfected with a non-specific control psiRNA-hH1 neoscr plasmid.
Blood Gas Analysis
Blood gas analysis was performed to assess oxygen partial pressure in supernatants of uninfected or infected A549 cells. The cells were cultured and infected at a MOI of 1 or 5. One hour after infection the cell-culture flasks were filled up with serum free media and were sealed gas-tight. Analysis of the supernatants was performed immediately after removal via the I-STAT Analyzer (Abbott, Wiesbaden, Germany) at different time points as described previously [19], [52].
Infection of mice with RSV and extraction of lung nuclear proteins
Female, 6- to 8-week-old BALB/c mice were purchased from Harlan (Houston, Texas, USA) and were housed in pathogen-free conditions in the animal research facility of the University Texas Medical Branch (UTMB), Galveston, Texas, in accordance with the National Institutes of Health and UTMB institutional guidelines for animal care. The Institutional Animal Care and Use Committee approved this protocol. Cages, bedding, food, and water were sterilized before use. Under light anesthesia, female, 6-8 weeks old BALB/c mice were infected intranasally with RSV at 1×107 plaque-forming units (PFUs), diluted in sterile PBS for a total inoculation volume of 50 µl. As mock treatment, control mice were inoculated in the same way with an equivalent volume of sucrose diluted in PBS. At the indicated time points after infection (12, 24 and 48 h) mice were anesthetized with an intraperitoneal injection of ketamine and xylazine before the thoracic cavity was opened [39]. Lungs were then removed, quick frozen in liquid nitrogen and stored at −80°C until nuclear protein was isolated. Nuclear proteins were isolated from the lung tissue using a modified method described by Bohrer and colleagues [46]. Lung tissue was homogenized in 5 ml ice-cold Buffer A (10 mM 2-hydroxyethyl-piperazine N′-2-ethanesulfonic acid [Hepes]–KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], 0.2 mM phemylmethyl sulfonyl fluoride [PMSF], 0.6% nonident P40 [NP-40]) and centrifuged at 350×g, 4°C for 30 seconds. The supernatant was kept on ice for 5 minutes and centrifuged for 5 minutes at 6,000×g at 4°C, and the pellet was resuspended in 200 µl Buffer B (10 mM Hepes–KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1.2 M sucrose, 0.5 mM DTT, 0.2 mM PMSF). After centrifugation (13,000×g, 4°C, 30 minutes), the pellet was resuspended in 100 µl Buffer C (20 mM Hepes–KOH, pH 7.9, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM ethylenediamine-tetraacetic acid, 0.5 mM DTT, 0.2 mM PMSF, 2 mM benzamidine, 5 µg/ml leupeptin, 25% glycerol), incubated on ice for 20 minutes, and centrifuged (6,000×g, 4°C, 2 minutes). The supernatant was quick frozen in aliquots at −80°C. HIF-1α stabilization was determined by Western blot analysis as described previously [56].
Statistical Analysis
Data collection and statistical analysis was performed using Microsoft Excel (Microsoft Office Professional Edition 2003) and Graph Pad Prism (GraphPad Software Inc., Prism 4 for Windows Version 4.03). All presented values were calculated as the mean from at least three separate experiments. The results in the control and the viral infection group were analyzed and compared by utilizing the Unpaired Student t Test and the Mann-Whitney Nonparametric Test. For all statistical analyses a P value<0.05 was considered significant.
Results
Immunolocalization of HIF-1α during RSV infection in vitro
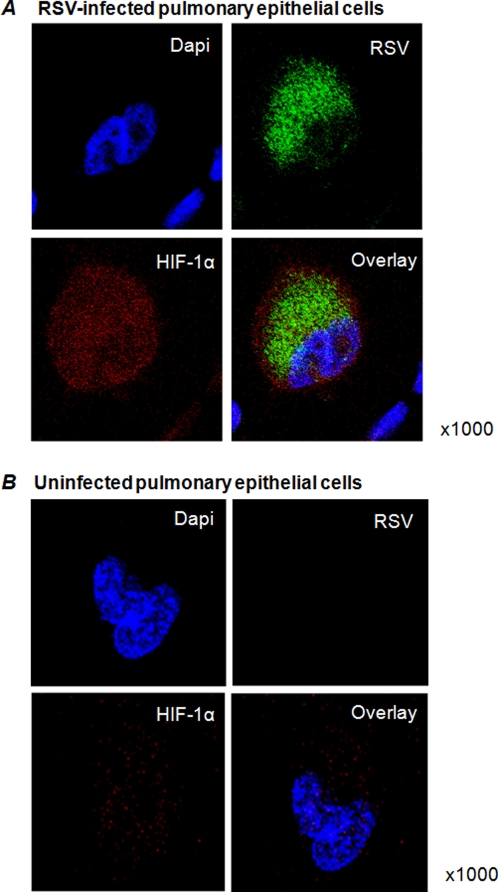
Recent evidence revealed that numerous parallels exist between inflammation and hypoxia, including changes in barrier function or inflammatory cell recruitment [5], [6], [55], [57]–[60]. In addition, recent studies have revealed that during infections with human pathogens, HIF-1α is activated [19], [29]. Previous studies demonstrated that RSV infections are characterized by a particularly prominent inflammation of the pulmonary mucosa-both in natural and experimental infections-and RSV is among the most potent biological stimuli that induce the expression of pro-inflammatory genes [36]. Therefore, we hypothesized that HIF-1α is stabilized during infections with RSV and may contribute to RSV-associated changes in gene expression. For our studies, we used A549 cells, a cell line derived from an alveolar cell carcinoma of the lung. As first step, we performed immunohistochemical staining with antibodies for RSV (green) or HIF-1α (red) using confocal laser scanning microscopy (Figure 1A). As counterstaining for the nuclei we used dapi staining (blue). As shown in Figure 1A, RSV infected cells also stained positive for HIF-1α, with localization of HIF-1α both in the cytosole and the nuclei. This is consistent with other studies demonstrating that HIF-1α is present in the cytosole and in the nucleus [61]. In contrast, uninfected A549 cells only had a very week signal for HIF-1α (Figure 1B). Isotype controls and staining of infected A549 cells with secondary antibody alone were negative (data not shown). Taken together, these data reveal that during RSV infection, HIF-1α accumulates in the cytosole and the nucleus of infected pulmonary epithelia, suggesting HIF-1α activation during RSV infection in vitro.
Figure 1. HIF-1α during RSV infected of pulmonary epithelia.
(A) ∼1,5×105 A549-cells were seeded on glass slides and infected with RSV (multiplicity of infection, MOI 3). After 24 h they were fixed, permeabilized and incubated with anti-HIF1α and anti-RSV IgG as primary antibodies. Alexa Fluor 488 and Alexa Fluor 594 were used for staining. In addition, slides were counter-stained with Dapi. The cells were visualized with confocal laser scanning microscopy. Uninfected cells were used as controls (B).
HIF-1α protein is stabilized during RSV infection
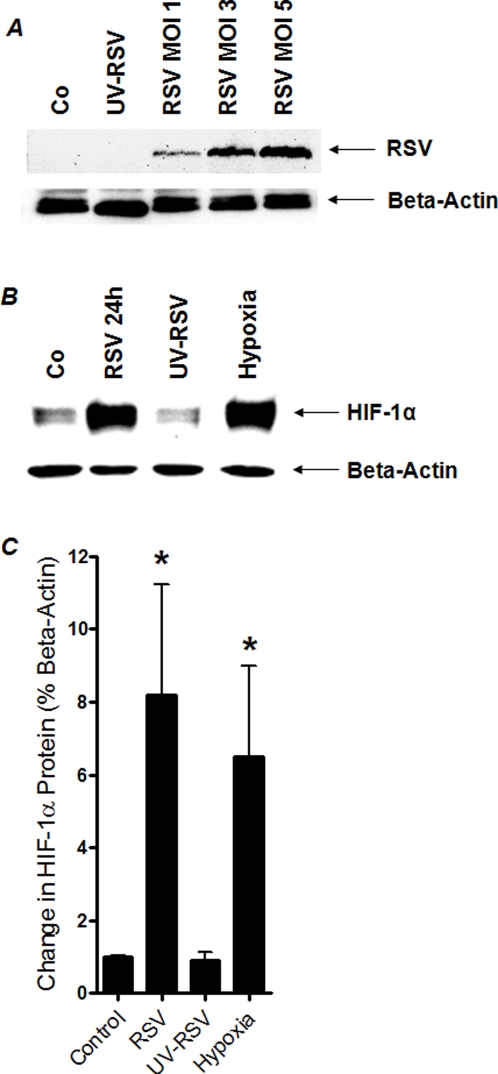
After having shown by confocal laser scanning microscopy that HIF-1α is stabilized during RSV infection, we next used Western blot analysis to confirm these results with a more quantitative approach. Here, we first confirmed successful infection of A549 cells using different infection doses (MOI1-5). As shown in Figure 2A, we found a close correlation of virus load with RSV G-protein after 24 h of infection (Figure 2A). In contrast, RSV pre-exposed to UV light source as previously described for RSV inactivation [43] showed no signal for intracellular RSV G-protein. Uninfected cells were used as negative control. As next step, we measured HIF-1α during RSV infection by Western blot analysis. These studies revealed an 8.2±5.2-fold increase in HIF-1α protein compared to non-infected A549 cells (Figure 2B and C, relative to beta-actin, MOI of 3). Interestingly, this HIF-response was completely attenuated when using UV-treated RSV virus (0.9±0.42-fold HIF-1α protein relative to beta-actin, Figure 2 B and C). In additional control studies, we exposed A549 cells to ambient hypoxia (2% oxygen over 24 h), which was associated with a robust increase in HIF-1α protein (6.5±4.3-fold increase in HIF-1α). Taken together, these studies reveal robust stabilization of HIF-1α during infection with life RSV in vitro.
Figure 2. HIF-1α protein measurements during RSV infection in vitro.
(A, B) Cultured pulmonary epithelia (A549) were grown to 80% confluency, infected with intact (multiplicity of infection, MOI 1, 3 or 5) or UV-inactivated RSV (MOI 3). In other studies A549 cells were exposed over 24 h to ambient hypoxia (2% oxygen). Cells were lysed and nuclear proteins were isolated, and Western immunoblotting for RSV G-protein (A) or HIF1α was performed. Uninfected cells were used as control (Co). The same blots were probed for β-actin expression as a control for protein loading. A representative blot of 3 is shown, in addition to densitometric analysis of HIF-1α protein levels relative to β-actin (C;*P<.01, different from control, n = 3).
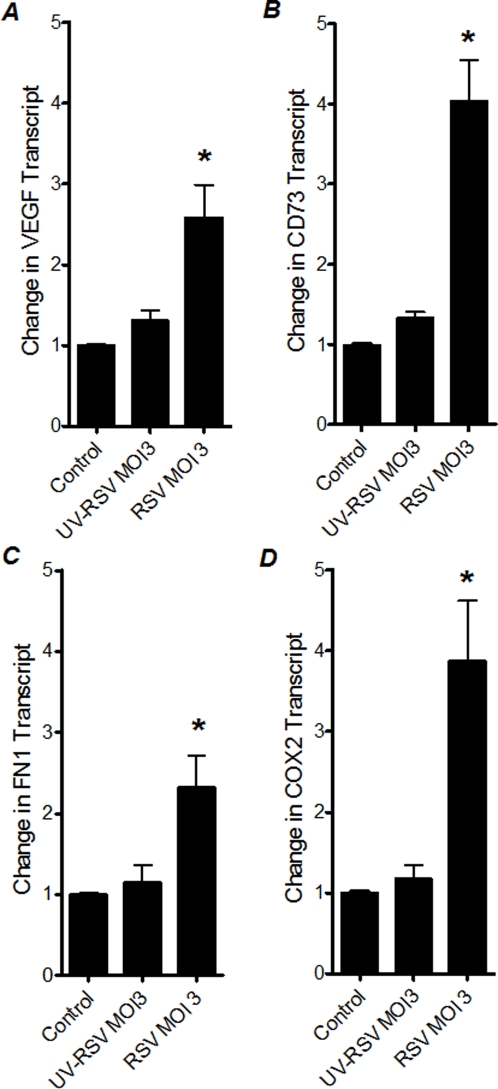
HIF-dependent genes are induced following RSV infection
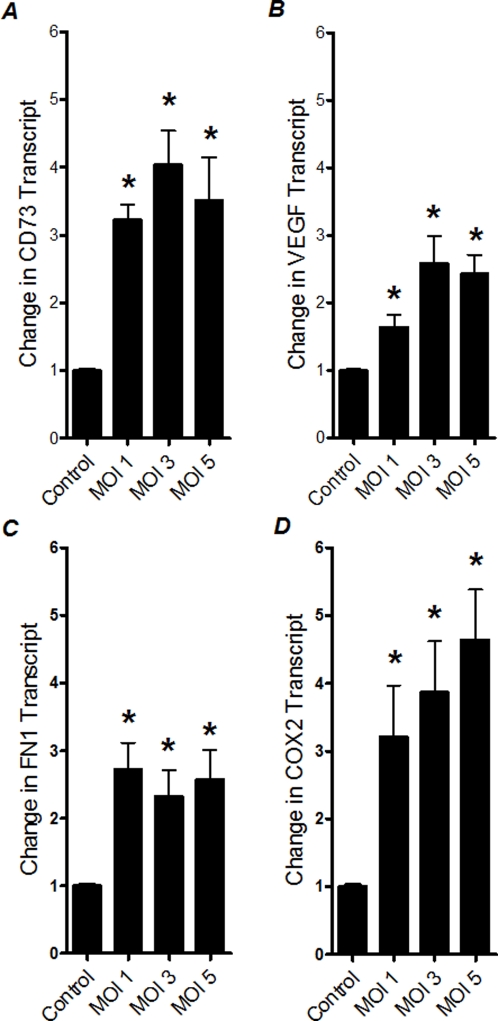
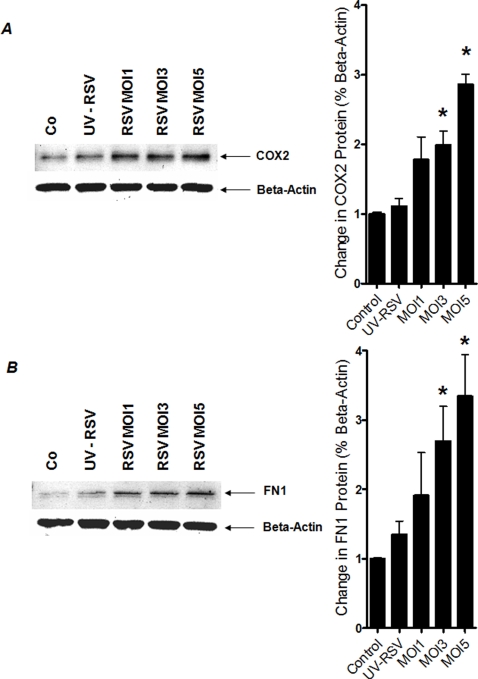
After having demonstrated HIF-1α protein stabilization during infection with RSV, we next pursued functional consequences of HIF-1α in transcriptional gene induction during RSV infection. For this purpose, we performed expressional studies of known HIF-1-dependent genes during RSV infection. Thus, we measured transcript levels of CD73 [62], VEGF [63], Fibronectin1 (FN1) [64] and COX2 [65] after 24 h of RSV infection of A549 pulmonary epithelial cells using different RSV infection doses (MOI1-5). As shown in Figure 3, analysis of transcript levels by real-time RT-PCR revealed induction of all tested HIF-1 target genes. Extensions of these findings at the protein level by Western blot confirmed significant induction of COX2 or FN1 protein levels (Figure 4A and B). Similarly, measurements of VEGF in the supernatants from RSV infected pulmonary epithelial cells revealed significantly elevated levels of VEGF (data not shown). Taken together, these studies demonstrate induction of HIF-1α -depedendent genes during RSV infection in vitro.
Figure 3. Transcript levels of HIF-1-dependent genes following RSV infection.
Total RNA was isolated from RSV-infected (multiplicity of infection, MOI 1, 3 and 5) or non-infected A549 cells (control) and (A) CD73, (B) VEGG, (C) FN1, (D) COX2 mRNA levels were determined by real-time RT-PCR. Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold increase over uninfected control-cells ±SEM at each infection dose (*P<0.05, different from uninfected control-cells).
Figure 4. Protein levels of HIF-1-dependent genes following RSV infection.
Total protein was isolated from RSV-infected (multiplicity of infection, MOI 1, 3 and 5) or non-infected A549 cells. Protein levels were determined by Western blot. The same blots were probed for β-actin expression as a control for protein loading. In addition, densitometric analysis of protein levels relative to β-actin were performed. Data are expressed as fold increase over uninfected control-cells ±SEM at each infection dose. (A) COX2; (B) FN1 (*P<0.01, different from control, n = 3).
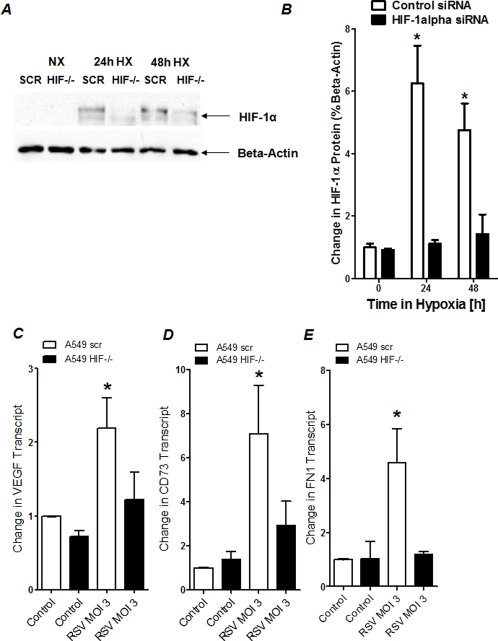
HIF-dependent gene expression during RSV infection in pulmonary epithelial cells following siRNA repression of HIF-1α
To demonstrate a functional role of HIF-1α in the observed induction of HIF-dependent genes induction during RSV infection, we next pursued HIF-1α-loss-of-function studies. For this purpose, we generated a pulmonary epithelial cell line (A549 cells) with stable repression of HIF-1α. This was achieved via hairpin siRNA technique as we have done previously in other cell lines [26], [53]–[56]. To demonstrate effective repression of HIF-1α in these cell lines, we utilized a model of ambient hypoxia. For this purpose, we exposed these cells to hypoxia over 24 or 48 h (2% oxygen), while growth-synchronized control cells were maintained at room air (21% oxygen). These studies revealed significant accumulation of HIF-1α protein in control transfected cells in conjunction with attenuated HIF-1 α stabilization in HIF-1 α-siRNA-transfected A549 cells (Figure 5A and B). We utilized this model to directly test the functional role of HIF-1α in transcriptional modulation during RSV infection. Here, we measured transcript levels of control transfected or HIF-1α-targeted pulmonary epithelia. These studies revealed significant induction of HIF-1-target genes (VEGF, CD73 or FN1) in control cells, while these responses were abolished in HIF-1α-targeted pulmonary epithelia. Taken together, these studies suggest a functional role of HIF-1 α in transcriptional induction of HIF-1α -targeted genes during infection with RSV.
Figure 5. Transcript levels of HIF-dependent genes following HIF-1α siRNA repression during RSV infection.
(A) HIF-1α protein levels in A549 cells following hairpin siRNA repression of HIF-1α (HIF-/-; A549 cells transfected with control siRNA:SCR). Cells were grown to 80% confluency and exposed to normoxia or hypoxia (2% oxygen) over indicated time period. Nuclear proteins were isolated and Western Blot analysis was performed for HIF-1α. The same blots were probed for β-actin expression as a control for protein loading. A representative blot of 3 is shown, in addition to densitometric analysis of HIF-1α protein levels relative to β-actin (B;*P<0.01, different from control, n = 3).(C, D, E) Total RNA was isolated from RSV-infected (multiplicity of infection, MOI 3) or non-infected A549 following HIF-1α repression (A549 HIF-/-) or transfection with control siRNA (A549 scr). (C) VEGF, (D) CD73, (E) FN1 transcript levels were determined by RT-PCR. Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold increase over uninfected control-cells ±SEM (*P<0.05).
Influence of UV-inactivation of RSV on HIF-dependent gene induction
In view of the above results, we hypothesized that only intact RSV is capable of HIF-1 α stabilization and induction of HIF-1α target genes. Therefore, we next investigated the effects of UV-inactivated virus on the HIF-1 α target genes. For this purpose, we measured VEGF, CD73, FN1 and COX2 transcript levels in A549 cells that were infected with intact or with UV-inactivated RSV (Figure 6 A-D). Consistent with our studies above, we found significant induction of HIF-1α target genes in RSV infected A549 cells. In contrast, induction of HIF-1α target genes was completely abolished after similar infection doses with UV-inactivated RSV. Taken together, these studies suggest that only functional RSV virus, capable of intracellular replication–is necessary to cause HIF-1α-dependent gene induction.
Figure 6. Transcript levels of HIF-dependent genes following infection with inactivated RSV.
Total RNA was isolated from uninfected, RSV-infected or UV-inactivated RSV infected A549 cells. (A) VEGF, (B) CD73, (C) FN1, (D) COX2 transcript levels were determined by real-time RT-PCR. Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold increase over uninfected control-cells ±SEM at each infection dose (*P<0.05, different from uninfected control-cells).
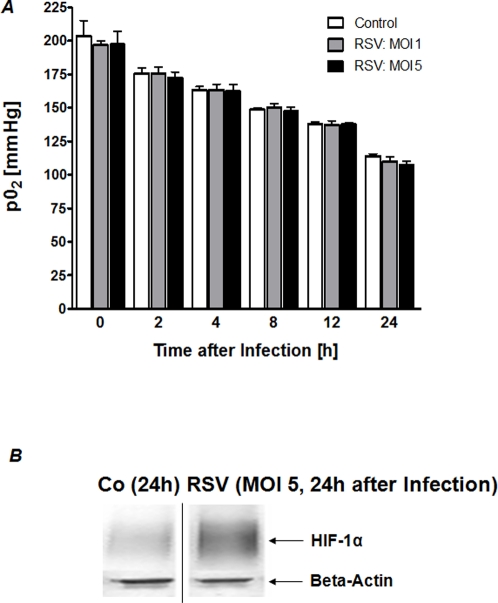
HIF1α stabilization after RSV infection occurs independent of hypoxia
Previous studies of infection and inflammation have revealed significant changes in metabolic supply and demand. For example, studies of murine colitis revealed convincing evidence that the mucosal surface is prone to inflammation-associated hypoxia [58], [66]. Our above studies showed that intracellular uptake and binding of RSV appears to be necessary for HIF-1α dependent gene induction. Therefore, we hypothesized that changes in metabolic supply and demand ratios during RSV infection may accompany RSF-infection and RSV-associated tissue hypoxia could lead to HIF-1α activation. To address this hypothesis in an experimental setting, we measured oxygen partial pressures (pO2) values in the supernatants from RSV infected pulmonary epithelia or controls that were maintained in an oxygen impermeable culture system. As shown in Figure 7A, no differences in PO2 values were observed between experimental groups. In fact, neither supernatant from control nor RSV-infected pulmonary epithelia showed significant degrees of hypoxia. Consistent with these findings, and as shown in Figure 7B, Western blot analysis confirmed that HIF-1α was not stabilized in control cells maintained under the above cell culture conditions at 24 h. Similarly, HIF-1α stabilization was not observed at earlier time points (date not shown). In contrast, we observed significant HIF-1α stabilization in RSV-infected pulmonary epithelia. Taken together, these studies suggest oxygen independent stabilization of HIF-1α during RSV infection.
Figure 7.
(a) Measurements of oxygen partial pressures (pO2) in the supernatants of RSV infected pulmonary epithelia. (A) A549 cells were cultured and infected at a multiplicity of infection (MOI) of 1 or 5 in gas-tight sealed flasks. Oxygen partial pressure was measured in the supernatants at indicated time points following infection. (B) A samples was assessed for HIF-1α protein levels by Western blot 24 h after RSV infection or control conditions. Blots were probed for β-actin expression as a control for protein loading
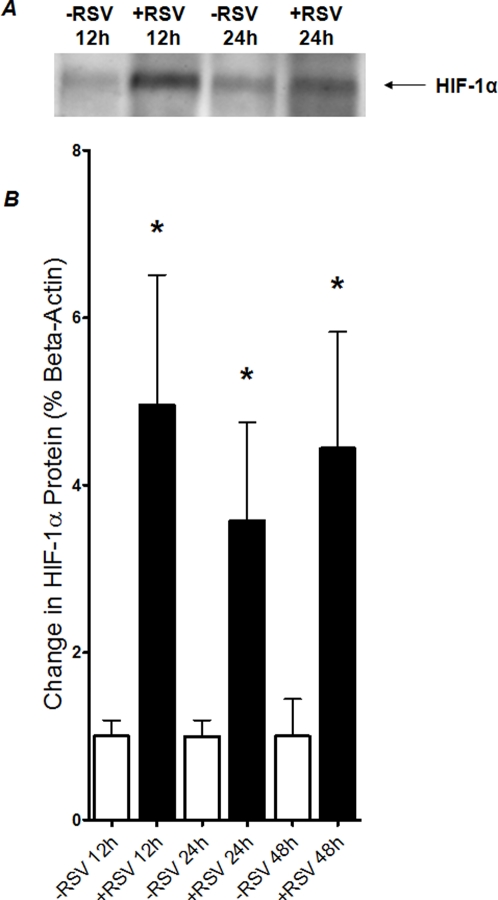
HIF-1 α is stabilized during murine RSV infection in vivo
As proof of principle for these concepts in vivo, we compared the influence of RSV infection on pulmonary HIF-1α stabilization using a previously described model [38]–[41]. This mouse model shows close similarity to the pathogenesis of RSV-induced lower airway disease in humans. In fact, recently established that the experimental infection of BALB/c mice with highly purified preparations of RSV A, at a dose of 107 PFU, induces a severe inflammatory response in lung tissue as early as 24 h after intranasal inoculation [39]. Lung inflammation was characterized by an excess of monocytes/macrophages, lymphocytes, and to a lesser extent, neutrophils surrounding bronchioles and vessels, with evidence of the involvement of alveolar spaces [39]. In the present studies, female BALB/c mice were inoculated intranasally with purified RSV. In control experiments, BALB/c mice matched in age, gender and weight were inoculated in the same way with an equivalent volume of vehicle. At the indicated time points after infection, mice were anesthetized, lungs were shockfrozen and HIF-1α was determined by Western blot analysis. As shown in Figure 8A and B, HIF-1 α was stabilized with RSV infection at all measurement time points. Taken together, these data confirm our in vitro findings and suggest that during murine RSV pneumonia HIF-1α is stabilized.
Figure 8. HIF-1α protein during murine RSV pneumonia in vivo.
Female, 6- to 8-week-old BALB/c mice were inoculated intranasally with purified RSV at 1×107 plaque-forming units (PFUs), diluted in sterile 0.9 % sodium chloride for a total inoculation volume of 50 µl. As mock treatment, control mice were inoculated in the same way with an equivalent volume of sucrose diluted in 0.9 % sodium chloride. Lungs were removed at indicated time-points, and HIF-1α protein levels were determined by Western blot analysis (A) or quantified by densitometry, relative to beta-actin (B; *p<0.01).
Discussion
Many studies during the last decade have demonstrated a central role of HIF-1 in mammalian oxygen homeostasis [3]. However, more recently many studies have also demonstrated a role of HIF-1α in the transcriptional coordination during inflammation and infection [6], [32], [54], [67]. In fact, previous studies have revealed that HIF-1α can be stabilized during infections with human pathogens via oxygen-dependent [67] or oxygen independent [54] pathways. In the present studies we pursued HIF-1α stabilization and gene-transcription during infection with RSV–one of the most potent biological stimuli to induce an inflammatory milieu [38]–[41]. In initial studies of cultured pulmonary epithelia infected with RSV revealed stabilization of HIF-1α protein. Moreover, transcription of known HIF-1α target genes was induced following RSV infection, while siRNA-dependent repression of HIF-1α abolished these responses. However, infection with RSV was not associated with increased oxygen consumption or cellular hypoxia, suggesting that HIF-1α stabilization and HIF-dependent gene induction during RSV infection occurs in an oxygen-independent fashion. Finally, studies of murine RSV pneumonia revealed significant HIF-1α stabilization throughout the course of the disease and suggest that RSV-associated HIF-1α activation also occurs in vivo.
Previous studies of HIF-1α during inflammation and infection have found oxygen-independent activation of HIF-1α during infections with human pathogens. For example, a recent study on molecular mechanisms of how bacteria activate HIF-1α found a role of bacterial siderophores in HIF-1α activation during infection with Enterobacteriaceae [54]. Here, the authors studied HIF-1α activation and HIF-1α -dependent gene induction in Peyer's patches that were analyzed after orogastric infection with Yersinia enterocolitica and orogastric Y enterocolitica infection in mice with a conditional deletion of HIF-1α [66] in the intestine. These studies demonstrated that infection of mice with Y enterocolitica led to functional activation of HIF-1α in Peyer's patches. Moreover, mice with conditional deletion of HIF-1α in the intestinal epithelium showed a significantly higher susceptibility to orogastric Y enterocolitica infections, suggesting HIF-1α activation as a host defense mechanism in this model. Additional studies with Y enterocolitica, S enterica subsp enterica, or E aerogenes, and, moreover, application of their siderophores (yersiniabactin, salmochelin, aerobactin) caused a robust, dose-dependent HIF-1α response in human epithelia and endothelia, independent of cellular hypoxia. Taken together, such studies demonstrate a role for bacterial siderophores in hypoxia-independent activation of HIF-1α during infection with human pathogenic bacteria [54].Similarly, previous studies on viral infections with RSV have demonstrated induction of HIF-1α in primary human bronchial epithelial cells via a nitric-oxide-dependent pathway [42]. Other studies have identified a crosstalk between viral genes and the HIF-1α pathway during infections with the human herpesvirus 8 (HHV-8) [32], [68]. Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) is the etiological agent of Kaposi's sarcoma, a highly vascularized, endothelial-derived tumor. A direct role for KSHV-mediated induction of angiogenesis has been proposed based upon the nature of the neoplasia and various KSHV gene overexpression and infection model systems. These studies revealed that KSHV infection of endothelial cells induces mRNA of HIF-1α and HIF-2α. While HIF is classically activated posttranscriptionally, these studies demonstrate that both alpha-subunits are up-regulated at the transcript level by KSHV infection. Here, the transcriptional activation of HIF leads to a functional increase in HIF activity under normoxic conditions, as shown utilizing luciferase reporter assays and HIF-dependent gene expression.
From the present studies it remains unclear whether HIF-1α activation during RSV infection represents a host-defense mechanism or is an essential part of the disease pathogenesis enabling virus uptake or replication. While some studies have identified a host-protective role of HIF-1α during inflammation [25]–[27], [53], [54], [69] or infections [54], other studies have found a contribution of HIF-1α activation in growth and survival of human pathogens. For example, Toxoplasma gondii is an obligate intracellular protozoan pathogen. Recent studies revealed that genes mediating cellular responses to hypoxia were upregulated in Toxoplasma -infected cells but not in cells infected with another intracellular pathogen, Trypanosoma cruzi [70], [71]. The inducible expression of these genes is controlled by the HIF-1α. Additional studies revealed that Toxoplasma infection rapidly increased the abundance of the HIF1α and activated HIF-1α reporter gene expression and survival was severely reduced in cells targeted for HIF-1α [72]. These studies also suggested that while HIF-1α was not required for parasite invasion, HIF-1α was required for parasite cell division and organelle maintenance, indicating that Toxoplasma activates HIF-1α and requires HIF-1α for growth and survival at physiologically relevant oxygen levels [70]–[72].
Taken together, the present studies reveal oxygen-independent stabilization of HIF-1α during RSV infection in vitro and in vivo. Future challenges will include the determination whether such responses elicited during RSV infections are host-protective or host-detrimental. In addition, it will be critical to gather a more thorough understanding of the mechanisms of HIF-1α induction during RSV infection (e.g. the role of RSV elicited TLR signaling in HIF stabilization) or the contribution of RSV-associated nitric oxide release [42]. Ongoing studies are currently testing HIF-activation or HIF-inhibition in different settings of medical therapy and novel therapeutics will soon become available in patient care to inhibit or to activate the HIF-1α pathway. Such compounds may comprise a novel approach during RSV infections.
Acknowledgments
We would like to acknowledge Marion Faigle and Stephanie Zug for excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This manuscript was supported in part by a Foundation for Anesthesia Education and Research (FAER) Grant to HKE.
References
- 1.Semenza GL. Regulation of erythropoietin production. New insights into molecular mechanisms of oxygen homeostasis. Hematol Oncol Clin North Am. 1994;8:863–884. [PubMed] [Google Scholar]
- 2.Bunn HF, Gu LJ, Huang E, Park JW, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 4.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annual Review of Immunology. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 6.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. O2 sensing: only skin deep? Cell. 2008;133:206–208. doi: 10.1016/j.cell.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular oxygen tension. Proc Natl Acad Sci. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 11.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Nat Acad Sci (USA) 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 15.Guillemin K, Krasnow M. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 16.Kvietikova I, Wenger RH, Marti HH, Gassmann M. The hypoxia-inducible factor-1 DNA recognition site is cAMP responsive. Kid Int. 1997;51:564–566. doi: 10.1038/ki.1997.80. [DOI] [PubMed] [Google Scholar]
- 17.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, et al. HIF-1{alpha} expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann H, Eltzschig HK, Wurz HKH, Rakin A, et al. Gastroenterology; 2007. Hypoxia-independent activation of HIF-1 by Enterobacteriaceae and their siderophores. p. in press. [DOI] [PubMed] [Google Scholar]
- 20.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1{alpha}-dependent NF-{kappa}B activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 24.Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia Inducible Factor (HIF)-1 Coordinates Induction of Toll-Like Receptors TLR2 and TLR6 during Hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 28.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008 doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempf VAJ, Lebiedziejewski M, Alitalo K, Walzlein J-H, Ehehalt U, et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: Evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]
- 30.van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, et al. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–38. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 31.van der Flier M, Stockhammer G, Vonk GJ, Nikkels PG, van Diemen-Steenvoorde RA, et al. Vascular endothelial growth factor in bacterial meningitis: detection in cerebrospinal fluid and localization in postmortem brain. J Infect Dis. 2001;183:149–153. doi: 10.1086/317643. [DOI] [PubMed] [Google Scholar]
- 32.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 33.Nasimuzzaman M, Waris G, Mikolon D, Stupack DG, Siddiqui A. Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. J Virol. 2007;81:10249–10257. doi: 10.1128/JVI.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Han HK, Han CY, Cheon EP, Lee J, Kang KW. Role of hypoxia-inducible factor-alpha in hepatitis-B-virus×protein-mediated MDR1 activation. Biochem Biophys Res Commun. 2007;357:567–573. doi: 10.1016/j.bbrc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, et al. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2004;24:5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garofalo RP, Haeberle H. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol. 2000;23:581–585. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- 37.Haeberle HA, Nohe B, Eltzschig HK, Unertl K, Dieterich HJ. Effect of synthetic colloids on major histocompatibility complex class II expression. J Clin Anesth. 2006;18:96–101. doi: 10.1016/j.jclinane.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Haeberle HA, Casola A, Gatalica Z, Petronella S, Dieterich HJ, et al. IkappaB kinase is a critical regulator of chemokine expression and lung inflammation in respiratory syncytial virus infection. J Virol. 2004;78:2232–2241. doi: 10.1128/JVI.78.5.2232-2241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, et al. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haeberle HA, Nesti F, Dieterich HJ, Gatalica Z, Garofalo RP. Perflubron reduces lung inflammation in respiratory syncytial virus infection by inhibiting chemokine expression and nuclear factor-kappa B activation. Am J Respir Crit Care Med. 2002;165:1433–1438. doi: 10.1164/rccm.2109077. [DOI] [PubMed] [Google Scholar]
- 41.Haeberle HA, Takizawa R, Casola A, Brasier AR, Dieterich HJ, et al. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J Infect Dis. 2002;186:1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 42.Kilani MM, Mohammed KA, Nasreen N, Tepper RS, Antony VB. RSV causes HIF-1alpha stabilization via NO release in primary bronchial epithelial cells. Inflammation. 2004;28:245–251. doi: 10.1007/s10753-004-6047-y. [DOI] [PubMed] [Google Scholar]
- 43.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, et al. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueba O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–272. [PubMed] [Google Scholar]
- 45.Kisch AL, Johnson KM. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med. 1963;112:583–589. doi: 10.3181/00379727-112-28111. [DOI] [PubMed] [Google Scholar]
- 46.Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 48.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. 2007 doi: 10.1182/blood-2007-10-117044. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 50.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 51.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 52.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 53.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology. 2008;134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. Faseb J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 56.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008 doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 57.Eltzschig HK, Karhausen J, Kempf VA. Acute oxygen-sensing mechanisms. N Engl J Med. 2006;354:975–977. [PubMed] [Google Scholar]
- 58.Karhausen J, Haase VH, Colgan SP. Inflammatory Hypoxia: Role of Hypoxia-Inducible Factor. Cell Cycle. 2005;4 [PubMed] [Google Scholar]
- 59.Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc Natl Acad Sci U S A. 2004;101:10440–10445. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 61.Wotzlaw C, Otto T, Berchner-Pfannschmidt U, Metzen E, Acker H, et al. Optical analysis of the HIF-1 complex in living cells by FRET and FRAP. FASEB J. 2007;21:700–707. doi: 10.1096/fj.06-6280com. [DOI] [PubMed] [Google Scholar]
- 62.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 64.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 65.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 66.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kempf VA, Lebiedziejewski M, Alitalo K, Walzlein JH, Ehehalt U, et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]
- 68.Carroll PA, Kenerson HL, Yeung RS, Lagunoff M. Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J Virol. 2006;80:10802–10812. doi: 10.1128/JVI.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, et al. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem. 2001;276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 71.Vaena de Avalos S, Blader IJ, Fisher M, Boothroyd JC, Burleigh BA. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J Biol Chem. 2002;277:639–644. doi: 10.1074/jbc.M109037200. [DOI] [PubMed] [Google Scholar]
- 72.Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, et al. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–352. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]