Abstract
Although genetic factors are known to be important in addiction, no candidate genes have yet been consistently linked to drug use or abuse. Brain-derived neurotrophic factor (BDNF), which has been implicated in the behavioral response to psychomotor stimulants and potentiates neurotransmitters that are strongly linked to addiction, is a logical candidate gene to study. Using a drug challenge approach, we tested for association between BDNF G196A (val66met) genotype and subjective responses to amphetamine (AMPH). Healthy volunteers participated in a double-blind, crossover design in which they received placebo, 10 mg, and 20 mg oral d-amphetamine in random order. Subjective and physical responses to ingestion of AMPH were measured at thirty minute intervals after drug ingestion. Each subject was genotyped for the BDNF G196A polymorphism and grouped and analyzed accordingly. The effects of AMPH on ratings of arousal, energy, and heart rate were compared in subjects with the val/val genotype (N = 67) and the subjects with either the val/met or met/met genotypes (N = 32). AMPH produced less pronounced self-ratings of arousal and energy, yet higher increases in heart rate, in the val/met and met/met compared to the val/val group. These results suggest that BDNF is related to the subjective and physical response to low doses of AMPH.
Keywords: brain-derived neurotrophic factor (BDNF), amphetamine (AMPH), polymorphism, drug response, addiction
Introduction
While psychomotor stimulant drugs such as amphetamine (AMPH) are widely recognized as having a high potential for abuse, there is significant inter-individual variability in liability to abuse of such drugs (Hiroi and Agatsuma, 2004). Inter-individual vulnerability to psychostimulant abuse may depend, at least partially, on differences in acute responses to the drug. Indeed, several controlled studies have shown that responses to fixed doses of AMPH vary markedly across individuals (de Wit et al., 1986; Mattay et al., 2000; Nurnberger et al., 1982; Sprague and Sleator, 1977). It is possible such interindividual variation may in part be explained by genetic variations in the many processes and pathways that mediate the effects of AMPH (Hohoff et al., in press; Lott et al., 2005; Mattay et al., 2003).
A potential candidate to help explain such variation is the gene that encodes brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, which is a group of structurally related secretory proteins widely expressed in neurons and their target cells (Barde, 1989). These proteins have been well-established as regulators of long-term survival and differentiation/development of the central and peripheral nervous systems (Lu and Figurov, 1997), and BDNF has been well implicated in the responses to psychomotor stimulants including AMPH. A single intraperitoneal injection of methamphetamine in mice increases striatal BDNF approximately 5-fold for up to twelve hours (Thomas et al., 2004). Exogenous infusions of BDNF enhance the behavioral effects of psychostimulants on experimental animals (Horger et al., 1999; Meredith et al., 2002). Drug cues presented during extinction in rats trained to self-administer cocaine increase BDNF levels in mesolimbic areas of the brain (Lu et al., 2004). Furthermore, cocaine elicits significantly reduced behavioral effects in heterozygous BDNF knockout mice (Hall et al., 2003). Such studies provide evidence of a link between concentrations of BDNF in the brain and the behavioral effects of psychostimulants.
This clear link between BDNF and ingestion of psychomotor stimulants raises the possibility that genetic variations in this protein may contribute to individual differences in response to psychomotor stimulants in humans. A common 196G/A polymorphism producing a nonconservative valine to methionine amino acid substitution at codon 66 (val66met) has been previously identified in the human BDNF gene (dbSNP number rs6265). Allelic variants at this locus have been shown to be associated with obsessive-compulsive disorder, mood disorders, human memory and hippocampal function, and alcoholism (Green and Craddock, 2003; Hall et al., 2003; Hariri et al., 2003; Matsushita et al., 2004). In addition, met/met homozygotes at this locus (in comparison with val/val homozygotes) show decreased activity-dependent secretion of BDNF by neurons, a finding also associated with decreased intracellular trafficking of synaptic vesicles (Egan et al., 2003). Interestingly, Itoh et al. recently reported that this polymorphism may not be associated with Japanese methamphetamine abusers. However, to date there is no available data regarding the potential relationship between the BDNF val66met polymorphism and acute drug response in human subjects.
Using a drug challenge approach under highly controlled laboratory conditions, the present study investigated the interaction between the BDNF polymorphism in question and the acute behavioral and subjective response to single doses of AMPH or placebo amongst healthy human volunteers. Despite the lack of data from studies involving human subjects, based on the findings reviewed above we hypothesized that subjective and physiological effects from a single moderate dose of AMPH would be related to val66met genotype.
Methods and Materials
Design
Subjects participated in three 4 h sessions in which they received d-amphetamine (10 and 20 mg) or placebo in random order, under double-blind conditions. Dependent measures were recorded at set intervals throughout each session and consisted of both physiological measures and standardized self-report measures of drug effects and mood states. Subjects’ responses on these measures were examined in relation to their genotype for the BDNF val66met polymorphism.
Volunteers
101 healthy male and female volunteers, aged 18-35, were recruited by posters, advertisements, and word-of-mouth referrals. Candidates underwent a structured clinical psychiatric interview to exclude individuals with psychiatric disorders according to DSM IV criteria (American Psychiatric Association, 1994). Candidates completed a psychiatric symptom checklist (SCL-90) (Derogatis, 1983), the Michigan Alcoholism Screening Test (Selzer, 1971) and a health questionnaire with a detailed section on current and lifetime drug use. In addition, all subjects obtained an electrocardiogram (ECG) and physical examination by a physician. In order to reduce variability due to withdrawal from nicotine or caffeine, volunteers were excluded if they smoked more than 10 cigarettes per week or consumed more than 3 cups of coffee per day. In order to control for variations in pharmacokinetics, volunteers with a BMI outside the range of 19-26 were also rejected. Other exclusion criteria include any current medical condition requiring medication or any current or past medical condition considered to be a contraindication for AMPH (e.g., hypertension, abnormal ECG), any current Axis I psychiatric disorder (American Psychiatric Association, 1994), treatment for a substance abuse disorder, history of legal, personal or employment problems related to drug use, less than a high school education, lack of fluency in English, night shift work, pregnant or lactating women, women planning to become pregnant during the study, or total abstention from all recreational drugs including alcohol.
Drugs
d-Amphetamine (10 mg or 20 mg; Dexedrine; GlaxoSmithKline) was placed in size 00 capsules with dextrose filler to mask the contents of the capsule. Placebo capsules contained dextrose only.
Procedure
The study was approved by the University of Chicago’s Institutional Review Board. Subjects first attended an orientation to explain the procedures of each study, practice the questions, provide informed consent and schedule the sessions. After providing consent, 20 ml (2 × 10 ml) of blood was drawn for genotyping.
The three experimental sessions were conducted from 09.00 to 13.00 h separated by at least 48h. Sessions were conducted in comfortably furnished-testing rooms. Upon arrival in the laboratory, subjects provided urine samples for urine toxicology and breath samples for blood alcohol level. Pre-drug subjective effects and vital signs were also recorded. At 09.30 h subjects ingested a capsule containing d-amphetamine (10 or 20 mg) or placebo. For blinding purposes, they were informed the capsules might contain a stimulant, sedative, or placebo. Post-capsule physiological and behavioral measures were obtained at five time points after ingestion of the capsule (30, 60, 90, 150, and 180 min). Physiological measurements included heart rate, temperature and blood pressure. Behavioral measurements included self-ratings of drug effects and mood (see below). At the end of each session subjects also completed an end of session questionnaire reporting on their overall experience with the drug they received. During times when no experimental events were scheduled, subjects were free to engage in recreational activities of their choice (e.g. read or watch movies). After completing all three sessions subjects were debriefed and paid a total of $120.
Measurements
Subjective mood states and drug responses were recorded using standardized self-report questionnaires. Drug effects were assessed using a locally-developed visual-analog questionnaire (Drug Effects Questionnaire, or DEQ) designed to assess the extent to which subjects experiences four subjective states: ‘Feel Drug,’ ‘Feel High,’ Like Drug,’ and ‘Want More’ and the Addiction Research Center Inventory (ARCI BG) (Martin et al., 1971), which is a 49-item true-false questionnaire containing five empirically-derived scales that are sensitive to the effects of a variety of classes of commonly abused drugs (e.g. stimulant, sedative). Mood measures included the a visual-analog scale (VAS) of adjectives describing mood states, and the Profile of Mood States (POMS) (McNair et al., 1971) a 72-item adjective checklist measuring momentary mood states (de Wit and Griffiths, 1991).
Physiological measurements providing objective indicators of the drug’s effects included heart rate, temperature, and blood pressure. Temperature was measured using an IVAC monitor (San Diego, CA) and blood pressure and heart rate were measured using a Digitronic monitor (New Brunswick, NJ).
Genotyping
20 ml (2 × 10 ml) of EDTA anticoagulated venous blood samples were collected from each volunteer in the Clinical Research Center at the University of Chicago Hospitals. Samples were coded for blinding and confidentiality. DNA was then extracted in the core laboratory in the Clinical Research Center using a standard DNA isolation kit. Concentrations were determined using the PicoGreen® dsDNA Quantitation Kit (Molecular Probes, Eugene, OR).
BDNF genotype was determined using the TaqMan® Assays-on-Demand™ SNP Genotyping Protocol (Applied Biosystems, Foster City, CA). Each reaction contained 1X TaqMan® Universal PCR mix, 1X SNP probe mix (ID# C_11592758_10) and 10-20 ng genomic DNA in a total volume of 5 μl in a 384-well plate. Genotyping was accomplished using a TaqMan® PCR protocol under the following conditions: 1 AmpErase® cycle at 50°C for 2 min, 1 cycle of enzyme activation at 95°C for 10 min, followed by 40 alternating cycles of denaturation and reannealing at 92°C for 15 sec and 58°C for 1 min, respectively. All PCR was performed using a Perkin Elmer 9700 Thermocycler (Applied Biosystems, Foster City, CA). PCR products were analyzed with an LJLAnalyst AD fluorescence microplate reader (LJL Biosystems, Sunnyvale, CA). Fluorescence intensity plots were generated and genotype calls were made using TaqManager software as part of the PAARManager database, a tool developed as part of the Pharmacogenetics of Anticancer Agents Research Group of the NIH Pharmacogenetic Research Network (PGRN). Genotyping was conducted blind to all phenotypic data, and the genotype completion rate was 100% (99/99). Twelve samples were genotyped on 2 different days with 100% agreement (12/12).
Statistical Analysis
Statistical analysis was done using SPSS® 12.0 for Windows® (SPSS Inc., Chicago, IL). Of the 101 recruited subjects, one was excluded because of a missing laboratory sample, and another due to incomplete acquisition of data. The subjects were initially categorized into three groups based on their observed genotype for the BDNF polymorphism (val/val, val/met, and met/met). However, because the met/met group was extremely small (N = 4), these subjects were included with the val/met subjects (N = 28) for purposes of statistical analysis. For demographic data, independent samples (Student’s) T-test was used to compare continuous data (e.g. age, weight), whereas Pearson’s chisquare test was used to compare dichotomous data (e.g. ethnicity, sex). The presence of Hardy-Weinberg equilibrium was examined using an online resource (http://www.kursus.kvl.dk/shares/vetgen/_Popgen/genetik/applets/kitest.htm). The significance level used for all statistical tests was a p < 0.05.
Responses to AMPH were examined using a General Linear Model Repeated Measures ANOVA, incorporating two within-subjects factors (drug (three levels) and time (five levels)) and one between-subjects factor (genotype group). Pre-drug scores for each measure from each session were used as a covariate in order to account for baseline (i.e. before capsule ingestion) differences within the genotype groups prior to drug administration. For post hoc analyses, an area under the curve (AUC) approximation was incorporated for measures showing a significant Drug*Group effect, while independent samples (student’s) T-test was used for measures where there was shown to be a significant Drug*Time*Group effect. There were no significant differences between the genotype groups at baseline.
Results
BDNF val66met polymorphism allele/genotype frequencies and relationship of genotype to demographic data
The allele/genotype frequencies and demographic data are reported for the 99 analyzed volunteers in Tables 1 and 2, respectively. The data in Table 1 displays the total allelic and genotypic distribution according to ethnicity. Three of the subjects in the val/met genotype group reported affiliation with two different ethnic groups, and so their data was incorporated into each ethnic grouping for the demographic analysis. This increased the overall number of subjects presented in Table 1 from 99 to 102; however, this discrepancy did not affect the primary findings reported here since drug responses were analyzed according to genotype group, and not by ethnicity. The results found in Table 1 are very similar to allele and genotype frequencies reported in previously published studies regarding this polymorphism (Egan et al., 2003; Hariri et al., 2003). Table 2 shows the demographic characteristics of the 99 analyzed subjects, grouped by genotype. The two groups were similar for most demographic measures, except for the proportion of Hispanic (p = 0.040), Caucasian (p = 0.047) and African-American (p = 0.003) subjects between groups. The genotype frequencies in the entire sample population (df = 1, χ2 = 0.24, p > 0.25) and the Caucasian population alone (df = 1, χ2 = 0.35, p > 0.25) are in Hardy-Weinberg equilibrium.
Table 1.
Allele and genotype frequencies. Data are presented as raw counts with percentage in parentheses
| Allele Frequency | Genotype Frequency | ||||
|---|---|---|---|---|---|
| Val | Met | Val/Val | Val/Met | Met/Met | |
| Total (N=102) | 165 (80.9) | 39 (19.1) | 67 (65.7) | 31 (30.4) | 4 (3.9) |
| Hispanic (N=12) | 17 (70.8) | 7 (29.2) | 5 (41.7) | 7 (58.3) | 0 (0) |
| Caucasian (N=57) | 87 (76.3) | 27 (23.7) | 34 (59.7) | 19 (33.3) | 4 (7.0) |
| African-American (N=20) | 39 (97.5) | 1 (2.5) | 19 (95) | 1 (5) | 0 |
| Asian (N=12) | 21 (87.5) | 3 (12.5) | 9 (75) | 3 25) | 0 |
| American Indian (N=1) | 1 (50) | 1 (50) | 0 | 1 (100) | 0 |
Table 2.
Demographic data. Differences between the two genotype groups are bolded
| Demographic Characteristic | Val/Val | Val/Met & Met/Met | Significance |
|---|---|---|---|
| N | 67 | 32 | |
| Age (mean) | 24.04 ± 0.53 | 23.56 ± 0.66 | NS |
| Weight (mean) | 152.54 ± 3.20 | 145.63 ± 3.60 | NS |
| BMI (mean) | 22.87 ± 0.29 | 22.03 ± 0.38 | NS |
| Education (mean yrs) | 14.96 ± 0.21 | 15.06 ± 0.27 | NS |
| Ethnicity | |||
| %Hispanic | 7 | 22 | p = 0.040 |
| %Caucasian | 51 | 72 | p = 0.047 |
| %African-American | 28 | 3 | p = 0.003 |
| %Asian | 13 | 9 | NS |
| %American Indian | 0 | 3 | NS |
| Gender | |||
| %Male | 51 | 50 | NS |
| Current Drug Use | |||
| Alcohol (mean d/wk) | 3.72 ± 0.39 | 4.94 ± 0.58 | NS |
| Cigarettes (mean cigs/wk) | 0.69 ± 0.24 | 1.55 ± 0.57 | NS |
| Caffeine (mean cups/d) | 1.22 ± 0.15 | 1.54 ± 0.26 | NS |
| Marijuana (%yes) | 25 | 22 | NS |
| Lifetime Substance Use (ever used) | |||
| Stimulants (% yes) | 16 | 28 | NS |
| Sedatives (% yes) | 6 | 3 | NS |
| Opiates (% yes) | 12 | 16 | NS |
| Hallucinogens (% yes) | 31 | 47 | NS |
| Marijuana (% yes) | 75 | 72 | NS |
| Inhalants (% yes) | 13 | 9 | NS |
BDNF val66met Polymorphism and Response to Amphetamine
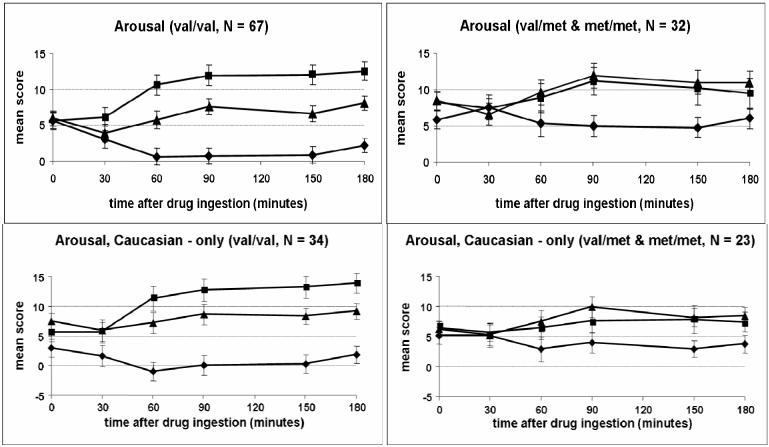
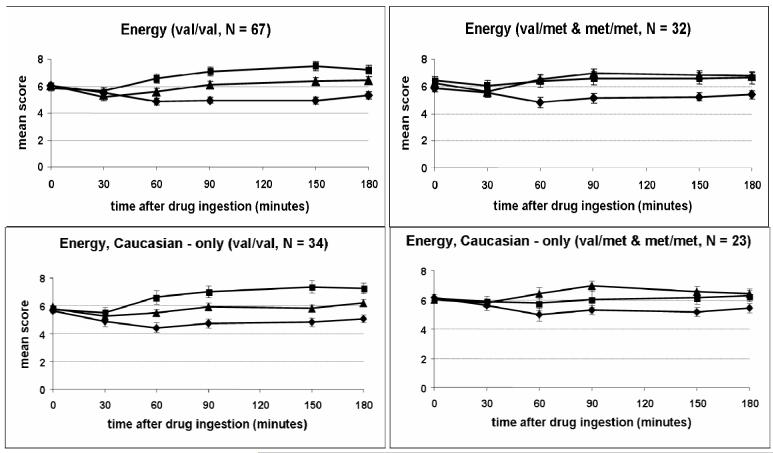
Responses to AMPH are reported based on subjects genotype at amino acid 66 of the BDNF gene (val/val group, N = 67, and val/met or met/met group, N = 32). Although the groups did not differ significantly for any subjective or physical measure at baseline (i.e. before capsule ingestion), minor variability prior to drug ingestion was corrected for in the statistical analysis by using baseline scores as covariates. The two groups differed significantly in their responses to AMPH on self-ratings of arousal (POMS; Drug*Group effect; p = 0.002; Figure 1) and energy (ARCI BG; Drug*Group effect; p = 0.018; Figure 2). Figure 1 shows the val/val group exhibited a greater decrease in arousal after placebo and a greater increase in arousal after 20 mg AMPH. Figure 2 suggests the onset of effects of AMPH on ratings of energy occurred at 60 min after ingestion of the capsule, and scores remained elevated throughout the remaining 3 h session. Similarly, the val/val group showed a greater increase in energy after ingestion of 20 mg AMPH. These same patterns on arousal and energy were apparent when data were examined for Caucasian subjects only (arousal: p = 0.002; energy: p = 0.032; Drug*Group effect; Figures 1 and 2).
Figure 1.
Time course of self-reported arousal (POMS) after drug administration for the two genotype groups (Drug*Group Effect: p = 0.002) and for caucasian subjects only (Drug*Group Effect: p = 0.002). Data shown are mean (± SEM) ratings. ◆ = Placebo; ▲ = 10 AMPH; ■ = 20 mg AMPH.
Figure 2.
Time course of self-reported energy (ARCI BG) after drug administration (placebo, 10 mg AMPH, 20 AMPH) for the two genotype groups (Drug*Group Effect: p = 0.018) and for Caucasian subjects only (Drug*Group Effect: p = 0.032). Data shown are mean (±SEM) ratings. ◆ = Placebo; ▲ = 10 AMPH; ■ = 20 mg AMPH.
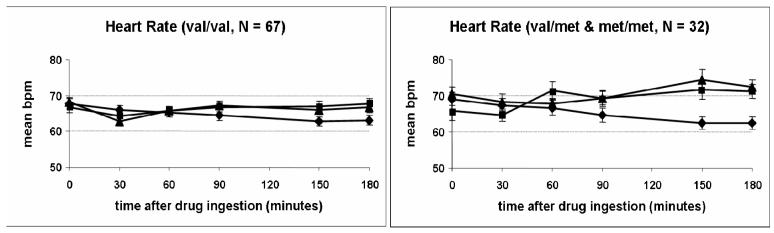
Group differences were also apparent on the objective physical measure of heart rate (Drug*Time*Group effect; p = 0.005; Figure 3). In this case, however, the val/val group exhibited a smaller increase in heart rate in response to administration of AMPH compared to the other group. Inspection of the mean heart rate across the session in Figure 3 suggests the peak drug, and peak differences between the genotype groups, occurred relatively late in the session, at 150 min. The differential effects of AMPH on heart rate were not observed when the analysis was conducted using only Caucasian subjects. The groups did not differ in their responses to AMPH on any other subjective or physiological measure of drug effect.
Figure 3.
Time course of heart rate after drug administration (placebo, 10mg AMPH, 20 AMPH) for the two genotype groups (Drug*Time*Group effect: p = 0.005). Data shown are mean (± SEM) values. ◆ = Placebo; ▲ = 10 AMPH; ■ = 20 mg AMPH.
Discussion
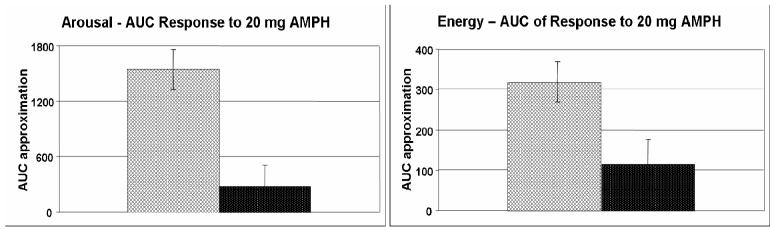
The primary finding in this study was that healthy adult volunteers homozygous for the val allele experienced more pronounced subjective responses and a less pronounced physiological response from a single moderate dose of AMPH than the other two genotypic groups. With respect to subjective response to AMPH, we found that individuals with the val/val genotype reported significantly greater increases in individual ratings of arousal (p = 0.002) and energy (p = 0.018; Figures 1 & 2). This effect was observed only at the 20 mg dose of AMPH (Figure 4); self-ratings of arousal and energy did not differ between the genotype groups in response to placebo or 10 mg AMPH, and the two groups did not significantly differ on baseline ratings of arousal or energy. Although Figure 1 suggests the val/val group exhibited a greater decrease in self-ratings of arousal on the placebo sessions, the difference between the groups on placebo sessions was not significant, and did not explain the group differences in responses to AMPH. Further, because the order of administration of the three drug conditions was counterbalanced, the apparent group differences on placebo sessions were not accounted for by difference in drug order. This was confirmed by an analysis using order as a factor.
Figure 4.
Area under the curve (AUC) approximation of self-ratings of arousal (POMS; p <0.001) and energy (ARCI BG; p = 0.015) in response to 20 mg AMPH for the two genotype groups ( = val/val;
= val/val; = val/met & met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
= val/met & met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
In contrast to the subjective responses to AMPH in the genotype groups, the physical effects of AMPH as measured by heart rate (Drug x Time x Group; p = 0.005) were less pronounced in individuals with the val/val genotype (see Figure 3). Post hoc analysis revealed that subjects with either the val/met or met/met genotype experienced greater increases in heart rate 150 min (p < 0.001) and 180 min (p = 0.001) after ingestion of 10 mg AMPH only. It is interesting to note that the time course of the subjective and heart rate effects were quite different (30-60 min and 120 min for the subjective and physiological measures, respectively), suggesting they were mediated by different processes. At this point, it is not clear why apparently group differences in apparently opposite directions were observed with the heart rate and subjective responses, at two different doses of AMPH (10 mg and 20 mg) and at different times after drug administration (1 hr vs 2 hr). Nevertheless, these results provide some support for our hypothesis that genetic variations in BDNF can influence acute responses to a challenge dose of AMPH on certain outcome measures.
Our findings on measures of arousal and energy are consistent with preclinical findings regarding the BDNF G196A (val66met) polymorphism and responses to AMPH. Treatment with psychostimulants such as AMPH is associated with increases in BDNF levels in neurons of the mesolimbic dopamine system, an area of the brain which is primarily implicated in the euphoric and reward/reinforcing actions of drugs of abuse (Lu et al., 2004; Thomas et al., 2004; Koob et al., 1998; Nestler, 2001). Furthermore, BDNF also appears to play a role in potentiating dopamine release in these areas, an effect which might be increased in response to AMPH via increased mobilization and/or docking of synaptic vesicles to presynaptic active zones (Narita et al., 2003; Pozzo-Miller et al., 1999). However, the met/met genotype (in comparison with the val/val genotype only) has been shown to significantly decrease activity-dependent secretion of BDNF by neurons, probably by decreasing intracellular trafficking of synaptic vesicles (Egan et al., 2003). Just as these findings would suggest individuals with the met/met genotype would be more resistant to release of dopamine and its associated reward/reinforcing actions in response to AMPH administration, we found that individuals with either the met/met or val/met genotype showed smaller increases in self-ratings of arousal and energy than individuals with the val/val genotype following administration of 10 or 20 mg AMPH. Considering this variant is located in the sequence responsible for encoding the pro-BDNF precursor peptide (Seidah et al., 1996), which is proteolytically cleaved to form the mature protein postranslationally, it seems plausible to suggest that differences in expression level of the mature BDNF protein might underlie these functional and behavioral differences. With respect to the finding reported by Itoh et al., like previous conflicting studies examining the association between the BDNF gene and bipolar illness (Neves-Pereira et al., 2002; Sklar et al., 2002; Nakata et al., 2003) it is very likely that ethnic differences may be contributing to the inconsistent findings between Japanese and American samples.
It is important to point out several limitations of this study. The total number of subjects studied (N = 99) was relatively small for a genotypic analysis. As a result, this raises the possibility the findings reported here simply occurred by chance, or that population differences related the BDNF val66met polymorphism are simply correlated with, but not predictive of, responses to ingestion of AMPH. Based on a statistical power cutoff of 80% for association studies, it appears that our study design lacked adequate power for the observed differences in self-reported measures of energy only (data not shown). Increasing the sample size of our study population would be an appropriate way in which to increase the power of the reported effect size above the 80% cutoff.
There also exists the issue of population stratification - which can lead to spurious associations between a candidate marker and a phenotype - especially considering the distribution of ethnicities between the two groups was found to be significantly different with respect to the percentage of Hispanic, Caucasian and African-American subjects (see Table 1). However, a Caucasian-only analysis revealed the same statistically significant Drug*Group differences for arousal (p = 0.002) and energy (p = 0.032; Figures 1 & 2). Despite attempting to adjust our model using a structured association method, it is still possible that occult population stratification within the Caucasian group could account for our findings reported here. Genomic control methods could be applied to correct for this issue in future replication studies of these findings in order to adjust for outliers due to population stratification. In addition, it is difficult to explain why a Caucasian-only analysis failed to reveal a significant difference in heart rate between the two genotype groups. However, it is possible that the smaller relative Caucasian sample size did not have adequate power.
Furthermore, because of the extremely small sample size of met/met subjects (N = 4), the responses of this group were analyzed with those of the val/met group for all primary outcomes reported in this paper. However, we performed an additional AUC analysis of arousal, energy and heart rate for all three genotype groups in response to 10 and 20 mg AMPH (Figures 5, 6 and 7). The figures suggest decreased self-ratings of arousal and energy with an increase in heart rate in the met/met group. ANOVA revealed these differences to be nonsignificant, although a nonsignificant trend was found to exist amongst all three groups for self-ratings of arousal in response to 10 mg AMPH (p = 0.079) as well as for self-ratings of energy in response to 20 mg AMPH (p = 0.074). Despite the lack of a significant difference amongst all three groups, based on previously reported functional differences it would be interesting to be able to analyze a larger group of subjects homozygous for the met allele.
Figure 5.
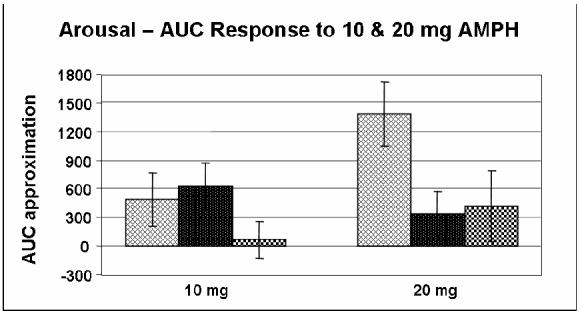
Area under the curve (AUC) approximation of self-ratings of arousal (POMS) in response to 10 mg (p = 0.772) & 20 mg (p = 0.079) AMPH for Caucasian-only subjects in all three genotype groups ( = val/val;
= val/val; = val/met;
= val/met;  = met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
= met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
Figure 6.
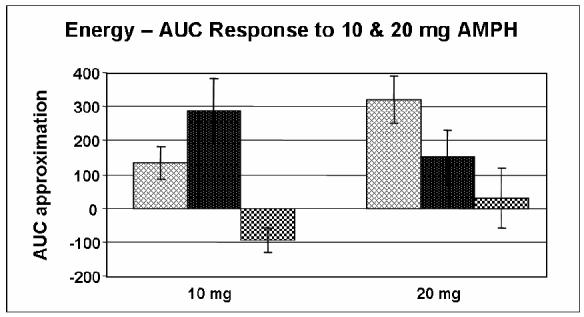
Area under the curve (AUC) approximation of self-ratings of energy (ARCI BG) in response to 10 mg (p = 0.074) & 20 mg (p = 0.163) AMPH for Caucasian-only subjects in all three genotype groups ( = val/val;
= val/val; = val/met;
= val/met;  = met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
= met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
Figure 7.
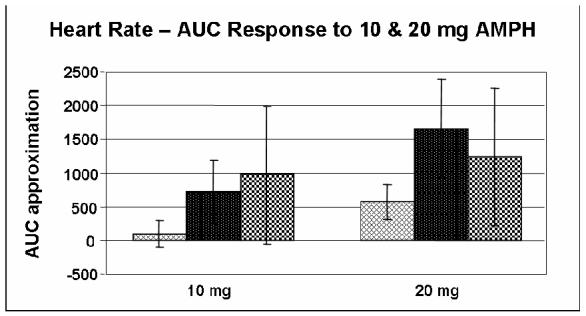
Area under the curve (AUC) approximation of measurement of heart rate in response to 10 mg (p = 0.279)& 20 mg (p = 0.237) AMPH for Caucasian-only subjects in all three genotype groups ( = val/val;
= val/val; = val/met;
= val/met;  = met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
= met/met). AUC was calculated relative to placebo. Data shown are the mean (± SEM) values.
An additional limitation of our study is also derives from the fact that serum levels of amphetamine were not determined at any time in any of the subjects taking part in this study. Therefore we can not be entirely certain that there exists no difference amongst the serum concentration of amphetamine between genotype groups. Furthermore, if there was a difference we are unable to account for any affect this might have had on the subjects’ subjective and physical responses to the different drug doses. However, in order to control for this potential confounding variable we only accepted subjects within a certain BMI (19-26) to control for variations in pharmacokinetics. We have looked at subjects responses to amphetamine in relation to their body weight within this range and found no relationship between weight and amphetamine response (data not published). Moreover, a previous study by Chait et al. supported this assertion by showing that body weight did not influence subjective responses to a 10 mg oral dose of amphetamine. In addition, BDNF is not linked to any loci which would have pharmacogenetic variants relevant to amphetamine metabolism, so any pharmacokinetic variation would only be expected to add random variance. Although this may have contributed to some false negative results, it would not be likely to lead to any false positive results. Although we acknowledge that this is indeed a shortcoming of our study we believe that we have properly controlled for the possibility of differences in response to low doses of amphetamine secondary to differences in pharmacokinetic profile as determined by BMI.
In summary, this study provides evidence that genetic variation in the BDNF gene is related to acute subjective and behavioral effects induced by AMPH in healthy, adult non-substance abusers. Finding the genetic basis for variations in the quality or magnitude of responses to commonly abused drugs may help researchers understand why some individuals are vulnerable to, or protected from, drug addiction.
Acknowledgements
This work was supported by the NIH (DA02812; MO1RR00055). We thank the entire staff of the Human Behavioral Pharmacology Laboratory at the University of Chicago, David Lott, Soo-Jeong Kim, Kathleen Hennessy and the Laboratory for Developmental Neuroscience for their central assistance in the success of the this project.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition American Psychiatric Press; Washington, DC: 1994. p. 886. [Google Scholar]
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. Individual differences in the discriminative stimulus effects of d-amphetamine in humans. Drug Dev Res. 1989;16:451–460. [Google Scholar]
- de Wit H, Griffiths RR. Methods of assessing the abuse liability of sedatives in humans. Drug Alcohol Depend. 1991;28:83–111. doi: 10.1016/0376-8716(91)90054-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the behavioral and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R: Administration, scoring and procedures manual-II. Clinical Psychometric Research; Towson, MD: 1983. p. 71. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Green E, Craddock N. Brain-derived neurotrophic factor as a potential risk locus for bipolar disorder: evidence, limitations, and implications. Curr Psychiatry Rep. 2003;5:469–476. doi: 10.1007/s11920-003-0086-1. [DOI] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Hall D, Dhilla A, Charalambous A, Gogos JA, Karayiorgou M. Sequence variants of the brain-derived neurotrophic factor (BDNF) gene are strongly associated with obsessive-compulsive disorder. Am J Hum Genet. 2003;73:370–376. doi: 10.1086/377003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Agatsuma S. Genetic susceptibility to substance dependence. Mol Psychiatry. 2004;10:1–9. doi: 10.1038/sj.mp.4001622. [DOI] [PubMed] [Google Scholar]
- Hohoff C, McDonald JM, Baune BT, Cook EH, Jr., Deckert J, de Wit H. Interindividual variation in anxiety response to amphetamine: Possible role for adenosine A(2A) receptor gene variants. Am J Med Genet B Neuropsychiatr Genet. 2005;139:42–4. doi: 10.1002/ajmg.b.30228. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH, Jr, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–9. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Kimura M, Miyakawa T, Yoshino A, Murayama M, Masaki T, Higuchi S. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol Clin Exp Res. 2004;28:1609–1612. doi: 10.1097/01.alc.0000145697.81741.d2. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman DL. Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Research. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Nakata K, Ujike H, Sakai A, Uchida N, Nomura A, Imamura T, Katsu T, Tanaka Y, Hamamura T, Kuroda S. Association study of the brain-derived neurotrophic factor (BDNF) gene with bipolar disorder. Neurosci Lett. 2003;337:17–20. doi: 10.1016/s0304-3940(02)01292-2. [DOI] [PubMed] [Google Scholar]
- Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119:767–775. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: Evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Gershon ES, Simmons S, Ebert M, Kessler LR, Dibble ED, Jimerson SS, Brown GM, Gold P, Jimerson DC, Guroff JJ, Storch FI. Behavioral, biochemical and neuroendocrine responses to amphetamine in normal twins and ‘well-state’ bipolar patients. Psychoneuroendocrinology. 1982;7:163–176. doi: 10.1016/0306-4530(82)90009-9. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: differences in dose effects on learning and social behavior. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment--an oligonucleotide microarray approach. J Neurochem. 2004;88:380–393. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]