Abstract
Estimates of the total number of species that inhabit the Earth have increased significantly since Linnaeus's initial catalog of 20,000 species. The best recent estimates suggest that there are ≈6 million species. More emphasis has been placed on counts of free-living species than on parasitic species. We rectify this by quantifying the numbers and proportion of parasitic species. We estimate that there are between 75,000 and 300,000 helminth species parasitizing the vertebrates. We have no credible way of estimating how many parasitic protozoa, fungi, bacteria, and viruses exist. We estimate that between 3% and 5% of parasitic helminths are threatened with extinction in the next 50 to 100 years. Because patterns of parasite diversity do not clearly map onto patterns of host diversity, we can make very little prediction about geographical patterns of threat to parasites. If the threats reflect those experienced by avian hosts, then we expect climate change to be a major threat to the relatively small proportion of parasite diversity that lives in the polar and temperate regions, whereas habitat destruction will be the major threat to tropical parasite diversity. Recent studies of food webs suggest that ≈75% of the links in food webs involve a parasitic species; these links are vital for regulation of host abundance and potentially for reducing the impact of toxic pollutants. This implies that parasite extinctions may have unforeseen costs that impact the health and abundance of a large number of free-living species.
Keywords: climate change, habitat loss, parasite biodiversity
The year 2008 marks the tercentenary of the birth of Linnaeus, the scientist who first provided a formal classification for biological diversity. In the initial edition of Systema Naturae (1), Linnaeus included a group of species—the Paradoxa—that confounded his classification or whose actual existence he questioned. Pelicans, for example, were placed in Paradoxa because Linnaeus thought they might reflect the over-fervent imaginations of New World explorers. Parasitic worms were also placed in Paradoxa because Linnaeus initially thought that they might be confused, or misplaced, earthworms. In later editions of Systema Naturae, Linnaeus revised his opinions about both pelicans and parasitic worms. We now know much about parasites but still rarely think of them as major components of biodiversity. One primary goal of this article is to revise this misconception and quantify the ubiquity of parasitism as a lifestyle. We then attempt to quantify how many parasite species are threatened with extinction.
To quantify the abundance and potential loss rates of parasite biodiversity, we initially need to quantify these measures for their host species. For this we have briefly synthesized the work of May (2, 3), Stork (4), Purvis and Hector (5), and Erwin (6). We then restrict our tally of parasite diversity to parasitic helminths of the vertebrates: trematodes, cestodes, acanthocephalans, and the parasitic nematodes. This tally will synthesize and update an excellent book-length treatment of this question by Poulin and Morand (7, 8). Although our approach uses the best available data for the most comprehensively studied groups of parasites and hosts, our attempts to quantify species numbers and extinction rates for parasites still provide underestimates of the true global values of these parameters for several taxonomic and pragmatic reasons: vertebrates are a small component of host diversity, vertebrates are parasitized by a subset of the helminths, and helminths are not the most fully described parasite taxa.
How Many Species Are There on Earth?
Beginning in 1988, Robert May (2, 3, 9) cogently argued that our inability to estimate the diversity of species on Earth provided a sad and somewhat self-centered testimony to human inquisitiveness. After collating data on the numbers of species in each major taxon, May (2) concluded that our knowledge of vertebrates far exceeded that of invertebrates and protists. The principle reason for the deficient quantitative assessment of diversity in invertebrates and protists was the limited number of trained taxonomists (especially in the tropics, where most of the world's biodiversity resides). Although strides have been made to build capacity in these areas over the last 20 years (10, 11), the number of taxonomists working in the museums of most tropical countries today is roughly comparable to the number that worked in Sweden's museums 250 years ago (in Linnaeus's time, at the dawn of taxonomy). Consequently, classifying and naming species continues to proceed at a slow and uneven rate.
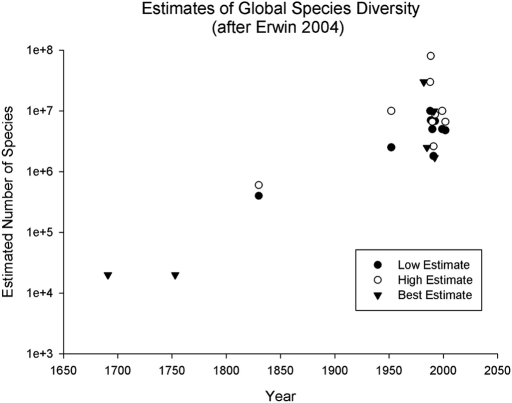
Erwin's (12) work on beetles in tropical forest canopies provided a dramatic illustration of our lack of comprehension of how many extant species exist. Erwin's initial estimates suggested there might be as many as 30 million species of beetles in the world's tropical forests [considerably more than the 20,000 species initially estimated by John Ray (1627–1705) and cataloged by Linnaeus in Systema Naturae (1)]. Erwin's estimate of global insect diversity stimulated a series of articles that used a variety of different approaches to estimate total species numbers. Erwin (6) recently reviewed this literature, and his summary table is illustrated in Fig. 1. Two key patterns emerge. First, estimates of global species diversity have increased almost exponentially since Linnaeus's and Ray's original estimates. Second, various numerical estimates of global biodiversity made during the past 20 years concentrate between 3 and 10 million species, of which only 1.4 million have been formally described. It seems unlikely that we will ever achieve a secure estimate of extant species, particularly because many species seem destined for extinction before they are counted, classified, and formally named.
Fig. 1.
Estimates since the time of Linnaeus of the number of metazoan species. Data are from Erwin (6), and the dates for Linnaeus (1735) and John Ray (1691) were estimated from time of publication of their major books on this topic (6). The most recent sets of estimates sometimes provide a range, or an upper bound, and less frequently a “best estimate” of total species numbers.
How Many Parasite Species?
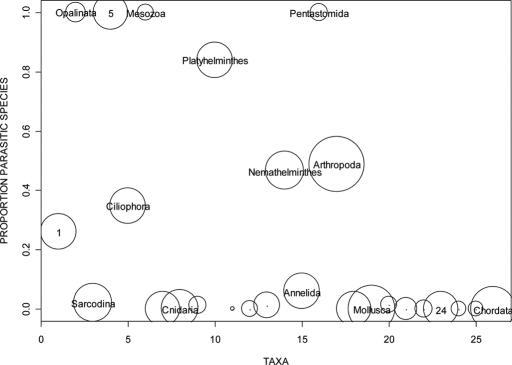
Rohde (23) provides an additional perspective on the ubiquity of parasitism as a lifestyle by estimating the numbers of parasitic species in each of the major taxa. A graphical representation of these data suggests that ≈40% of known species are parasitic, with parasitism ubiquitous in some taxa and either absent or rare in others (Fig. 2).
Fig. 2.
Relative abundance of different taxa, and the proportion of parasitic species in those taxa (data from Rohde, 1982, Ref. 23). Taxa are numbered along the x axis as follows: 1, Mastigophora; 2, Opalinata; 3, Sarcodina; 4, Apicomplexa/Microspora; 5, Ciliophora; 6, Mesozoa; 7, Porifera; 8, Cnidaria; 9, Ctenophora; 10, Platyhelminthes; 11, Priapulida; 12, Entoprocta; 13, Nemertina; 14, Nemathelminthes; 15, Annelida; 16, Pentastomida; 17, Arthropoda; 18, Tentaculata; 19, Mollusca; 20, Echiurida; 21, Sipunculida; 22, Hemichordata; 23, Echinodermata; 24, Pogonophora; 25, Chaetognatha; 26, Chordata. The area of a circle corresponds to the natural log of the total number of species in a taxon, and the center of the circle corresponds to the proportion of parasitic species in that taxon.
Poulin and Morand (7, 8) have used several approaches to further examine the potential diversity of parasitic helminths. They point out that many of the problems that beset estimates of free-living biodiversity also confound estimates of parasite diversity. In particular, the rate of discovery of new parasite species has grown linearly or exponentially in some well studied helminth taxa. In contrast, sampling of parasite diversity from the most diverse parts of the world is thin at best. For example, Cribb et al. (13) estimated that in groupers (Epinephelinae)—one of the largest and most common groups of marine fish—parasitic trematodes have been recorded from only 62 of the 159 species, and from only 9 of 15 genera. The absences reflect a paucity of sampling; most species were examined at only one location. Moreover, not only are most host species unstudied, but no tropical species of grouper has been exhaustively sampled for trematodes. This creates a significant problem for estimating global species richness of parasites based on extrapolations from known patterns of host specificity.
While acknowledging these problems, Poulin and Morand (7, 8) extrapolated estimates of specificity from studies of parasites in the relatively well surveyed vertebrates. Their summary table suggests that there are at least 50% more parasitic helminth species (≈75,000) than there are vertebrate hosts (45,000) (Table 1). [The number of parasite species could actually be much higher, especially because fish species are hugely undersampled (13, 14), as are the reptiles, amphibians, and indeed all vertebrate groups in the tropics (15).]
Table 1.
Estimates of mean number of parasite species per host, mean host specificity, and global species richness for the parasitic trematodes, cestodes, nematodes, and acanthocephalans that parasitize each of the major vertebrate taxa of hosts (after ref. 7)
| Parasite species | Host species (known no. of host species) |
||||||
|---|---|---|---|---|---|---|---|
| Chondrichthys (843) | Osteichthys (18,150) | Amphibia (4,975) | Reptilia (6,300) | Aves (9,040) | Mammalia (4,637) | Total (43,945) | |
| Mean parasite species per host species | |||||||
| Trematoda | 0.12 | 2.04 | 1.27 | 1.06 | 3.24 | 1.61 | |
| Cestoda | 2.71 | 1.57 | 0.27 | 0.39 | 3.67 | 1.89 | |
| Acanthocephala | — | 1.01 | 0.19 | 0.42 | 0.72 | 0.28 | |
| Nematoda | 0.48 | 1.49 | 2.82 | 2.15 | 3.32 | 3.90 | |
| Mean host specificity | |||||||
| Trematoda | 2.00 | 6.35 | 5.40 | 1.77 | 2.97 | 2.01 | |
| Cestoda | 1.69 | 6.38 | 4.75 | 2.21 | 2.36 | 1.89 | |
| Acanthocephala | — | 14.95 | 6.74 | 12.50 | 8.35 | 4.32 | |
| Nematoda | 2.67 | 10.28 | 5.27 | 2.12 | 3.28 | 6.07 | |
| Estimated global species richness | |||||||
| Trematoda | 51 | 5,831 | 1,170 | 3,773 | 9,862 | 3,714 | 24,401 |
| Cestoda | 1,352 | 4,466 | 283 | 1,112 | 14,058 | 4,637 | 25,908 |
| Acanthocephala | — | 1,226 | 140 | 212 | 779 | 301 | 2,658 |
| Nematoda | 152 | 2,631 | 2,662 | 6,389 | 9,150 | 2,979 | 23,963 |
| Total | 1,555 | 14,154 | 4,225 | 11,486 | 33,849 | 11,631 | 76,930 |
Modern molecular methods have revealed a further bias that suggests that we have underestimated parasite species richness. These methods have revealed significant numbers of “cryptic-species” of parasite that look morphologically similar but are sufficiently genetically distinct so as to represent different species (e.g., see refs. 16–19). The number of cryptic parasite species previously classified as a single morphologically recognized species can sometimes be disconcertingly high [for example, Miura et al. (20) distinguished eight genetic species for a single morphospecies]. The issue of cryptic species will significantly distort estimates of global parasite species richness based on extrapolations from host specificity and mean numbers of parasites observed per host species. One of the basic elements of Poulin and Morand's extrapolation is the number of hosts used by a parasite (Table 1). As parasites use more hosts, estimates of global diversity go down. However, many studies have found that cryptic species parasitize only a subset of the species originally recognized as hosting a parasite morphospecies (e.g., see refs. 17 and 21). Thus, considerations of cryptic species might well lead to a further doubling of the estimates of global parasite richness, suggesting that there could be >300,000 parasitic helminth species using vertebrates as hosts.
How Many Parasite Species per Host Species?
In the best-studied taxa, an average mammalian host species appears to harbor two cestodes, two trematodes, and four nematodes, and an acanthocephalan is found in every fourth mammalian species examined. Each bird species harbors on average three cestodes, two trematodes, three nematodes, and one acanthocephalan (7, 8, 22). None of these estimates take possible unrecognized cryptic species into account, but, in general, helminths that parasitize avian species seem to be less host-specific than those that parasitize mammals. Ultimately, the parasitic fauna of any host species reflects its interaction with the host's feeding niche, latitudinal range, and social system.
The survey of parasite diversity provided by Poulin and Morand raises many unanswered questions. Do host species from monospecific genera harbor more specialized parasites than do species from more diverse genera or families? What is the status of parasite diversity in the tropics? Nearly all parasite data for non-human hosts have been collected from the commonest species of the temperate zone.
Studies of helminth parasites of fishes suggest that latitudinal gradients of diversity are more complex than are those of their hosts. There are many more fish species in the tropics, so we might initially expect there to be more parasite species as well. But, if high host diversity in the tropics leads to low densities of each host species, then some host-specific parasites might be unable to maintain viable populations in their low-density tropical hosts, in which case host-specific parasites and their hosts could exhibit reverse gradients of species diversity. Empirically, the two best studied parasite taxa show opposite trends: tropical fish species have more monogenean parasites per host species than do those in temperate zones (23–25), whereas tropical fish species have less diverse gut parasites than do their temperate counterparts (26, 27). The monogeneans predominantly live on the skin and gills of fish and are either transmitted directly by physical contact between hosts (in the case of the Gyrodactyloidea, the most speciose monogenean group) or via short-lived infectious stages known as oncomiracidia. Thus, monogeneans may be more host-specific, assuming that transmission occurs primarily between individuals living in conspecific social groups. In contrast, the gut parasites may tend to be host-generalists because they characteristically enter a host via predation on infected prey species that may be a component of the diet of many host species. More research is needed to understand how these differences in habitat and transmission mode drive the different gradients of parasite species diversity.
How Many Parasites and What Is Their Role in an Ecological Food Web?
An alternative approach to ascertain global estimates of parasite diversity is simply to examine how many parasites are in a specific habitat or ecosystem. We have been undertaking this for salt marshes along the coasts of California and Baja, Mexico (28, 29, 64). The initial results confirm that ≈40% of the species in any location are parasitic on the 60% of species that are free-living. However, consideration of the trophic links of the parasitic species significantly changes our perception of how ecological food webs are structured.
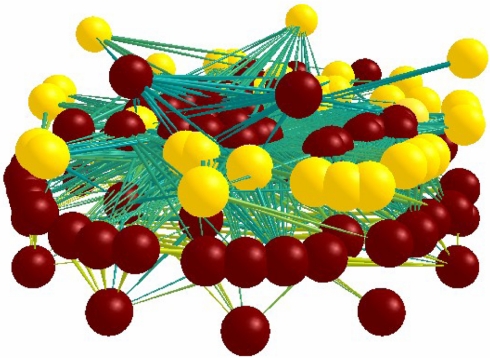
The standard ecological food web is normally considered to be a trophic pyramid, with primary producers on the bottom, fewer species of herbivores on the next level, and even fewer predatory species higher up (30). When parasites are included, this pattern is almost literally “turned on its head” (Fig. 3); essentially, a second web appears around the free-living web, and this completely changes the level of connectivity. The addition of ≈40% more species to the community leads to four times the number of trophic connections between species, thus creating a web that is much more tightly coupled. In many ways, parasite species appear as hidden “dark matter” that holds the structure of the web together, and in ways that are very different from those of free-living species (Fig. 3). Furthermore, the web's structure changes from a pyramid to an inverted rhomboid. Predatory species at high trophic levels are now seen to be consumed from within by a diversity of parasites. Animals at lower trophic levels have fewer parasites, but they are often essential hosts for specific stages of parasite that need hosts from two or three different trophic levels to complete the life cycle. When transmitting between trophic levels, only a minority of parasites successfully infect a host; most parasite individuals are consumed as planktonic prey items by many of the species they are trying to parasitize.
Fig. 3.
Three-dimensional visualization of the complexity of a real food web with parasites from the Carpinteria Salt Marsh web using WoW software. Balls are nodes that represent species. Parasites are the light-shaded balls, and free-living species are the dark-shaded balls. Sticks are the links that connect balls through consumption. Basal trophic levels are on the bottom, and upper trophic levels are on the top. Figure from Lafferty et al. (65).
Even if a parasite successfully establishes in a host, it is often consumed when the host becomes a prey item in the diet of a predator. Natural selection has made considerable use of this resource–consumer link and allowed parasites to continue their life cycle in the viscera of predatory species. In many cases, the parasites have evolved to modify the behavior of the prey to make it more accessible to the predator, thus significantly increasing transmission efficiency through this stage of the life cycle (31, 32). We suspect that the food-web structure observed in salt-marsh communities is common to most natural ecological communities, with parasite species comprising ≈40% of the local species diversity but exerting significant stabilizing forces that hold together the structure of much of the free-living web.
How Rapidly Are We Losing Hosts and Parasites?
Estimates for the loss of biodiversity use a variety of methods to compare current rates of species extinction against background rates (33, 34). All of these methods suggest that we are entering a period of mass extinction that is directly comparable to the mass extinctions recorded in the fossil record. Poulin and Morand (7) used the proportion of threatened hosts in each major vertebrate taxon to estimate the potential threatened number of parasitic species. We have modified their projection to consider different levels of host specificity (Table 2). Poulin and Morand's original calculation assumed a direct correspondence between the proportion of parasites threatened and the proportion of hosts threatened. This figure was then adjusted by the degree of host specificity of the parasites. Koh et al. (35) performed a similar analysis, using more sophisticated models on select groups of hosts and parasites for which they acquired good data on host-use patterns. All of their data suggest that the relationship between host extinction and parasite species extinction is concave, with parasites (and other dependent species) lost more rapidly than their free-living host species. However, the two groups of parasites that they examined (lice and pinworms of primates) both have very high host specificities, so we would expect quite a tight matching between host extinction and parasite extinction.
Table 2.
Percentage of vertebrate species listed as threatened by IUCN Red List and the estimated numbers of parasitic helminth species that this puts at risk of extinction (upper) [Poulin and Morand (7), who assume that the proportion of parasite species at risk equals the proportion of hosts at risk], and proportion of parasites at risk when corrected for different levels of hosts specificity exhibited by each parasite taxa in each host taxa (lower)
| Parasite species | Hose species (% of host species listed as threatened) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Chondrichthys (2) | Osteichthys (2) | Amphibia (2) | Reptilia (3) | Aves (11) | Mammalia (11) | Total | ||
| No. of parasite species at risk | ||||||||
| Trematoda | 1 | 117 | 23 | 113 | 1,085 | 409 | 1,748 | |
| Cestoda | 27 | 89 | 6 | 33 | 1,546 | 510 | 2,211 | |
| Acanthocephala | — | 25 | 3 | 6 | 86 | 33 | 153 | |
| Nematoda | 3 | 53 | 53 | 192 | 1,007 | 328 | 1,636 | |
| Trematoda | 1 | 18 | 4 | 64 | 365 | 203 | 656 | |
| Cestoda | 16 | 14 | 1 | 15 | 655 | 270 | 971 | |
| Acanthocephala | — | 2 | 0 | 1 | 10 | 8 | 20 | |
| Nematoda | 1 | 5 | 10 | 90 | 307 | 54 | 468 | |
| Totals (%) | 18 (1.13) | 39 (0.28) | 16 (0.38) | 170 (1.48) | 1,338 (3.95) | 535 (4.60) | 2,115 (2.75) | |
The estimates of parasite species extinction rate that Poulin and Morand initially produced failed to account for patterns of host specificity (upper section of Table 2) and produced high estimates for loss rates of parasite diversity. When we take host specificity into account, parasitic species seem to go extinct at a lower rate than the host species (lower section of Table 2); only ≈3% of helminths (≈2,000 species among 75,000 total) would then seem to be endangered. If our estimates of net parasitic helminth diversity are low by as much as a factor of four, then there could be as many as 10,000 threatened parasitic helminth species. All of this suggests that we are likely to lose considerable numbers of parasitic helminth species before we have had time to obtain specimens that might be identified and classified.
The numbers for parasitic helminth diversity calculated by Poulin and Morand (Table 1) suggest that the bulk of parasitic helminth diversity occurs in birds. The majority of these species will have complex life cycles and thus will also depend on host species at lower trophic levels to complete their life cycles. For example, most of the trematode species also require a snail species in which they undergo asexual reproduction, and many will then pass through another intermediate host that will be a prey item in the diet of the bird that acts as the definitive host in which the parasite reproduces sexually. Although the trematode may be able to use a diversity of different bird species as a definitive host, it will most likely be specific to the snail host. As we will show in the next section, projected avian extinctions imply that the spatial patterns of avian loss will be a major driver of the loss of parasite diversity.
Where Do Avian Hosts Occur?
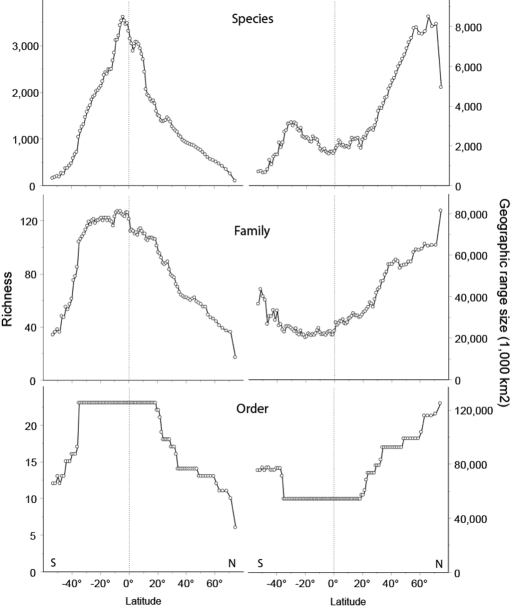
We have used a nearly complete, geo-referenced database of the geographical distributions of all of the world's 8,750 land-bird species to illustrate the geographic patterns of potential avian host diversity (sea birds and mainly pelagic species are excluded). These data reveal a range of patterns for avian diversity (Fig. 4) that are not only fascinating from the perspective of avian evolutionary radiations, but also raise an intriguing set of questions about patterns of parasite geographical diversity.
Fig. 4.
Latitudinal relationship between taxonomic richness (left) and geographical range size (right) for all 9,754 bird species at three different taxonomic levels: species, family, and order. Only breeding distributions were included, and range sizes were measured over dry land and averaged across all species, families, or orders occurring at a given latitudinal band.
For example, avian species diversity peaks in the tropics and declines rapidly toward the poles. Broadly similar patterns occur at higher taxonomic levels, but the rates of latitudinal decline are less rapid, because many of the bird orders and families that evolved in the tropics have representatives that radiated into the temperate and arctic zones. In contrast, few evolutionary radiations in the temperate (or arctic) zones have spread back into the tropics. A major future challenge is to examine how the pattern of parasitic helminth diversity maps onto this pattern of host diversity. Our null expectation is that the two patterns should be concordant, but the high levels of host species diversity per order (and per family) in the tropics would suggest we are likely to see more generalist parasites (using closely related host species) in the tropics and more specialist species in the taxonomically poorer temperate and arctic zones. However, studies from Beringia (in the high Arctic) suggest that significant levels of parasite interchange occur during intermittent periods of climatic warming when host species from the arctic regions of different continents disperse across the poles and provide new host opportunities for their parasites (36).
If the range size of avian species, orders, and families increases with distance from the equator (Fig. 4), might we see a similar effect with the range size of parasites? If so, then this will have caused us to further underestimate the diversity of parasites in the tropics, because the area sampled by tropical parasite taxonomists is tiny. Similarly, do the nested patterns of geographical diversity for the hosts reflect pulses of radiation and speciation between the tropics and temperate zones after past periods of climate change, and would we see similar radiations of diversity if we traced the phylogenies and geographical distributions of avian parasites at different taxonomic levels? Surveys suggest that the diversity of human parasites is significantly higher in the tropics (37, 38), but as we saw above, this is less clearly the case for fish parasites. If similar latitudinal patterns occur in avian orders and genera, and if parasites are responsible for driving significant components of sexual selection that lead to host speciation, then we might expect complex patterns of geographical variation in parasite diversity at the taxonomic level of host order and family. Unfortunately, the parasite data with which to test these hypotheses are unavailable.
Loss of Avian Diversity: Climate Change Versus Habitat Loss
We have used the geographic distribution database for birds described above to evaluate potential impacts of projected environmental change on each of the major continents (39). The Millennium Ecosystem Assessment (MEA) used four quantitative scenarios to examine how land cover would change across the land surface of the Earth over the next 50 and 100 years (40, 41). The scenarios were driven by quantitative climate models derived from the IPCC (Intergovernmental Panel on Climate Change) and projections of human population growth, wealth, and other socioeconomic parameters across regions (42). In these projections, rates of land conversion would be driven either by climate change or by the need for new agriculture land. The four MEA scenarios were defined by whether or not governments take a proactive or reactive response to environmental management, and by whether the world's nations become more unified and interactive or they become more protectionist and isolated (43). Jetz et al. (39) used the output from the scenarios to examine the potential impact on the world's land-bird species under the simplifying assumption of stationary geographic ranges.
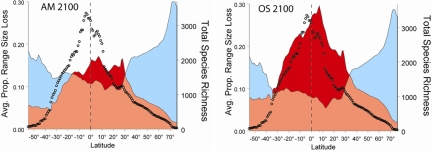
Projections of land-use change based on the different MEA Scenarios have revealed consistent geographical patterns of impact. The projections differ mainly in the magnitude of their impacts, with the reactive and isolationist scenarios experiencing about twice the rate of habitat conversion as the scenarios for proactive and connected worlds (40). In all cases, the impacts of climate change in the next 50 to 100 years are largest in polar regions. Although climate change also has effects in the temperate and tropical zones, these are almost completely masked by human agricultural expansion, particularly in the tropics. This pattern of land-use change will interact directly with the geographical variation in the range sizes of bird species. In particular, bird species with small ranges are at a much greater risk of extinction than those with large geographical ranges (39). Unfortunately, most avian species living in the tropics have small ranges and a significant number will experience large declines in range size due to agricultural habitat conversion. In contrast, the minority of species that live in the polar zones are projected to experience large potential loss of range due to climate change, but they usually have sufficiently large geographical ranges that some of their environment remains habitable (Fig. 5) (39).
Fig. 5.
The relationship between geographic range size and percentage range transformations for all of the world's 8,750 land birds under two MEA Scenarios of future land-use change. (Left) “Adaptive mosaic” (which assumes a world with open political dialogue that deals proactively with environmental problems). (Right) “Order from strength” scenario (which assumes a more insular political world that only deals retroactively with environmental problems). Jetz et al. (39) provide complete detail for how the analyses were developed. The dots illustrate number of avian species, blue shading denotes range change due to climate change, and red illustrates land use change due to agricultural expansion.
How Many Bird Parasites Have We Lost?
Parasite species ultimately depend on their host species for persistence (35, 44). The analysis of future bird extinctions described above suggests that rare and specific tropical parasite species will be lost rather rapidly as tropical bird species decline in range and abundance, or go extinct. However, common parasite species that can use a range of host species in the temperate zone may be significantly buffered against extinction (45, 46). This suggests that the relationship between loss of host species and loss of parasite species will tend to be concave (35). At best, the relationship between host extinction rate and parasite extinction rate may be sigmoidal in shape, with the point of inflexion determined by the relative proportion of species that are specific to individual host species. Unfortunately, insufficient data exist to accurately examine the shape of these relationships. In general, we expect that inefficiently transmitted parasites (or pathogens) will tend to be lost first, whereas efficiently transmitted species with low host specificity (due to their use of vectors or trophic transmission) will persist at low host densities.
Although a parasite species that can use a range of host species will not go extinct if one of its hosts species declines to extinction, it is likely that the abundance and geographical range of a parasite species will decline as each potential host species is lost or itself declines in range and abundance. This suggests that parasitic species will tend to decline at a faster rate than their hosts. Furthermore, as noted by Poulin and Morand (7) and Koh et al. (35), parasites with complex life cycles that require multiple host species will be more prone to extinction as natural habitats are destroyed or disrupted than will be pathogens with direct life cycles. Additionally, given the existence of minimum thresholds of host density below which many parasites cannot sustain recruitment (47), many parasites will go extinct even before their hosts disappear. Arguably the least endangered parasites will be sexually transmitted pathogens and pathogens transmitted from infected females to their offspring (48, 49). Although highly host-specific, such pathogens can persist in smaller host populations than the normal directly transmitted pathogens (48, 50).
Ecosystems Services Lost?
It may be that the loss of a significant proportion of the world's parasitic helminth species is a tragedy only for parasitologists. Indeed, once a host species loses its parasite species, it might experience an increase in population size that could prevent it from declining to extinction. However, this perspective ignores several important “ecosystem services” that parasites perform. For example, parasites often act as regulators of host abundance, which in the case of generalist pathogens may lead to strong frequency-dependent control over relative host abundance throughout the host community (51). Another example involves parasitic helminths that may play a major role in buffering levels of pollution in natural communities (52).
Regulation of Host Populations and Relative Abundance in Communities
Parasites create a diversity of links in food webs that at first site may appear atypical, but they are not unusual in nature—more than 75% of links in natural food webs probably involve parasites (28). Because many parasites use multiple competing hosts on the same trophic level, their population dynamics may be modeled by sets of coupled differential equations that take the general form
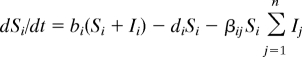 |
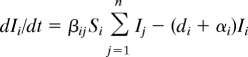 |
where we assume that each host species i has species-specific birth and death rates (b and d) and experiences transmission of the pathogen at a rate βij from infected individuals of species j. Infection converts each susceptible host, S, into an infectious individual, I, that experiences an increased pathogen-induced mortality rate, α. When compared with single species infectious disease models, the presence of interspecific transmission is usually strongly stabilizing for a wide range of interspecies transmission rates that are less than the rates of within-species transmission (51). However, when rates of interspecific transmission approach rates of within-species transmission, the pathogen acts as a powerful mechanism of indirect competition [as a shared natural enemy (53)] that can drive some host species extinct.
We can examine the potential consequences of this for more complex systems by recasting the differential equation models within the matrix framework that describes the initial trajectory of a perturbation to the whole food web. Thus, each element of the matrix represents a pairwise interaction between each pair of species in the food web (54, 55). If we retain our classification of each host as susceptible and infected, then the parasite in effect enters the food web as two species. Both have the phenotype of the host (although the feeding preferences might change after infection). However, the infected hosts now effectively have the genotype of the pathogen, and transmission acts as a birth process converting susceptible hosts into infected individuals that can also be considered as “shared natural enemies” of uninfected hosts of all susceptible species. We can briefly examine a submatrix of food web interactions for specialist and generalized pathogens within a food web.
Specialist parasites and competing host species
 |
Pathogens shared between competing host species
 |
In these two matrices of species interaction, host species A and B compete with each other for resources such as food or space, and each host species has a pathogen associated with it (thus infected hosts of species A are characterized as “species” Ia). In the case of specialist parasites (upper matrix), infected hosts of species A cannot infect species B; the complementary case operates for the lower matrix, where both species of pathogen infect both species of host. The main consequence of host species sharing nonspecific parasite species is that several elements of the interactions matrix have to be converted (across the man diagonal) from “zeros” into “plus–minus” consumer–resource relationships. If we are concerned with the stability properties of the web, then May (56) has shown that the dominant eigenvalues of this matrix have to be negative if there is to be any hope of web stability. In May's initial formulation, increased species diversity and hence increased connectance should reduce the probability that the web is stable. However, although the net effect of shared pathogens is to increase the connectance of the food web, this occurs in a subtle and important way. Namely, the conversion of specific pathogens to generalized pathogens greatly increases the proportion of “across-diagonal” plus–minus links in the web. Because the product of their interaction is always negative, adding more summed negative terms increases the chances that this eigenvalue will be negative (57). More specifically, adding shared pathogens to the food web significantly increases the proportion of negative cross-product terms relative to positive product terms produced by competition (where negative times negative = positive!). This effect generalizes: As we increase the species diversity of the web, destabilizing competitive interactions will increase at a maximum rate of (n2 − n)/2, whereas potentially stabilizing shared pathogen interactions increase at the significantly faster maximum rate of n2.
Similar effects arise when we consider parasites with complex multiple host life cycles. These infectious agents confound traditional concepts of food-web structure because they feed on several different trophic levels within different host species during the course of their life cycle. They also act as food resources to species on different trophic levels as they pass through their free-living stages. Usually, <1% of the energy-rich, free-living infective stages of a parasite ever manage to infect a host; the other 99% are eaten by planktivorous species. Parasites with this type of life cycle can again be incorporated into food-web models. Initial results with matrix models of the form described above suggest that such parasites will also have key stabilizing effects on the structure of food webs because they also add pairwise sequences of “plus–minus” resource–consumer interactions at every stage of their life cycle, and these will consistently increase the probability that the dominant eigenvalues of the linearized system will be negative. Furthermore, generalist parasites with complex multihost life cycles also introduce long circular loops of relatively weak links into the web; theoretical analysis by Neutel (58) suggests that these may also be important in imparting stability to food webs.
Thus, generalist parasites and those with complex life cycles potentially play important roles in regulating the relative abundance of their free-living host species. Whereas generalist species with direct transmission are likely to be buffered from extinction by the rescue effect of at least one host remaining abundant, parasites with complex life cycles will depend highly on the host species in the life cycle to which they are most specifically adapted. The trematode and acanthocephalan species that are recorded as adult worms from scores of vertebrate host species are often depend entirely on a single species of mollusk or amphipod that serves as their intermediate host. Thus, snails or other invertebrates that invade natural ecosystems and replace crucial host species within the complex life cycles of parasites may lead to losses of parasite diversity that cascade throughout the food web.
Regulation of Concentration of Pollutants
Recent work by Sures (52, 59) and colleagues has shown that parasitic helminths may play a substantial role in concentrating and ultimately removing heavy metals and other pollutants from their hosts. They can concentrate and withstand levels of cadmium, zinc, and other heavy metals that are up to 2,000% above background levels (and ≈1,000 times greater than the levels sustained by snails and other host species widely used as monitors of toxicants and pollutants). Parasites achieve this level of concentration through their preference for absorbing bile from the guts of their hosts. Most vertebrates attempt to minimize the impact of harmful substances in their gut by surrounding the offensive items with bile and passing them out in their feces. However, significant amounts of the substance are reabsorbed with bile in the lower intestine. This occurs to a much lesser extent in hosts parasitized by parasitic helminths; many of these parasites selectively absorb bile as a food source, thereby removing the pollutants from the host's gut and concentrating them in the worm (52).
Results from studies of salt-marsh ecosystems suggest that metazoan parasites constitute up to ≈3% of the biomass of major animal groups in the system (64). If parasites are 3% of the animal biomass, then their ability to superconcentrate pollutants may mean that they contain 30–50% of the mass of pollutants in the system. This would amount to a formidable ecosystem service! We note, however, that this assumes that the many different groups of metazoan parasites studied in ref. 64 are as efficient at absorbing pollutants as the adult stages of helminths in the guts of vertebrates studied by Sures (52, 59). Nevertheless, a relatively small biomass of adult worms in vertebrates may sequester a significant proportion of the pollutants that would otherwise disrupt the viability of host populations. This suggests that if parasites are lost via extinction of their hosts, or via replacement of intermediate hosts by nonviable invasive host species, then the free-living host species may experience enhanced levels of pollutants. Parasitic helminths of humans supply a similar ecosystem service when they selectively remove both pollutants and allergens from human guts. This provides a viable explanation for why allergies are much more common in human societies that have successfully reduced their parasite loads than in those that still bear a significant burden of parasitic helminths (60).
In conclusion, we suggest that there is reason to join Sprent (61) and Windsor (62) in mourning the loss of the parasitic species that disappear when their hosts go extinct (35, 44). If significant increases in extinction rates now apply to birds, mammals, amphibians, and fish, then it is almost inevitable that extinction rates in host-specific parasite species are increasing at least concomitantly. As we develop a deeper understanding of food-web structure and dynamics, it seems increasingly likely that parasitic helminths play a major role in ecosystem function and may even supply important economic services to humans. Understanding the structure of food webs remains among the deepest scientific challenges of the 21st century. Parasites will play a key role in developing this understanding, yet they are at least as threatened by mass extinction as are many other species—potentially even more so. A healthy functioning ecosystem will have a full complement of parasitic species (63). Fully determining the role that parasites play in regulating natural systems remains a major challenge for ecologists and evolutionary biologists. If the major job of conservation biologists is to maintain fully functional food webs, then it is crucial that we consider parasites as a vital and necessary component of biodiversity. It is then but a small step to acknowledge that these animals are well worth conserving.
Acknowledgments.
A.D.'s thinking about this whole topic was hugely shaped by many conversations with Robert M. May; we are very grateful to him for the insights provided by these discussions and to John Avise, Doug Erwin, Micheal Donoghue, Nadia Talhouk, and Alejandra Jaramillo for comments on an earlier draft. The first draft of the article was written in Kilimanjaro, Nairobi, and Heathrow Airports; A.D. thanks British Airways and Precision Air for the patience, care, and attention of their ground staff. This work was supported by National Institutes of Health National Science Foundation Ecology of Infectious Disease Program Grant DEB-0224565.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution II: Biodiversity and Extinction,” held December 6–8, 2007, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_biodiversity.
An initial report of this work was presented in November 2007 at the Royal Swedish Academy during a symposium to celebrate the tercentenary of the birth of Linnaeus.
The authors declare no conflict of interest.
References
- 1.Linnaeus C. Systema Naturae: Sive Regna Tria Naturae Systematice Proposita per Classes, Ordines, Genera, and Species. Leiden, The Netherlands: Theodorum Haak; 1735. [Google Scholar]
- 2.May RM. How many species are there on earth? Science. 1988;241:1441. doi: 10.1126/science.241.4872.1441. [DOI] [PubMed] [Google Scholar]
- 3.May RM. How many species? Philos Trans R Soc London Ser B. 1990;330:293–304. [Google Scholar]
- 4.Stork NE. How many species are there? Biodiversity Conserv. 1993;2:215–232. [Google Scholar]
- 5.Purvis A, Hector A. Getting the measure of biodiversity. Nature. 2000;405:212–218. doi: 10.1038/35012221. [DOI] [PubMed] [Google Scholar]
- 6.Erwin TL. The biodiversity question: How many species of terrestrial arthropods are there? Forest Canopies. In: Lowman MD, Rinker HB, editors. Amsterdam: Elsevier; 2004. pp. 259–269. [Google Scholar]
- 7.Poulin R, Morand S. Parasite Biodiversity. Washington, DC: Smithsonian; 2004. p. 216. [Google Scholar]
- 8.Poulin R, Morand S. The diversity of parasites. Q Rev Biol. 2000;75:277–293. doi: 10.1086/393500. [DOI] [PubMed] [Google Scholar]
- 9.May RM. How many species inhabit the Earth? Sci Am. 1992;267:42–48. [Google Scholar]
- 10.Janzen DH. Priorities in tropical biology. Trends Ecol Evol. 1994;9:365–367. doi: 10.1016/0169-5347(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 11.Smith R, Rogo L. Partnerships for the Global Taxonomy Initiative, CBD Technical Series. 2005. Available at www.bionet-intl.org/opencms/export/sites/default/resourceCentre/pdf/BioNET_SBSTTA11_Extended_Abstract.pdf.
- 12.Erwin TL. Tropical forests: their richness in Coleoptera and other arthropod species. Coleoptera Bull. 1982;36:74–82. [Google Scholar]
- 13.Cribb TH, et al. The trematodes of groupers (Serranidae: Epinephelinae): Knowledge, nature and evolution. Parasitology. 2002;124:S3–S42. doi: 10.1017/s0031182002001671. [DOI] [PubMed] [Google Scholar]
- 14.Hoberg EP, Klassen GJ. Revealing the faunal tapestry; Co-evolution and historical biogeography of hosts and parasites in marine systems. Parasitology. 2002;124:S2–S22. doi: 10.1017/s0031182002001841. [DOI] [PubMed] [Google Scholar]
- 15.Brooks DR, Hoberg EP. Triage for the biosphere: The need and rationale for taxonomic inventories and phylogenetic studies of parasites. Comp Parasitol. 2000;67:1–25. [Google Scholar]
- 16.Hung GC, et al. Molecular evidence for cryptic species within Cylicostephanus minutus (Nematoda: Strongylidae) Int J Parasitol. 1999;29:285–291. doi: 10.1016/s0020-7519(98)00203-3. [DOI] [PubMed] [Google Scholar]
- 17.Jousson O, Bartoli P, Pawlowski J. Cryptic speciation among intestinal parasites (Trematoda: Digenea) infecting sympatric host fishes (Sparidae) J Evol Biol. 2000;13:778–785. [Google Scholar]
- 18.Haukisalmi V, et al. Molecular and morphological evidence for multiple species within Paranoplocephala omphalodes (Cestoda, Anoplocephalidae) in Microtus voles (Arvicolinae) Zool Scripta. 2004;33:277–290. [Google Scholar]
- 19.Perrot-Minnot MJ. Larval morphology, genetic divergence, and contrasting levels of host manipulation between forms of Pomphorhynchus laevis (Acanthocephala) Int J Parasitol. 2004;34:45–54. doi: 10.1016/j.ijpara.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Miura O, et al. Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse) Int J Parasitol. 2005;35:793–801. doi: 10.1016/j.ijpara.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Reversat J, Renaud F, Maillard C. Biology of parasite populations, the differential specificity of the genus Helicometra Odhner, 1902 (Trematoda, Opecoelidae) in the Mediterranean Sea demonstrated by enzyme electrophoresis. Int J Parasitol. 1989;19:885–890. [Google Scholar]
- 22.Poulin R. The intra- and interspecific relationships between abundance and distribution in helminth parasites of birds. J Anim Ecol. 1999;68:719–725. [Google Scholar]
- 23.Rohde K. In: Ecology of Marine Parasites, Australian Ecology Series. Heatwole H, editor. St Lucia, QLD, Australia: Univ of Queensland Press; 1982. p. 245. [Google Scholar]
- 24.Rohde K. Ecology and biogeography of marine parasites. Adv Mar Biol. 2002;43:1–86. doi: 10.1016/s0065-2881(02)43002-7. [DOI] [PubMed] [Google Scholar]
- 25.Rohde K. Latitudinal gradients in species diversity and Rapoport's rule revisited: A review of recent work and what parasites teach us about the causes of the gradients. Ecography. 1999;22:593–613. [Google Scholar]
- 26.Choudary A, Dick TA. Richness and diversity of helminth communities in tropical freshwater fishes: Empirical evidence. J Biogeogr. 2000;27:935–956. [Google Scholar]
- 27.Poulin R. Another look at the richness of helminth communities in tropical freshwater fish. J Biogeogr. 2001;28:737–743. [Google Scholar]
- 28.Lafferty KD, Dobson AP, Kuris AM. Parasites dominate food web links. Proc Natl Acad Sci USA. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafferty KD, et al. Food webs and parasites in a salt marsh ecosystem. In: Collinge SK, Ray C, editors. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford: Oxford Univ Press; 2006. pp. 119–134. [Google Scholar]
- 30.Lindeman RL. The trophic-dynamic aspect of ecology. Ecology. 1942;23:399–418. [Google Scholar]
- 31.Dobson AP. The population biology of parasite-induced changes in host behavior. Q Rev Biol. 1988;63:139–165. doi: 10.1086/415837. [DOI] [PubMed] [Google Scholar]
- 32.Lafferty K. Foraging on prey that are modified by parasites. Am Nat. 1992;140:854–867. [Google Scholar]
- 33.May RM, Lawton JH, Stork NE. Assessing extinction rates. In: Lawton JH, May RM, editors. Extinction Rates. Oxford: Oxford Univ Press; 1995. [Google Scholar]
- 34.Regan HM, et al. The currency and tempo of extinction. Am Nat. 2001;157:1–10. doi: 10.1086/317005. [DOI] [PubMed] [Google Scholar]
- 35.Koh LP, et al. Species coextinctions and the biodiversity crisis. Science. 2004;305:1632–1634. doi: 10.1126/science.1101101. [DOI] [PubMed] [Google Scholar]
- 36.Hoberg EP, Adams AM. Phylogeny, historical biogeography, and ecology of Anophrycephalus spp. (Eucestoda: Tetrabothriidae) among pinnipeds of the Holarctic during the late Tertiary and Pleistocene. Can J Zool. 1992;70:703–719. [Google Scholar]
- 37.Guernier V, Hochberg ME, Guegan JFO. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2004;2:740–746. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low BS. Marriage systems and pathogen stress in human societies. Am Zool. 1990;30:325–339. [Google Scholar]
- 39.Jetz W, Wilcove D, Dobson AP. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007;5:1–8. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter SR, et al., editors. Scenarios, Ecosystems and Human Well-being. Vol 2. Washington, DC: Island; 2005. p. 560. [Google Scholar]
- 41.Alcamo J, et al. Methodology for developing the MA scenarios. In: Carpenter SR, editor. Scenarios, Ecosystems and Human Well-being. Vol 2. Washington, DC: Island; 2005. pp. 145–172. [Google Scholar]
- 42.Image_Team. The IMAGE 2.2 Implementation of the SRES Scenarios: A Comprehensive Analysis of Emissions, Climate Change, and Impacts in the 21st Century. Bilthoven, The Netherlands: Rijksinstituut voor Volksgezondheid en Milieu/National Institute of Public Health and Environment; 2001. Vol CD-ROM 481508018 (CD-ROM). [Google Scholar]
- 43.Cork S, et al. Four scenarios. Scenarios, Ecosystems and Human Well-being. In: Carpenter SR, editor. Vol 2. Washington, DC: Island; pp. 223–296. [Google Scholar]
- 44.Stork NE, Lyal CHC. Extinction or ‘co-extinction’ rates? Nature. 1993;366:307. [Google Scholar]
- 45.Bush AO, Kennedy CR. Host fragmentation and helminth parasites: Hedging your bets against extinction. Int J Parasitol. 1994;24:1333–1343. doi: 10.1016/0020-7519(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 46.Brooks DR, Hoberg EP. How will global climate change affect parasite-host assemblages? Trends Parasitol. 2007;23:571–574. doi: 10.1016/j.pt.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RM, May RM. The invasion persistence and spread of infectious diseases within animal and plant communities. Philos Trans R Soc London Ser B. 1986;314:533–570. doi: 10.1098/rstb.1986.0072. [DOI] [PubMed] [Google Scholar]
- 48.Smith G, Dobson AP. Sexually transmitted diseases in animals. Parasitol Today. 1992;8:159–166. doi: 10.1016/0169-4758(92)90010-y. [DOI] [PubMed] [Google Scholar]
- 49.Kuris AM, Blaustein AR, Alio JJ. Hosts as islands. Am Nat. 1980;116:570–586. [Google Scholar]
- 50.Altizer S, et al. Social organization and parasite risk in mammals: Integrating theory and empirical studies. Annu Rev Ecol Syst. 2003;34:517–47. [Google Scholar]
- 51.Dobson AP. Population dynamics of pathogens with multiple hosts. Am Nat. 2004;164(Suppl):S64–S78. doi: 10.1086/424681. [DOI] [PubMed] [Google Scholar]
- 52.Sures B. Accumulation of heavy metals by intestinal helminths in fish: An overview and perspective. Parasitology. 2003;126:S53–S60. doi: 10.1017/s003118200300372x. [DOI] [PubMed] [Google Scholar]
- 53.Holt RD, Lawton JH. The ecological consequences of shared natural enemies. Annu Rev Ecol Syst. 1994;25:495–520. [Google Scholar]
- 54.Pimm SL. Food Webs. London: Chapman & Hall; 1982. [Google Scholar]
- 55.Pascual MM, Dunne JA. Ecological Networks: Linking Structure to Dynamics. Oxford: Oxford Univ Press; 2005. [Google Scholar]
- 56.May RM. Stability and Complexity in Model Ecosystems. Princeton: Princeton Univ Press; 1973. p. 265. [Google Scholar]
- 57.Allesina S, Pascual M. Network structure, predator-prey modules, and stability in large food webs. Theor Ecol. 2008;1:55–64. [Google Scholar]
- 58.Neutel A-M, Heesterbeek JAP, de Ruiter PC. Stability in real food webs: Weak links in long loops. Science. 2002;296:1120–1123. doi: 10.1126/science.1068326. [DOI] [PubMed] [Google Scholar]
- 59.Sures B. Environmental parasitology: Relevancy of parasites in monitoring environmental pollution. Trends Parasitol. 2004;20:170–177. doi: 10.1016/j.pt.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 61.Sprent JFA. Parasites lost? Int J Parasitol. 1992;22:139–151. doi: 10.1016/0020-7519(92)90095-3. [DOI] [PubMed] [Google Scholar]
- 62.Windsor DA. Equal rights for parasites. Conserv Biol. 1995;9:1–2. doi: 10.1353/pbm.1997.0011. [DOI] [PubMed] [Google Scholar]
- 63.Hudson PJ, Dobson AP, Lafferty KD. Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Kuris AM, et al. Parasites and free-living biomass in three estuaries: Implications for ecosystem energetics. Nature. 2008 doi: 10.1038/nature06970. in press. [DOI] [PubMed] [Google Scholar]
- 65.Lafferty KD, et al. Parasites in food webs: The ultimate missing links. Ecol Lett. 2008;II:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]