Abstract
The fossil record amply shows that the spatial fabric of extinction has profoundly shaped the biosphere; this spatial dimension provides a powerful context for integration of paleontological and neontological approaches. Mass extinctions evidently alter extinction selectivity, with many factors losing effectiveness except for a positive relation between survivorship and geographic range at the clade level (confirmed in reanalyses of end-Cretaceous extinction data). This relation probably also holds during “normal” times, but changes both slope and intercept with increasing extinction. The strong geographical component to clade dynamics can obscure causation in the extinction of a feature or a clade, owing to hitchhiking effects on geographic range, so that multifactorial analyses are needed. Some extinctions are spatially complex, and regional extinctions might either reset a diversity ceiling or create a diversification debt open to further diversification or invasion. Evolutionary recoveries also exhibit spatial dynamics, including regional differences in invasibilty, and expansion of clades from the tropics fuels at least some recoveries, as well as biodiversity dynamics during normal times. Incumbency effects apparently correlate more closely with extinction intensities than with standing diversities, so that regions with higher local and global extinctions are more subject to invasion; the latest Cenozoic temperate zones evidently received more invaders than the tropics or poles, but this dynamic could shift dramatically if tropical diversity is strongly depleted. The fossil record can provide valuable insights, and their application to present-day issues will be enhanced by partitioning past and present-day extinctions by driving mechanism rather than emphasizing intensity.
Keywords: biogeography, macroevolution, recovery
The inventory of life on Earth has always been determined at the most basic level by the difference between origination and extinction. This fundamental macroevolutionary equation, richness = origination − extinction, has been formally applied in many ways and with many elaborations, but takes on special consequence when attempting to evaluate the processes shaping present-day biodiversity, where neither term in the right side of the equation can be observed directly. Some progress has been made in modeling these parameters, but most approaches involve strong assumptions, require very large datasets and carry large uncertainties (e.g., refs. 1–3). The spatially explicit form of this equation, where richness is a local or regional pool of species or higher taxa, and immigration and emigration terms enter the right side of the equation (4), is important in many situations, from the biotic response to Pleistocene climate cycles and ongoing climate changes to the recovery from mass extinctions. However, this form is even more difficult to apply rigorously without historical data, and my emphasis here will be on the fossil record. Few would argue against the idea that the spatial fabric of extinction has shaped, and will continue to shape, the biosphere in profound ways, but spatial effects have been neglected relative to temporal patterns (partly because documentation is so challenging). I will argue that the insights beginning to emerge from spatially explicit approaches to ancient extinctions have significant implications for the dynamics of diversity of the past and in the future.
This discussion will encompass a range of extinction intensities and focus on marine systems, where the fossil record is richest: application of these generalizations to terrestrial realms requires more study. Opinions are divided on whether the handful of mass extinctions of the geologic past are a separate class of intensities from the “background” extinction that constitutes the great bulk of geologic time (and the bulk of total extinction; ref. 5), but this is a secondary issue that can probably be resolved by factoring out the well known secular decline in background extinction rates (6–8). As discussed below, extinction selectivities evidently shift between episodes of low and high extinction rates, and this selectivity is the more important issue for understanding the role of extinction in shaping past and future biotas. I will corroborate previous evidence for a strong spatial component to survivorship during major extinction events, present a multifactorial analysis of the end-Cretaceous (K-T) mass extinction in which geographic range emerges as the best predictor of survivorship in marine bivalves, and argue that such indirect effects are probably more important than generally appreciated. I will also discuss regional variations in the balance of invasions and local origination in the aftermath of the K-T event, which are somewhat unexpected given that the extinction itself tended to increase biotic homogenization on a global scale by preferentially removing the more localized taxa. Invasions and extinctions are also important during times of “normal” extinction intensities, as I will illustrate with reference to the dynamics of the latitudinal diversity gradient. I will conclude with some implications for integrating insights for past and present-day extinctions and suggest that a powerful approach might involve comparative dissection in extinction patterns according to likely drivers. Throughout I will note gaps in our understanding that would benefit from combined study of modern and ancient systems.
In this article, I will focus mainly on marine bivalves such as mussels, scallops, and cockles. Bivalves are becoming a model system for the analysis of large-scale biogeographic and evolutionary patterns (4, 9–14) for several reasons. They are taxonomically rich but not unmanageable (≈3,000 living and fossil genera), and their systematics are increasingly understood, so that taxonomic standardization and phylogenetic treatment of heterogeneous data are feasible. They have diverse life habits, from filter-feeding to photosymbiosis and chemosymbiosis to carnivory. They occur at all depths from the intertidal zone to deep-sea trenches and from the tropics to the poles. They are abundant and often well preserved as fossils (although not all habitats and clades are equally represented; ref. 13). and they have diverse shell mineralogies and microstructures, which allows analyses to control statistically for, and thus factor out, some, although not all, of the biases in the fossil record (12, 13). These favorable attributes do not mean that the bivalve fossil record is perfect, and preservation and sampling biases must always be considered in large-scale analyses (see, for example, the variety of approaches in refs. 4 and 15–19). However, our growing knowledge of living and fossil bivalves, including the taxonomic, preservational, and geographic factors that can distort their fossil record, makes this group an excellent vehicle for integrating present-day and paleontological diversity dynamics.
Extinction Selectivity Changes at the Most Extreme Events
A broad array of organismic and clade-level traits enter into extinction risk for present-day species. For example, in evaluating extinction risk in present-day terrestrial vertebrates, Purvis and colleagues (20, 21, 22) found mixed, but significant, effects for body size, a consistent inverse relation between both abundance and geographic range and extinction risk and either a positive relation or no effect for habitat specialization. Similar patterns are seen in the fossil record. For example, the geographic range is a significant determinant of Cretaceous and Cenozoic molluscan species duration or survivorship (refs. 23 and 24 and references therein), and Paleozoic crinoids show a significant positive relation between habitat breadth and species duration (25). Predictable interactions among factors can also be seen, although this aspect needs much more work. Molluscan genera containing many widespread species tend to be more extinction-resistant, with a median duration of 130 million years (Myr), than genera having just a few, localized species, which show a median duration of 32 Myr, and the genera with the other combinations give intermediate values (7). These are not theoretically surprising results, but it is encouraging that the paleontological outcomes so clearly match expectations.
Extinction selectivity appears to change significantly at the most severe mass extinctions, however. The rules of survivorship changed during the K-T extinction, such that species-richness and species-level range failed to predict genus survivorship, singly or in concert (refs. 7 and 26 and see ref. 27 for comparable results for K-T corals). In fact, survivorship of marine invertebrates in the K-T mass extinction is unrelated to a number of factors that have been shown or hypothesized to be important during more normal times. Besides the two already mentioned, these factors include local abundance, mode of larval development (which is in turn related to fecundity and species-level dispersal capability), estimated generation time, living position relative to the sediment-water interface, and trophic strategy (7).
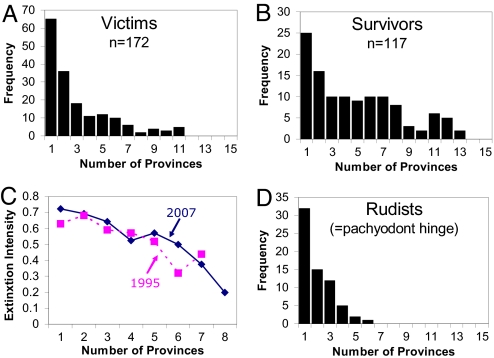
Despite this loss in effectiveness of a variety of organismic, species- and even clade-level traits, survivorship at mass extinction boundaries is not random. Every event seems to show some degree of selectivity, but one factor that seems to have promoted survival for most major groups and most mass extinctions is broad geographic distribution at the clade level (i.e., genera), regardless of species-level geographic ranges. This effect, which has been recorded for many groups and all of the major mass extinctions (see ref. 7 for a tabulation), is again further corroborated in an extensively revised version of Jablonski and Raup's (28) data on K-T bivalves (Fig. 1A and B). This is more than a simple binary effect: bivalve genus extinction is inversely related to geographic range, with strong concordance between the new and old data (Fig. 1C). The 70% extinction suffered by the genera found in just one or two biogeographic provinces is significantly higher than the 20% losses seen for genera found in eight or more provinces (of a global total of 16; following ref. 28). That said, even 20% represents a major, and highly unusual, drawdown of diversity in this most extinction-resistant part of the biota, equivalent to losing 20% of the most widespread genera in the sea today, such as the mussels (Mytilus, Modiolus) and the scallops (Pecten, Chlamys). [Although not ideal in some respects, analyses were conducted at the provincial scale rather than based on occurrences at individual localities, because clades are distributed not along simple linear coastlines, thereby undermining the use of linear distances or simple latitude/longitude extremes. Binning by province also damps some aspects of sampling and taxonomic uncertainty at the genus level, the range-endpoints of present-day molluscan genera tend to cluster at province boundaries (29, 30), and the results are robust to different approaches to quantifying province-based range sizes.]
Fig. 1.
Spatial effects in the end-Cretaceous (K-T) mass extinction for marine bivalve genera. (A, B, and D) Victims of the K-T extinction (A) tended to be significantly less widespread than surviving bivalve genera (B), as measured by the number of biogeographic provinces they occurred in during the Maastrichtian stage just before the event [Mann–Whitney U test, P = 0.00001; new analysis based on an extensive, in-progress revision and update of ref. 27, omitting rudist bivalves (D) as before, note that their inclusion as narrow-ranging victims would strengthen this result]. Adding provinces to fill gaps in observed geographic ranges strengthens the separation between victims and survivors (75% of victims are unchanged in range size and their median range is unchanged at two provinces; 60% of survivors are unchanged and their median increases from four to five provinces). Some caution is needed, because the proportion of survivors is likely to increase with phylogenetic analysis and further taxonomic standardization of early Cenozoic bivalves, but the major pattern is unlikely to change. (C) Significant inverse relation between extinction intensity and the number of biogeographic provinces occupied by bivalve genera during the K-T extinction (Spearman rank test, P < 0.01). Solid line indicates analysis of revised dataset (n = 289 genera). Broken line indicates analysis of previous version of dataset (ref. 28; n = 297 genera; 28 genera were added and 36 genera were removed in the revision). (D) Loss of a major adaptation (the pachyodont hinge) by hitchhiking on geographic distribution. The unique pachyodont hinge structure disappeared with the extinction of these genera at the K-T boundary, signaling the termination of the rudist bivalves (order Hippuritoida).
Multifactorial analyses corroborate the importance of clade-level distribution in determining survival during mass extinctions and show the value of testing for interaction among factors. For example, if variables are treated independently in the updated K-T dataset, geographic range remains the most important factor in clade survivorship, but species richness also appears to play a significant role (and body size is insignificant as a survivorship predictor). However, multiple logistic regression models taking the three variables simultaneously into account, using Akaike's Information Criterion (AIC) as a basis for model selection (31), shows species richness to covary with range such that when range is factored out, richness has an insignificant effect on survivorship (P = 0.85, as opposed to P = 0.002 for clade range, in the multifactor model). Body size also enters into the multiple-factor model as a weak, but significant, variable, but the multiple-factor model does not have significantly more explanatory power than the geographic range model alone, according both to the similar AIC weights (Table 1) and a likelihood ratio test (P = 0.09; see ref. 32). Multivariate approaches will help clarify patterns of extinction selectivity, even if, as here, they show that survivorship virtually collapses to the single variable of geographic range for K-T bivalves. The overlapping variation in range size among the victims and survivors suggests, however, that additional factors, or strong stochastic elements, enter into the fates of individual clades.
Table 1.
Testing models for bivalve genus survivorship during the K-T mass extinction
| Models | No. of parameters | AIC | Weight | P |
|---|---|---|---|---|
| G** + R + B* | 4 | 347.9 | 0.58 | 0.002/0.85/0.03 |
| Geographic range*** | 2 | 348.6 | 0.40 | e-6 |
| Species richness*** | 2 | 356.2 | 0.02 | 0.0001 |
| Body size | 2 | 373.5 | e-6 | 0.94 |
When geographic range (G), species richness (R), and body size (B) are analyzed as independent factors, G is the most important factor, but R is also significant. When the three are analyzed together, R is not a significant factor. Note that the combined model is not significantly better than geographic range alone according to the AIC (for model selection, which essentially weighs the adding of parameters against the improved explanatory power of each model). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
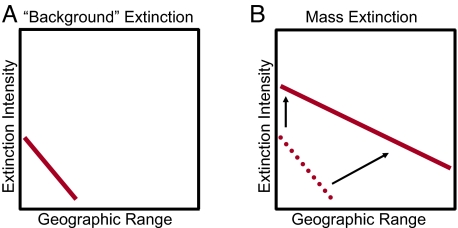
Widespread clades are probably always extinction-resistant compared with narrow-ranging relatives. However, during times of low extinction intensity, range is evidently just one significant feature among many, becoming increasingly important as the crowd of factors influencing taxon duration falls away as intensity mounts. How the relation between range and extinction risk varies with extinction intensity is not known and is difficult to assess. The few available data suggest that the relation between extinction probability has a steep slope during times of low extinction intensity, with the most widespread genera suffering negligible extinction at those times (33, 34) (Fig. 2A). The simplest view would be that perturbations generally operate at too small a spatial scale to affect these most widespread elements of the global biota. In the major mass extinctions, the y-intercept increases, so that a greater fraction of taxa are lost from all range classes, and the slope probably decreases (Fig. 2B). This configuration is much more demanding of the data, so that sparse or noisy data may fail to capture that shallower slope. We know little about whether the slope and intercept change continuously or shift abruptly at thresholds. For obvious reasons, including some very practical ones relating to the present-day biota, this would be good to know.
Fig. 2.
The inverse relation between geographic range and extinction risk appears to vary with severity of extinction. Conceptual model for this variation, such that both slope and intercept may change between times of background (A) and mass extinction (B).
We also know relatively little about the determinants of geographic range size at the clade level. Organismic traits such as dispersal ability and ecological strategy must play a role, but interactions with biogeographic context, clade history, and many other factors, including the way that clades extend their ranges by speciation across barriers, serve to decouple geographic ranges at the species and clade levels. For example, the geographic ranges of the 213 marine bivalve genera present today at shelf depths on the eastern Pacific margin from Point Barrow, Alaska to Cape Horn, Chile are not significantly related to the median or maximum ranges of their respective constituent species (7). Genera can attain broad ranges via a few widespread species, a mosaic of nonoverlapping but narrow-ranging species, or any combination thereof, each apparently equivalent in a mass-extinction event (although this equivalence deserves further study). Genus ranges are not simply species attributes writ large, but involve a dynamic that is set at another hierarchical level, by the complexities of speciation, species extinction, and range expansion.
This strong spatial component to extinction selectivity suggests that survival can be determined by features that are not tightly linked to the organismic and species-level traits that are favored, indeed shaped, during times of lower extinction intensities. Even well-established clades or adaptations could be lost simply because they are not associated with those few features that enhance survivorship during unusual, and geologically brief, high-intensity events. As discussed below, the removal of incumbents and the subsequent diversification of formerly marginal taxa are essential elements of the evolutionary dynamic fuelled by major extinctions (see also refs. 7, 35, 36, and 37).
These results also suggest that hitchhiking effects may be mistaken for direct selectivity more often than generally appreciated. Biological traits tend to covary, even across hierarchical levels, and so selection on one feature can drag others along with it, hampering efforts to pinpoint cause and byproduct. Such hitchhiking was detected for bivalve species richness in Table 1. Whenever widespread or restricted taxa tend to occupy nonrandom regions of phenotype space, for example in body sizes, trophic habits, or metabolic rates within a major group, hitchhiking becomes a real possibility, an interesting interaction across hierarchical levels where the extinction probabilities of organism-level characters are conditioned by clade-level properties (38). For example, the rudist bivalves of the Cretaceous seas (Order Hippuritoida) represent an extreme repatterning of the bivalve body plan, with highly modified conical shells and a unique, pachyodont hinge structure (39, 40). This clade and its bizarre growth form, including the pachyodont hinge, disappeared in the K-T mass extinction (41), but this loss was probably related less to any disadvantage inherent in the remarkable hinge apparatus than to the restricted ranges of rudist clades (Fig. 1D), and perhaps to their reliance in at least some instances on photosymbionts (ref. 40 but see ref. 42), as seen in modern reef-building corals. If the range-frequency distribution of rudists played a role in their demise, with correlated morphologies carried along, then we would expect a similar pattern for the other bivalve orders. This is in fact the case: the five bivalve orders with median genus ranges of one or two provinces suffered significantly more severe K-T bottlenecks (median = 93% genus extinction) than the four orders with median genus ranges of three or more provinces (median = 32% genus extinction; Spearman's rank correlation of median genus range and extinction intensity for orders = 0.74, P = 0.02), as predicted by the hitchhiking argument for rudists. More detailed analyses must await a morphometric or discrete-character study combined with a well-resolved phylogeny of bivalve genera, and these results suggest that such studies would be worthwhile.
The hitchhiking of such striking adaptations on the less flamboyant features that actually determine extinction resistance is probably pervasive, both during background times (hence the large literature on comparative methods and phylogenetic autocorrelation; e.g., refs. 43 and 44) and during mass extinctions. For example, marine bryozoan genera with complex colonies suffer more severely during mass extinctions than simple genera, but colony complexity is also inversely related to genus-level geographic range (45, 46), which may well be the ultimate basis for differential survival during the end-Ordovician mass extinction. The end-Ordovician extinction also preferentially removed snails with broad selenizones providing access to the mantle cavity, and planktonic graptolites with multiple stipes creating complex pendant colonies; the K-T extinction also preferentially removed bivalves with schizodont hinges (trigonioids), echinoids with elongate rostra (a clade of holasteroids), cephalopods with complex sutures (ammonites), and a major clade of birds with foot bones that fused from the ankles to the toes (Enantiornithes). All of these losses or severe bottlenecks are more likely to represent correlations, not necessarily with geographic range, but with some other organismic or higher-level factor, rather than direct selectivity on the most striking morphology or functional trait. These extinctions nonetheless truncated or rechanneled evolutionary trajectories through morphospace, and additional examples are plentiful.
Some Extinctions Are Spatially Complex
The K-T extinction is remarkably homogenous on a global scale, except perhaps for greater intensity in tropical carbonate settings (7, 47). However, other extinction events, particularly those that are less severe on a global scale, tend to show more spatial structure. For example, the mid-Cretaceous (end-Cenomanian) marine extinction appears to have been concentrated in northern Europe and the Western Interior seaway of North America. The smaller events in the geologic record must be interpreted critically, because at least some of them may represent, or at least be heavily overprinted by, sampling variations (e.g., refs. 16 and 48). However, a seemingly genuine extinction pulse or regional series of pulses occurs in the oceans near the start of the Pleistocene. These regional extinctions are generally taken to represent a culling of taxa unable to cope with the onset of rapid climate swings and oceanographic shifts that typify the Pleistocene. They vary in intensity and occur at slightly different times among regions (e.g., refs. 49–53), perhaps owing to regional variations in the timing of oceanographic transitions toward a glacial state (e.g., ref. 54); the many subsequent glacial-interglacial cycles evidently drove few extinctions in marine or terrestrial settings (e.g., ref. 55).
This spatial structuring of Plio-Pleistocene extinctions is interesting from many perspectives, but perhaps the most urgent need is to understand the dynamical consequences of these extinctions, which bear directly on the path of future biodiversity. Do these events reflect the setting of a new regional diversity level, such that taxa capable of weathering the volatile Pleistocene climate regime are more generalized and thus structure a biota capable of accommodating fewer species and clades? (See refs. 56 and 57 for a view of diversity-dependent factors that would favor this explanation.) Or do some regions incur a “diversification debt,” a more positive analog to the extinction debt sometimes inferred for modern biotas squeezed into refugia too small to accommodate their present richness? The rapid recovery of diversity in the Caribbean, which evidently suffered more severely than the tropical eastern Pacific just on the other side of the Panama Isthmus (51), suggests that at least some Plio-Pleistocene extinctions involve diversification debts rather than diversity resettings. [By this argument, the anomalously low diversity of the southeast Pacific molluscan fauna (e.g., ref. 53) is a transient effect rather than a permanent biogeographic feature, attributable perhaps to the lower rates of diversity accumulation in extratropical regions; cf. ref. 4).] However, the spatially explicit form of the fundamental macroevolutionary equation shows that regional diversity can accrue either by in situ origination or invasion (immigration). The high present-day rate of anthropogenic introductions in marine systems will likely outstrip regional evolutionary recovery by an order of magnitude or more, exacerbated of course by other anthropogenic stresses (e.g., refs. 58–60). This paleontological perspective on regional marine biodiversity adds another element to the urgency of slowing anthropogenic homogenization of marine biotas, if the diversification debt of some regions indeed makes them more susceptible to invasions (and see below).
Recoveries Are Also Spatially Complex
Most research on spatial dynamics has focused on extinctions, but evidence is accumulating for a spatial component to recoveries as well. The raw evolutionary material that survives the mass extinction filter is crucial in shaping the postextinction world. However, the evolutionary novelties and the ecological restructuring that emerge in the postextinction interval, including the little-appreciated process of sorting survivors into winners and losers (61), may be as important as the extinction filter in determining the long-term trajectory of individual clades.
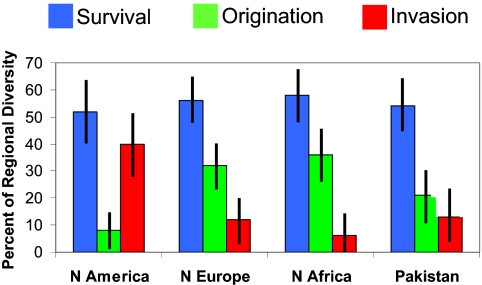
Returning to the spatially explicit form of the fundamental macroevolutionary equation, we can partition a regional biota after, e.g., the K-T mass extinction, into survivors of the event, locally evolved new taxa, and invaders. The four regions with the best marine molluscan records in the 10 Myr after the K-T event differ significantly in their recovery dynamics (62). For example, the North American biota was much more subject to invasions during the early stages of the post-Cretaceous recovery, and contains significantly fewer novel taxa, than the other regions. However, the survivor components (the regional extinction intensities) are indistinguishable among regions (Fig. 3).
Fig. 3.
Regional variation in molluscan recovery dynamics after the K-T extinction. Four post-extinction biotas are partitioned into their surviving, newly originating, or invading components. The Gulf and Atlantic Coastal Plain was subject to significantly more intense invasion than the other three regions (95% binomial confidence intervals). See ref. 62 for details.
Spatial heterogeneity has recently being detected for other recoveries as well. For example, after the end-Ordovician extinction ≈445 Myr ago, Baltica, a continental plate centered ≈30° south, had the lowest extinction intensity and the lowest invasion intensity among marine invertebrates, whereas Laurentia, which was straddling the equator, showed a tighter bunching of the three faunal components (63). Spatial heterogeneity in recovery from the huge end-Permian extinction has been reported for some groups [e.g., brachiopods and bivalves (64, 65)] but not others [e.g., ammonoids (ref. 66, but see ref. 67)].
The K-T example is striking in its failure to show the inverse relation between local survivorship and invasion that is generally expected (e.g., ref. 68) and is observed for the end-Ordovician. One can speculate that this difference is attributable to the greater proximity of North America to the K-T impact site in Yucatan, but how does that proximity translate to great invasions without imposing exceptional extinction? Qualitative, rather than quantitative, losses might account for the greater invasibilty of North America, but this hypothesis has not been tested. Alternatively, the correlation between extinction intensity and subsequent invasion may begin to break down above some threshold extinction level. One clue may come from another spatial difference: the short-lived evolutionary burst of a few taxa in North America (“bloom taxa” of ref. 69) that is evidently absent in northern Europe, North Africa, or Pakistan (62). North America's bloom taxa and the invasion pulse are almost certainly linked, presumably indicating a more profound ecological and evolutionary disturbance in North America than elsewhere, but this needs to be examined more closely. Whatever the ultimate cause, the fossil record pinpoints a theoretically interesting but pragmatically disquieting gap in our understanding of the extinction–invasion relationship. Given the current acceleration of both processes, and the pressure to establish reserves for remaining biodiversity, this relationship deserves more attention.
Extinction Influences Spatial Dynamics Across Latitude
Extinctions apparently promote not only invasion but evolutionary diversification in the fossil record, the classic case being the impressive radiation of mammals after the demise of the (nonavian) dinosaurs and other marine and terrestrial vertebrates at the end of the Cretaceous (e.g., refs. 15, 70, and 71; for general discussions, see refs. 7, 35, 36, and 72). These macroevolutionary observations are often seen as two sides of the same coin, as they intersect nicely with ecological work on the potential for incumbency or priority effects to resist extinction or damp diversification (38, 72). The three most prevalent explanations for both invasions and diversifications today and in the geologic past are (i) extinction or at least suppression of incumbents, already mentioned, (ii) superior competitive ability of the invaders, not least owing to their escape from their own competitors, predators, and pathogens when they enter a new area (73) (although this may be a transient effect and therefore less likely to play a macroevolutionary role), and (iii) changing climatic and other environmental conditions, such as those that drove the extensive invasions and reshuffling of Pleistocene biotas.
One of the most pervasive spatial patterns of invasions, seemingly independent of mass extinction events, underlies the marine latitudinal diversity gradient, wherein morphologies, species, and higher taxa are richest in the tropics and decline toward the poles. Although the gradient has been known for a long time and is documented for many groups and regions, the processes underlying this pervasive biodiversity pattern remain poorly understood (74, 75). The “out of the tropics” model for the marine gradient has taxa preferentially originating in the tropics, and then expanding their latitudinal ranges over time without actually abandoning their tropical cradle (4). The tropics are thus a diversity source, containing both young and old taxa, which accumulate to high richness. The poles are a diversity sink, mainly containing older taxa that have moved in from lower latitudes, and the temperate zones have intermediate richness and taxon ages, at least for the marine invertebrates where direct fossil evidence is available. If this model is generally true, then invasion is a basic factor in the latitudinal deployment of life on Earth.
The out of the tropics model is strongly supported in the marine bivalve fossil record. For each of three time slices (Pleistocene, Pliocene, and late Miocene), roughly twice as many bivalve genera first occur in the tropics than in higher latitudes (4). Because the extratropical fossil record is far better sampled than that of the tropics (4, 13, 17, 76, 77), the tropical values must be underestimates of their true origination rates, and the extratropical values must be overestimates. Further, most of the genera that first appeared in the tropics over the past 11 Myr have since spread to higher latitudes. This dynamic accounts for the striking inverse relation between diversity in a latitudinal bin and the median age of the genera in that bin: most of the geologically old genera at high latitudes also occur in the tropics, but the young genera are concentrated at low latitudes, decreasing the low-latitude median value significantly.
The number of taxa that expand out of the tropics is impressive, particularly given that these clades are invading new climate zones, traversing a gradient of increasing physical challenges for most taxa. Further, these extratropical expansions occurred in the face of progressive global refrigeration, culminating in the full-blown glacial cycles of the Pleistocene. However, while clades regularly left the tropics, few, if any, of the analyzed cohort have expanded above ≈50° north or south latitude. If the relative invasibility of the temperate and polar zones over the past 11 Myr was underlain by regional variation in background extinction intensity (E), we would expect, not the usual two-bin model, tropical E < extratropical E, or low-latitude E < polar E (4, 78, 79), or the monotonic latitudinal trend in extinction rates assumed by many others, but a hump-shaped pattern with an extinction maximum at midlatitudes.
A preliminary test of these alternatives did find a humped extinction pattern with latitude for Northern Hemisphere bivalve genera in the latest Cenozoic, with global plus regional extinction totaling ≈9% in the tropics, ≈20% in the temperate zone, and ≈12% in the Arctic (57). This result suggests that the temperate zones are invasible on geological time scales because they suffer the highest extinction rates, at least in global climate states approaching our own. Thus, even if climate does not directly set standing diversity, its fluctuations, which are greatest both in temperate latitudes today and during Pleistocene climate swings (e.g., refs. 54, 80, and 81), may set the pattern of extinction intensities. The data are not yet sufficient to study these dynamics in detail, but the relation between midlatitude thermal variability (which coincides with fluctuations in many additional factors) and extinction patterns clearly deserve further scrutiny. The poles are doubtless demanding places to live, but taxa evolve to cope with the challenges; Valentine and colleagues (56, 57) suggest they do this by becoming highly generalized trophically and argues that these broad niche dimensions are what tend to block invasions and allow them to weather glacial episodes subtidally, as they avoid seasonal extremes today. In any case, invasion resistance is apparently not a function of standing diversity alone, but of regional extinction rates, suggesting a significant role for incumbency (see also ref. 82). The polar mollusks may ultimately have come out of the tropics as well, but if so this must have occurred before the 11-Myr window presently available (which would be consistent with the much older genus ages seen at the poles). The interplay of extinction, origination, and immigration is complex, and of course it need not be in a steady state.
Terrestrial animals may well follow a different dynamic (83–85). Marine organisms can move down the continental shelf when ice forms at the surface, but terrestrial animals, plants and fungi do not have that luxury when confronted with a kilometer-thick ice sheet. High-latitude extinction and recolonization are thus almost certainly more important factors on land. Whatever the spatial dynamic near the poles, however, the tropics appear to be a crucial reservoir for biodiversity, with a subset of low-latitude clades expanding out of the tropics over geological time scales. Although this pattern is most readily detected in the shallowest part of the geologic record, thus falling entirely within times away from the major mass extinctions, some evidence suggests that postextinction recoveries are also fueled by the tropics, on land (86) and in the oceans (62, 63, 67). The tropics thus appear to be key to the generation and maintenance of global biodiversity across a wide range of boundary conditions.
Integrating Paleontological and Neontological Perspectives
I have touched on four spatial aspects of ancient extinctions that should be integrated with theoretical and applied approaches to the present-day biota. The fossil record amply demonstrates that the spatial fabric of extinction has profoundly shaped the biosphere. First, broad geographic range probably always buffers clades from extinction, but it becomes most important and clear-cut as the suite of other factors that enhance species and genus survival during normal times become ineffective. It is not yet clear whether the selectivity regime changes steadily with increasing extinction intensity or as a step function (7). More intense extinctions may tend to be less selective, which might explain the failure of intrinsic factors to predict extinction risk in the some of the most heavily stressed elements of the modern biota, such as freshwater fishes, amphibians, and Australian marsupials (87, 88).
This shift to a strong spatial component in survivorship during major extinction events greatly increases the likelihood of hitchhiking effects. Organismic traits can rise or fall according to the strength of their linkage to broad geographic range or other factors promoting survivorship through those bottlenecks, lending a highly stochastic element to the expansion or demise of individual adaptations or clades. Thinking about the present day, these linkages are unlikely to promote factors beneficial to, or even desirable for, humans or the ecosystems they hope to conserve (see ref. 89). Given that narrow-ranging genera cannot have wide-ranging species, the net effect must be to deplete specialists in favor of weedy generalists, but this pattern can be ameliorated by survival of clades whose broad ranges arise from the far-flung deployment of individually localized species.
Second, the fossil record is rich in regional extinction events of intermediate intensities, and these can provide insights into present-day biodiversity issues. For example, the Cenozoic history of today's biodiversity hotspots and coldspots (relative to expectations for their latitudes, for example) may help to predict the potential of these regions to accommodate further diversification, or alternatively to be subject to biotic invasions.
Third, the rules of successful recovery are poorly known, but are important for our understanding of both the larger outlines of the history of life and the future of modern diversity. The inordinate production of evolutionary novelties during recoveries suggests that postextinction dynamics do not simply involve an immediate return to business as usual. At the same time the spatial heterogeneity of recoveries, with significant invasions driving some of the regional patterns, requires a more careful look at the dynamics if we want to avoid biotic homogenization even after the reduction of the pressures on the modern biota. This could be another highly fruitful area at the intersection of paleontology and conservation biology.
Fourth, invasion has always been an evolutionary fact of life (82), even across biogeographic barriers and against climate gradients. The out-of-the-tropics model suggests an evolutionary approach to modeling biotic responses to future climate changes and attests to the evolutionary consequences of the stresses on tropical biotas today. If the tropics are the engine of global biodiversity, then driving tropical populations into extinction will have a global effect, by cutting off the primary source of new taxa for all latitudes. Further, if invasibility is more closely tied to extinction than to diversity per se, then there is the possibility of a reversal of the diversity flow, increasing the influx of invaders from higher latitudes. A tropical diversity crisis, now or in the geologic past, has profound long-term evolutionary consequences at a truly global scale.
Simply comparing the magnitude of the extinction occurring today, which is undoubtedly severe, with ancient intensities is not the most fruitful way to draw on the insights of the fossil record, or catalyze integrative research. A better approach might be to recognize that present-day extinctions have many drivers, and then to test for common patterns of selectivity on that basis: partitioning present-day extinction mechanisms should permit a clearer application of insights from the fossil record. Extinction selectivity probably does vary with driving mechanism to some extent. In birds, for example, habitat loss preferentially removes specialized and small-bodied taxa (but does not select on generation time), whereas exploitation and introduced predators preferentially remove large-bodied and long-lived taxa (90). Such systematic variations in selectivity help explain the apparent contradictions in and among analyses of present-day extinction risk (e.g., refs. 20 and 91), and similar arguments can be made for differences among ancient extinctions as well. As several authors have noted, extinction drivers have probably compounded over human history, with exploitation perhaps the most important in early phases, species invasions rising in frequency with the era of European exploration, and finally habitat alteration on a global scale accelerating with increased human population pressure, pollution, and climate change (e.g., refs. 20 and 59).
Many paleontological perturbations are probably most analogous to present-day habitat loss and could be explored in comparative fashion on that basis. Others will more closely correspond to the introduction of enemies, as when provinces collide or novel predation mechanisms evolve (see ref. 92). The particular combination of pressures seen today may be unique, just as they may have been for the K-T or end-Permian events. For example, today the ordinary biotic response to climate change (range translocation) is disallowed or at least severely curtailed over much of the planet owing to occupation or conversion of suitable habitat or migration corridors by humans and their artifacts. The unique combination of forces behind each major extinction puts a premium on focusing on first principles rather than extinction-specific patterns, underscoring the need for integrative research. It also underscores the need to take a hard look at the roles of incumbency and hitchhiking effects, to separate large-scale artifacts or byproducts from the underlying drivers. Extinction thresholds presumably exist for today's biota, beyond which whole systems collapse and most selectivity factors drop out, as seen for major events of the geologic past. Identifying such thresholds among environments, clades, and regions using fossil data, as another basis for avoiding them in the future, would be a valuable undertaking.
More generally, spatially explicit approaches to the fossil record have great potential for new insights into diversity dynamics, not just in the geologic past, but in the present day as well. The integration of paleontological and neontological insights takes on special urgency with the acceleration of extinction rates in the modern world, and the incorporation of the spatial dimension offers a powerful vehicle for that integration.
Acknowledgments.
I thank John Avise, Francisco Ayala, and Stephen Hubbell for inviting me to participate in this colloquium; J. Alroy, J. C. Avise, P. G. Harnik, S. M. Kidwell, M. J. Novacek, and J. W. Valentine for manuscript reviews; K. Roy and J. W. Valentine for many fruitful discussions and collaborations; A. Z. Krug and P. G. Harnik for discussions; and P. G. Harnik for assistance with multiple logistic regression. The National Science Foundation, the National Aeronautics and Space Administration, and the John Simon Guggenheim Foundation supported this research and synthesis.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution II: Biodiversity and Extinction,” held December 6–8, 2007, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_biodiversity.
The author declares no conflict of interest.
References
- 1.Paradis E. Can extinction rates be estimated without fossils? J Theor Biol. 2004;229:19–30. doi: 10.1016/j.jtbi.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Ricklefs RE. History and diversity: Explorations at the intersection of ecology and evolution. Am Nat. 2007;170(Suppl):S56–S70. doi: 10.1086/519402. [DOI] [PubMed] [Google Scholar]
- 3.Ricklefs RE. Estimating diversification rates from phylogenetic information. Trends Ecol Evol. 2007;22:601–610. doi: 10.1016/j.tree.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Jablonski D, Roy K, Valentine JW. Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]
- 5.Raup DM. The role of extinction in evolution. Proc Natl Acad Sci USA. 1994;91:6758–6763. doi: 10.1073/pnas.91.15.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bambach RK, Knoll AH, Wang SC. Origination, extinction, and mass depletions of marine diversity. Paleobiology. 2004;30:522–542. [Google Scholar]
- 7.Jablonski D. Mass extinctions and macroevolution. Paleobiology. 2005;31(Suppl):92–210. [Google Scholar]
- 8.Stanley SM. An analysis of the history of marine animal diversity. Paleobiology. 2007;33(Suppl):1–55. [Google Scholar]
- 9.Crame JA. Evolution of taxonomic diversity gradients in the marine realm: Evidence from the composition of recent bivalve faunas. Paleobiology. 2000;26:188–214. [Google Scholar]
- 10.Crame JA. Evolution of taxonomic diversity gradients in the marine realm: A comparison of Late Jurassic and Recent bivalve faunas. Paleobiology. 2002;28:184–207. [Google Scholar]
- 11.Jablonski D, Roy K, Valentine JW, Price RM, Anderson PS. The impact of the pull of the recent on the history of bivalve diversity. Science. 2003;300:1133–1135. doi: 10.1126/science.1083246. [DOI] [PubMed] [Google Scholar]
- 12.Kidwell SM. Shell composition has no net impact on large-scale evolutionary patterns in mollusks. Science. 2005;307:914–917. doi: 10.1126/science.1106654. [DOI] [PubMed] [Google Scholar]
- 13.Valentine JW, Jablonski D, Kidwell SM, Roy K. Assessing the fidelity of the fossil record by using marine bivalves. Proc Natl Acad Sci USA. 2006;103:6599–6604. doi: 10.1073/pnas.0601264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krug AZ, Jablonski D, Valentine JW. Contrarian clade confirms the ubiquity of spatial origination patterns in the production of latitudinal diversity gradients. Proc Natl Acad Sci USA. 2007;104:18129–18134. doi: 10.1073/pnas.0709202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alroy J. New methods for quantifying macroevolutionary patterns and processes. Paleobiology. 2000;26:707–733. [Google Scholar]
- 16.Foote M. Origination and extinction through the Phanerozoic: A new approach. J Geol. 2003;111:125–148. [Google Scholar]
- 17.Bush AM, Bambach RK. Did alpha diversity increase during the Phanerozoic? Lifting the veils of taphonomic, latitudinal, and environmental biases. J Geol. 2004;112:625–642. [Google Scholar]
- 18.Bush AM, Markey MJ, Marshall CR. Removing bias from diversity curves: The effects of spatially organized biodiversity on sampling standardization. Paleobiology. 2004;30:666–686. [Google Scholar]
- 19.Smith AB. Marine diversity through the Phanerozoic: Problems and prospects. J Geol Soc (London) 2007;164:731–745. [Google Scholar]
- 20.Purvis A, Jones KE, Mace GM. Extinction BioEssays. 2000;22:1123–1133. doi: 10.1002/1521-1878(200012)22:12<1123::AID-BIES10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Purvis A, Cardillo M, Grenyer R, Collen B. In: Phylogeny and Conservation. Purvis A, Gittleman JL, Brooks T, editors. Cambridge: Cambridge Univ Press; 2005. pp. 295–316. [Google Scholar]
- 22.Purvis A. Phylogenetic trees and the future of mammalian biodiversity. Proc Natl Acad Sci USA. 2008;105(Suppl):11556–11563. doi: 10.1073/pnas.0801917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt G, Roy K, Jablonski D. Heritability of geographic range sizes revisited. Am Nat. 2005;166:129–135. doi: 10.1086/430722. [DOI] [PubMed] [Google Scholar]
- 24.Jablonski D, Hunt G. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: Organismic vs. species-level explanations. Am Nat. 2006;168:556–564. doi: 10.1086/507994. [DOI] [PubMed] [Google Scholar]
- 25.Kammer TW, Baumiller TK, Ausich WI. Evolutionary significance of differential species longevity in Osagean-Meramecian (Mississippian) crinoid clades. Paleobiology. 1998;24:155–176. [Google Scholar]
- 26.Jablonski D. Background and mass extinctions: The alternation of macroevolutionary regimes. Science. 1986;231:129–133. doi: 10.1126/science.231.4734.129. [DOI] [PubMed] [Google Scholar]
- 27.Kiessling W, Baron-Szabo RC. Extinction and recovery patterns of scleractinian corals at the Cretaceous-Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;214:195–223. [Google Scholar]
- 28.Jablonski D, Raup DM. Selectivity of end-Cretaceous marine bivalve extinctions. Science. 1995;268:389–391. doi: 10.1126/science.11536722. [DOI] [PubMed] [Google Scholar]
- 29.Campbell CA, Valentine JW. Comparability of modern and ancient marine faunal provinces. Paleobiology. 1977;3:49–57. [Google Scholar]
- 30.Roy K, Jablonski D, Valentine JW. Higher taxa in biodiversity studies: Patterns from eastern Pacific marine mollusks. Phil Trans R Soc London Ser B. 1996;351:1605–1613. [Google Scholar]
- 31.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. 2nd ed. New York: Springer; 2002. [Google Scholar]
- 32.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd Ed. New York: Wiley; 2000. [Google Scholar]
- 33.Payne JL, Finnegan S. The effect of geographic range on extinction risk during background and mass extinction. Proc Natl Acad Sci USA. 2007;104:10506–10511. doi: 10.1073/pnas.0701257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell MG. Geographic range and genus longevity of late Paleozoic brachiopods. Paleobiology. 2007;33:530–546. [Google Scholar]
- 35.Erwin DH. Lessons from the past: Biotic recoveries from mass extinctions. Proc Natl Acad Sci USA. 2001;98:5399–5403. doi: 10.1073/pnas.091092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jablonski D. Lessons from the past: Evolutionary impacts of mass extinctions. Proc Natl Acad Sci USA. 2001;98:5393–5398. doi: 10.1073/pnas.101092598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erwin DH. Extinction as the loss of evolutionary history. Proc Natl Acad Sci USA. 2008;105(Suppl):11520–11527. doi: 10.1073/pnas.0801913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jablonski D. Scale and hierarchy in macroevolution. Palaeontology. 2007;50:87–109. [Google Scholar]
- 39.Skelton PW. Preadaptation and evolutionary innovation in rudist bivalves. Spec Pap Palaeontol. 1985;33:159–173. [Google Scholar]
- 40.Seilacher A. In: Bivalves, an Eon of Evolution. Johnston PAg, Haggart JW., editors. Calgary, Canada: University of Calgary; 1998. pp. 423–436. [Google Scholar]
- 41.Steuber T, Mitchell SF, Buhl D, Gunter G, Kasper HU. Catastrophic extinction of Caribbean rudist bivalves at the Cretaceous/Tertiary boundary. Geology. 2002;30:999–1002. [Google Scholar]
- 42.Steuber T. Skeletal growth rates of Upper Cretaceous rudist bivalves: Implications for carbonate production and organism-environment feedbacks. Geol Soc London Spec Publ. 2000;178:21–32. [Google Scholar]
- 43.Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: A test and review of evidence. Am Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 44.Paradis E. Statistical analysis of diversification with species traits. Evolution (Lawrence, Kans) 2005;59:1–12. [PubMed] [Google Scholar]
- 45.Anstey RL. Taxonomic survivorship and morphologic complexity in Paleozoic bryozoan genera. Paleobiology. 1978;4:407–418. [Google Scholar]
- 46.Anstey RL. Bryozoan provinces and patterns of generic evolution and extinction in the Late Ordovician of North America. Lethaia. 1986;19:33–51. [Google Scholar]
- 47.Raup DM, Jablonski D. Geography of end-Cretaceous marine bivalve extinctions. Science. 1993;260:971–973. doi: 10.1126/science.11537491. [DOI] [PubMed] [Google Scholar]
- 48.Smith AB, McGowan AJ. The shape of the Phanerozoic marine palaeodiversity curve: How much can be predicted from the sedimentary rock record of western Europe? Palaeontology. 2007;50:765–774. [Google Scholar]
- 49.Kitamura A, Omote H, Oda M. Molluscan response to early Pleistocene rapid warming in the Sea of Japan. Geology. 2000;28:723–726. [Google Scholar]
- 50.Monegatti P, Raffi S. Taxonomic diversity and stratigraphic distribution of Mediterranean Pliocene bivalves. Palaeogeogr Palaeoclimatol Palaeoecol. 2001;165:171–193. [Google Scholar]
- 51.Todd JA, et al. The ecology of extinction: Molluscan feeding and faunal turnover in the Caribbean Neogene. Proc R Soc London Ser B. 2002;269:571–577. doi: 10.1098/rspb.2001.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith JT, Roy K. Selectivity during background extinction: Plio-Pleistocene scallops in California. Paleobiology. 2006;32:408–416. [Google Scholar]
- 53.Rivadeneira MM, Marquet PA. Selective extinction of late Neogene bivalves on the temperate Pacific coast of South America. Paleobiology. 2007;33:455–468. [Google Scholar]
- 54.Ravelo AC, Andreasen DH, Lyle M, Lyle AO, Wara MW. Regional climate shifts caused by gradual global cooling in the Pliocene epoch. Nature. 2004;429:263–267. doi: 10.1038/nature02567. [DOI] [PubMed] [Google Scholar]
- 55.Huntley B. In: Climate Change and Biodiversity. Lovejoy TE, Hannah L, editors. New Haven, CT: Yale Univ Press; 2005. pp. 109–124. [Google Scholar]
- 56.Valentine JW. In: Biotic Interactions in Recent and Fossil Benthic Communities. Tevesz MJS, McCall PL, editors. New York: Plenum; 1983. pp. 121–156. [Google Scholar]
- 57.Valentine JW, Jablonski D, Krug AZ, Roy K. Incumbency, diversity, and latitudinal gradients. Paleobiology. 2008;34:169–178. [Google Scholar]
- 58.Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH. Invasion of coastal marine communities in North America: Apparent patterns, processes, and biases. Annu Rev Ecol Syst. 2000;31:481–531. [Google Scholar]
- 59.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 60.Lötze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas worldwide. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 61.Jablonski D. Survival without recovery after mass extinctions. Proc Natl Acad Sci USA. 2002;99:8139–8144. doi: 10.1073/pnas.102163299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jablonski D. Geographic variation in the molluscan recovery from the end-Cretaceous extinction. Science. 1998;279:1327–1330. doi: 10.1126/science.279.5355.1327. [DOI] [PubMed] [Google Scholar]
- 63.Krug AZ, Patzkowsky ME. Geographic variation in turnover and recovery from the Late Ordovician mass extinction. Paleobiology. 2007;33:435–454. [Google Scholar]
- 64.Chen ZQ, Kaiho K, George AD. Early Triassic recovery of the brachiopod faunas from the end-Permian mass extinction: A global review. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;224:270–290. [Google Scholar]
- 65.Bonuso N, Bottjer DJ. A test of biogeographical, environmental, and ecological effect on Middle and Late Triassic brachiopod and bivalve abundance patterns. Palaios. 2008;23:43–54. [Google Scholar]
- 66.McGowan AJ. Ammonoid recovery from the Late Permian mass extinction event. Comptes Rendus Palevol. 2005;4:517–530. [Google Scholar]
- 67.Brayard A, et al. The Early Triassic ammonoid recovery: Paleoclimatic significance of diversity gradients. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;239:374–395. [Google Scholar]
- 68.Fridley JD, et al. The invasion paradox: Reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:tiprpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 69.Hansen TA. Early Tertiary radiation of marine molluscs and the long-term effects of the Cretaceous-Tertiary extinction. Paleobiology. 1988;14:37–51. [Google Scholar]
- 70.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48:107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 71.Cifelli RL, Gordon CL. Recrowning mammals. Nature. 2007;447:918–920. doi: 10.1038/447918a. [DOI] [PubMed] [Google Scholar]
- 72.Jablonski D. Biotic interactions and macroevolution: Extensions and mismatches across scales and levels. Evolution (Lawrence, Kans) 2008;62:715–739. doi: 10.1111/j.1558-5646.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- 73.Sax DF, et al. Ecological and evolutionary insights from species invasions. Trends Ecol Evol. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 74.Hillebrand H. On the generality of the latitudinal diversity gradient. Am Nat. 2004;163:192–211. doi: 10.1086/381004. [DOI] [PubMed] [Google Scholar]
- 75.Mittelbach GG, et al. Evolution and the latitudinal diversity gradient: Speciation, extinction, and biogeography. Ecol Lett. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 76.Allison PA, Briggs DEG. Paleolatitudinal sampling bias, Phanerozoic species diversity, and the end-Permian extinction. Geology. 1993;21:65–68. [Google Scholar]
- 77.Jackson JBC, Johnson KG. Measuring past biodiversity. Science. 2001;293:2401–2404. doi: 10.1126/science.1063789. [DOI] [PubMed] [Google Scholar]
- 78.Goldberg EE, Roy K, Lande R, Jablonski D. Diversity, endemism, and age distributions in macroevolutionary sources and sinks. Am Nat. 2005;165:623–633. doi: 10.1086/430012. [DOI] [PubMed] [Google Scholar]
- 79.Roy K, Goldberg EE. Origination, extinction, and dispersal: Integrative models for understanding present-day diversity gradients. Am Nat. 2007;170(Suppl):S71–S85. doi: 10.1086/519403. [DOI] [PubMed] [Google Scholar]
- 80.Jansson R, Dynesius M. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu Rev Ecol Syst. 2002;33:741–777. [Google Scholar]
- 81.Lyle MW, et al. The Pacific Ocean and Cenozoic evolution of climate. Rev Geophys. 2008 10.1029/2005RG000190. [Google Scholar]
- 82.Vermeij GJ. In: Species Invasions. Sax DF, Stachowicz JS, Gaines SD, editors. Sunderland, MA: Sinauer; 2005. pp. 315–339. [Google Scholar]
- 83.Weir JT, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1574–1576. doi: 10.1126/science.1135590. [DOI] [PubMed] [Google Scholar]
- 84.Hawkins BA, Diniz-Filho JAF, Jaramillo CA, Soeller SA. Climate, niche conservatism, and the global bird diversity gradient. Am Nat. 170(Suppl):S16–S27. doi: 10.1086/519009. [DOI] [PubMed] [Google Scholar]
- 85.Wiens JJ. Global patterns of species richness and diversification in amphibians. Am Nat. 2007;170(Suppl):S86–S106. doi: 10.1086/519396. [DOI] [PubMed] [Google Scholar]
- 86.Kerp HAAH, Vörding B, Bandel K. Typical Triassic Gondwanan floral elements in the Upper Permian of the paleotropics. Geology. 2006;34:265–268. [Google Scholar]
- 87.Duncan JR, Lockwood JL. Extinction in a field of bullets: A search for causes in the decline of the world's freshwater fishes. Biol Conserv. 2001;102:97–105. [Google Scholar]
- 88.Fisher DO, Blomberg SP, Owens IPF. Extrinsic versus intrinsic factors in the decline and extinction of Australian marsupials. Proc R Soc London Ser B. 2003;270:1801–1808. doi: 10.1098/rspb.2003.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson J. Ecological extinction and evolution in the brave new ocean. Proc Natl Acad Sci USA. 2008;105(Suppl):11458–11465. doi: 10.1073/pnas.0802812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owens IPF, Bennett PM. Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. Proc Natl Acad Sci USA. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fréville H, McConway K, Dodd M, Silvertown J. Prediction of extinction in plants: Interaction of extrinsic threats and life history traits. Ecology. 2007;88:2662–2672. doi: 10.1890/06-1453.1. [DOI] [PubMed] [Google Scholar]
- 92.Barnosky AD. Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc Natl Acad Sci USA. 2008;105(Suppl):11543–11548. doi: 10.1073/pnas.0801918105. [DOI] [PMC free article] [PubMed] [Google Scholar]