Abstract
Phylogenies describe the origins and history of species. However, they can also help to predict species' fates and so can be useful tools for managing the future of biodiversity. This article starts by sketching how phylogenetic, geographic, and trait information can be combined to elucidate present mammalian diversity patterns and how they arose. Recent diversification rates and standing diversity show different geographic patterns, indicating that cradles of diversity have moved over time. Patterns in extinction risk reflect both biological differences among mammalian lineages and differences in threat intensity among regions. Phylogenetic comparative analyses indicate that for small-bodied mammals, extinction risk is governed mostly by where the species live and the intensity of the threats, whereas for large-bodied mammals, ecological differences also play an important role. This modeling approach identifies species whose intrinsic biology renders them particularly vulnerable to increased human pressure. We outline how the approach might be extended to consider future trends in anthropogenic drivers, to identify likely future battlegrounds of mammalian conservation, and the likely casualties. This framework could help to highlight consequences of choosing among different future climatic and socioeconomic scenarios. We end by discussing priority-setting, showing how alternative currencies for diversity can suggest very different priorities. We argue that aiming to maximize long-term evolutionary responses is inappropriate, that conservation planning needs to consider costs as well as benefits, and that proactive conservation of largely intact systems should be part of a balanced strategy.
Keywords: extinction risk, latent risk, mammals
The Tree of Life—phylogeny—is a powerful metaphor for life's diversity, showing all species, including our own, as part of an interrelated whole. But phylogeny is more than a metaphor. It is also a research tool—the result of evolutionary processes integrated over the history of life, it can be analyzed for insights into how those processes have shaped today's biota (1). This approach is becoming increasingly powerful as the trees become ever more inclusive, built from rapidly accumulating databases using methods that continue to be improved (2, 3).
Species' histories are of interest, but their futures are of more pressing concern. The Tree of Life is currently under sustained attack, as people increasingly dominate landscapes (4). Comparisons of extinction rates between today and geological history are difficult for many reasons (5), but the Tree of Life is already being pruned more quickly than it is growing (6), and extinction rates are projected to rise by at least another order of magnitude over the next centuries (7). This article describes how phylogeny has a role to play in understanding the pattern of survivors and casualties and how it can help us both to predict species' futures and to estimate some of the biodiversity value that would be lost if they went extinct.
We focus on mammals as a model system. They are much better known than almost any other group, with a mature species-level taxonomy (8), a largely complete evaluation of species' extinction risk (9), a large database of ecological, life history, and geographical information (K.E.J., J.B., A.P., C.D.L.O., S.A.F., Christina Connolly, Amber Teacher, J.L.G., R.G., Elizabeth Boakes, Michael Habib, Janna Rist, Chris Carbone, Christopher A. Plaster, O.R.P.B.-E., Janine K. Foster, Elisabeth A. Rigby, Michael J. Cutts, Samantha A. Price, Wes Sechrest, Justin O'Dell, Kamran Safi, M.C., and G.M.M., unpublished data), and a comprehensive species-level estimate of phylogeny (11). [Many node ages in ref. 11 were slightly affected by a software bug; all our analyses use corrected dates (O.R.P.B.-E., M.C., K.E.J., Ross D. E. MacPhee, Robin M. D. Beck, R.G., Samantha A. Price, Rutger A. Vos, J.L.G., and A.P., unpublished data).] As always with a model system, advantages come at a price. Mammals are atypical (e.g., they are much larger than most other species), so results from them cannot necessarily be extrapolated more broadly. However, mammals are a charismatic group of special interest to many people, so results have value even if they cannot be generalized. We start with a snapshot of present mammalian diversity and the (overwhelmingly anthropogenic) pressures that species face, before going on to describe recent and ongoing attempts to understand the present and possible future consequences of those pressures.
A Snapshot of Mammalian Biodiversity
Mammalian species are distributed very unevenly among genera, families, and orders (6, 8). Differences in age among taxa of a given rank (13) confound evolutionary interpretation of the pattern, but the phylogeny permits a test of whether the chances of diversification have indeed varied among lineages. Under the equal-rates Markov model (ERM), in which chances are equal, phylogenies should have a weighted mean I [the degree to which species are partitioned unequally between sister clades (14)], not significantly >0.5. The estimate of phylogeny (11) has a weighted mean I of 0.657 (SE = ±0.0131), well above 0.5 (weighted t test vs. 0.5: t848 = 11.98, p ≪ 0.001), indicating that lineages have had different propensities to diversify. Such inequality is common throughout the Tree of Life (15, 16) and prompts the search for traits that might be responsible. Phylogenetic analyses reveal that large litter size and high abundance are both linked with high richness in sister-clade comparisons pooled across four orders (primates, carnivores, marsupials, and bats), whereas small body size and short gestation period also predict high richness within carnivores (17). In common with most comparable studies on other taxa (18), however, the biological traits leave most of the variance in richness unexplained, suggesting a possible role for the environment.
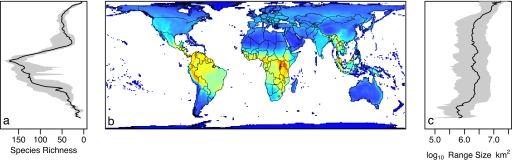
The geographic distribution of mammalian species is also very uneven (Fig. 1). Mammals follow global trends for higher tropical diversity, with a strong latitudinal diversity gradient (Fig. 1a). Within the tropics, richness seems to correlate with productivity and water-energy dynamics, peaking in Amazonia at the base of the Andes, in the Great Rift Valley in Africa (where richness exceeds 250 species per 10,000 km2), and in an arc running from the Himalayas into southeastern Asia (Fig. 1b). These peaks suggest that topographical heterogeneity has also shaped species-richness patterns (19). At higher latitudes, the relationship between richness and productivity is much weaker, and ambient energy is a better predictor of richness (19). The geographic pattern of mammalian richness is highly congruent with those of birds and amphibians (20), which are also well predicted by the same environmental variables (21, 22). All these groups have many narrow-range species on the Neotropical mainland, but other “hotspots of endemicity” differ—continental shelf island systems for mammals, oceanic archipelagoes for birds, and mainland locations for amphibians (20). The latitudinal gradient in median geographic range size in mammals (Fig. 1c) correlates strongly with the land area available within the same latitudinal bands (r = 0.68, although spatial autocorrelation complicates significance testing). However, median range size remains high in the northernmost bands despite the rapid decline in land area toward the Pole. Bird range sizes show similar patterns (23).
Fig. 1.
Geographic patterns in mammalian biodiversity. (a) The latitudinal gradient in species-richness. The solid line shows the median; gray bands demarcate the interquartile range; species are counted in every latitudinal band (100 km north to south) in which they occur. (b) A map of species richness within 100 × 100-km grid cells, ranging from deep blue (minimum = 0 species) to deep red (maximum = 258 species). (c) The latitudinal pattern of mean geographic range size; details as for a.
The tropics have been described as an evolutionary powerhouse, acting as a “cradle” for diversity (24). Might Fig. 1, therefore, reflect long-standing geographic differences in diversification rates? Cradles of diversity could be of particular interest if conservation actions are to be targeted toward conserving the processes that generate diversity, a point to which we return below. The geographic pattern of recent diversification can be examined in terms of taxonomy or phylogeny, although both have their problems. Boundaries to higher taxa can be arbitrary (13), whereas both the relative and absolute divergence times in molecular phylogenies can be controversial (25). Because of these reservations, we use both approaches.
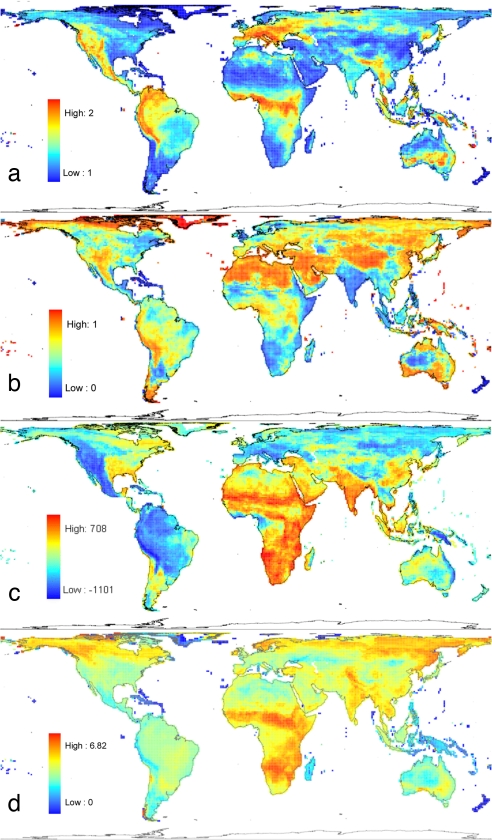
Taxonomically, the ratio of species to genera should indicate the regional diversification rate over the past several million years, if genera are approximately equal in age. This ratio correlates strongly with log(species richness) among equal-area grid cells (Pearson's r = 0.61; Fig. 2a). This correlation remains highly significant (corrected F = 78.49, corrected P < 0.001, n = 4,152: based on a subsample of cells and excluding single species occurrences) when degrees of freedom are reduced to account for spatial autocorrelation (26). Moving to phylogeny, places where a high proportion of species are on short terminal branches in the tree are likely to have rapid diversification, turnover, or immigration in their recent history (27). However, analyses are complicated by the low resolution (uncertain relationships) at the tips of the phylogeny, which introduces overestimates of the respective branch lengths. We ameliorated this problem by reducing ages of terminal polytomies using the correction suggested by Nee in ref. 28 and by assuming that the descendants from each polytomy diversified under a Yule process (29). Fig. 2b highlights the Andean and Himalayan diversity peaks, but not the African great lakes, as recent evolutionary crucibles. Much of the temperate north stands out more than much of the tropics in this map, and there is a negative overall correlation between the proportion of short branches and log(species richness) (r = −0.38, corrected F = 21.01, corrected P < 0.001, n = 4,210, analyses as above), although this depended on how we corrected for terminal polytomies. This result partially echoes recent findings of higher recent speciation and extinction rates in temperate than in tropical mammals (30). These maps also imply that some regions have seen marked shifts in net diversification rate, whereas others may have remained steady.
Fig. 2.
Maps showing four aspects of mammalian diversification and diversity. (a) Mean numbers of species per genus within cells. (b) Proportion of species with shorter terminal branch lengths than the overall median (phylogeny from ref. 11, with branch lengths corrected and modified as explained in the previous page). (c) Residuals from a loess regression of cell total evolutionary history against cell species number (phylogeny as above). (d) Variance in log(body mass) (unpublished data), with missing data inferred as the mean of all closest relatives with data.
Areas of “old” and “young” diversity can be identified from the residuals of a loess regression across cells of total evolutionary history (i.e., total branch length in the phylogeny of a cell's species) on species number. The African diversity peak emerges as old, whereas much of Andean diversity is young (Fig. 2c). Character disparity—among-species variation in morphology—also shows geographic pattern: Fig. 2d maps one index of disparity, the variance in log(body mass). Disparity tends to be high where diversity is old (r = 0.29, corrected F = 5.10, corrected P < 0.05, n = 4,210, analyses as above), although tropical regions drive this relationship.
Mammalian biodiversity, then, shows complex geographic and phylogenetic patterns of richness, recent diversification, and character variation. The African diversity peak is old and disparate, that in Asia is young and disparate, and the Andean peak is young with low disparity. These patterns reflect a complex history of speciation, extinction, anagenesis, and dispersal, with each factor probably shaped by biological traits and both biotic and abiotic environmental features in ways that have changed through time. Environmental features could provide much of the missing explanation for phylogenetic asymmetry (31), whereas traits may help to complete the explanation of geographic patterns. Other, less well known taxa doubtless have different patterns, but there is no particular reason to expect the patterns to be any simpler.
Natural diversity patterns increasingly bear the stamp of widespread anthropogenic system change. The biota we see today is already affected by anthropogenic extinction. Large, slowly reproducing mammals went extinct almost everywhere (with the notable exception of Africa, perhaps linking to the patterns in Fig. 2 c and d) ≈7,000 to 50,000 years ago (32). In the past few centuries, mammalian extinctions have mostly been on islands, notably the West Indies, with continental extinctions largely confined to Australia (9). Our analyses are therefore of an already reduced fauna. The next section considers the main ways in which human actions continue to reduce and reshape mammalian biodiversity.
Threats Facing Mammalian Biodiversity
The terrestrial environment is now dominated by people—1/4 to 1/3 of the land area has been transformed for human use (33). Additionally, human population density tends to be higher in species-rich areas, probably because productivity shapes both (34). Only a few mammal species fare well in human-dominated environments; the vast majority are vulnerable to the widespread and rapid anthropogenic changes. The main direct human-induced drivers that impact biodiversity now are habitat loss and fragmentation (the most important present threat), alien invasive species, overutilization, disease, pollution, and climate change (9). The International Union for Conservation of Nature (IUCN) assessed how these drivers are affecting mammals. Nearly all mammal species have been evaluated and, provided enough information was available, placed in one of the following extinction risk categories: least concern (LC), near-threatened (NT), vulnerable (VU), endangered (EN), critically endangered (CR), extinct in the wild (EW), and extinct (EX). The resulting IUCN Red List (35) lists 74 mammal species as having gone extinct in their native range since 1500 A.D., 1,094 (22.5% of those evaluated) as being threatened (i.e., VU, EN, or CR), and only 2,652 (54.5%) as giving no cause for concern (i.e., as being LC).
Does extinction risk show any patterns that might help us to understand the processes affecting biodiversity loss? The “field of bullets” scenario, in which extinction strikes completely at random, is a widely used null model for extinction (36, 37). The metaphor comes from trench warfare, where soldiers' survival may have depended more on luck than skill. This scenario predicts that threatened species should constitute a stochastically constant fraction of any sample. Mammalian extinction risk is not a simple field of bullets but shows both geographic and phylogenetic patterns (9, 38, 39). The prevalence of risk is higher in the Old World than in the New World and higher on islands than on continents (9, 38). It varies among clades too, being higher than average in primates and perissodactyls and lower than average in rodents (9). Species with few close relatives are also more likely to be at risk (39, 40).
These patterns reject the original field of bullets model, but the model lacks a geographic dimension because there may have been fields that were near to the battle but that nevertheless had no bullets. Likewise, human pressures have changed some places beyond recognition but left others almost untouched. Because closely related species often live in the same region, geographic heterogeneity in threat intensity could, by itself, cause taxonomic selectivity in extinction risk (39). Alternatively, the selectivity could arise because biological differences among clades affect species' abilities to withstand threats (41). How important for species' survival is staying out of the firing line, and how important is being bullet proof?
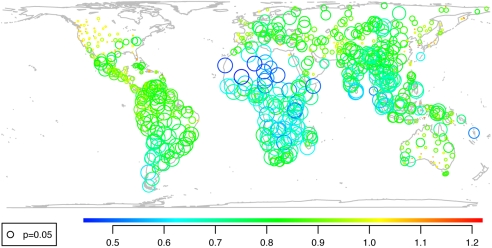
Human population density predicts proportions of threatened mammal species among continental countries (42), supporting the “firing-line” model. However, an analysis of extinction risk within terrestrial World Wildlife Fund (WWF) ecoregions shows that phylogenetic nonrandomness is common within single ecoregions, where pressures may be more even than across the globe. Within each ecoregion, we generated phylogenetically independent contrasts (essentially, differences between sister clades) (43) in extinction risk (0 for LC species, 1 for species having a higher status; data-deficient and unevaluated species were excluded) on the phylogeny (with polytomies resolved arbitrarily and branch lengths set to unity) and compared the sum of the absolute values of standardized contrasts with the sums obtained from 1,000 randomizations of the risk data among the ecoregion's species. If high-risk species are strongly clumped in the phylogeny, the observed sum will be lower than in 95% of the simulations. Of 691 ecoregions with at least three higher-risk species and some variance in extinction risk, 386 (54%) showed significant clumping. Interestingly, the strength (rather than the significance) of the clumping is high in most realms apart from the Nearctic (Fig. 3). It also appears to be stronger in ecoregions with high disparity (Spearman's ρ = 0.316) and with relatively old diversity (Spearman's ρ = 0.195). These correlations should not be taken as evidence of a functional syndrome unless confirmed at more local scales: Some of the signal probably derives from differences among, rather than within, major biogeographic realms. The prevalence of clumping of risk implies that, faced with approximately equal pressures, species differ in their ability to persist because of lineage-specific characteristics. This finding invites a search for biological correlates of extinction risk.
Fig. 3.
Strength and significance of clumping in extinction risk within WWF ecoregions. Scale bar below the map indicates clumping strength. A value of 1 indicates randomness, and clumping is stronger for lower values. Circle size indicates the p value [radius is proportional to −ln(p)]; circle size for P = 0.05 is shown at the lower left.
Comparative Analyses of Mammalian Extinction Risk
Perhaps the most obvious proposed risk factor for extinction is large body size. The end-Pleistocene mass extinction of mammals removed mostly large species (32), and declining mammals are an order of magnitude heavier, on average, than are nonthreatened species (44). There are several possible reasons: Large-bodied species are more tempting targets than small ones for hunters; they are, on average, less abundant; and they take longer to reach sexual maturity, have smaller litters of larger offspring, and have larger individual home ranges. Narrow ecological tolerances are also a plausible risk factor—habitat specialists may be more at risk than generalists from habitat loss (45). A small geographic range size may reflect narrow tolerances and increase the risk that the whole of the species' range is in the firing line. Which of these features matter most for extinction risk, and are any associations consistent across all mammals?
Cardillo et al. (44) carried out the most comprehensive investigation to date. Threatened species were included only if they were on the IUCN Red List because of observed decline, to avoid autocorrelation with predictor variables. Red List status, on a 0–5 scale, was used as the response variable. Many facets of geography (including human population density), ecology, and life history were tested as predictors of extinction risk, by using phylogenetically independent contrasts. A phylogenetic approach is needed because, although extinction risk and some of the possible predictors listed above (e.g., geographic range size) do not evolve along the phylogeny's branches like, say, body size does, they nonetheless tend to show phylogenetic signal [i.e., they tend to take more similar values in close relatives than in species chosen at random; (41, 45, 46)]. Minimum adequate models were derived from a large initial set of predictors. This approach helps exclude variables that correlate only indirectly with extinction risk, for example, because another variable shapes both them and risk.
The predictors of risk were significantly different for smaller and larger species, with the importance of many predictors changing markedly at a body size of ≈3 kg. Species smaller than this fit the firing-line model: They are more likely to be threatened if they have small geographic ranges, live in temperate areas, face high human population densities, and live where a high proportion of the other mammal species are also threatened. Larger species, however, face multiple jeopardy: Biology matters as well as geography, with high abundance, small neonates, and many litters per year all independently helping to bullet-proof species. High abundance is predicted to bullet-proof species if the field of bullets model operates at the level of individuals rather than species (47), but such a model also predicts that no other biological traits would independently predict risk.
For both large and small mammals, the most important single risk factor is small geographic range size. The firing-line model predicts that small-ranged species will be most at risk because a single localized threat can impact their entire distribution. However, range size itself varies systematically among clades [although it shows weaker phylogenetic signal than, e.g., body size (48, 49)], suggesting that it is shaped, at least in part, by organismal traits such as dispersal ability (50) or niche breadth as well as by circumstances of geography. For example, small-ranged species are more common at low latitudes and within climatically stable regions. Any traits, including geographic location, that confer large species ranges also help make species bullet-proof (although geographical variation in species' range sizes again complicates separation of geographical variability in threat intensity from intrinsic biological vulnerability).
Large-scale analyses can find general predictors of extinction risk but can miss interesting variation among regions or clades, which more narrowly focused models might pick up (45). Order-specific models typically have higher explanatory power than the large-scale models. These models have some common features, such as the importance of geographic range size, but also differ considerably (51). For example, body size is a predictor in bats but not in rodents, whereas different life history traits predict risk in carnivores (gestation length) and ungulates (weaning age, interlitter interval). Likewise, different environmental factors and measures of human impact are implicated in different taxa. The models also vary regionally, with life history mattering less in North temperate regions than elsewhere (51).
One likely source of variation in models is that different drivers may select against different characteristics and show spatial variation in intensity. Broad-scale analyses may therefore lump competing signals together (52). Within artiodactyls, predictors of extinction risk differ between hunted and nonhunted species: Late weaning age was the sole risk factor among the former, whereas low income levels among local people and small range size predicted risk among the latter (53). More generally, low reproductive rates and large size are likely risk factors for overexploitation, but a specialized habitat may matter more under habitat loss (52). Analyses focused more tightly on driver-specific responses often tend to consider far fewer species, in which case far fewer predictor variables can be considered simultaneously without overfitting, and statistical power may be lower. On the plus side, the tighter focus can reduce the chance of mixed signals [although interaction terms can also do this (51)], and more precise measures of driver intensity and extinction risk might be available than can be had globally (54, 55). Broad- and narrow-scale analyses each give part of an obviously very complex picture. Furthermore, we have focused on phylogenetic nonindependence, but to fully consider the interaction between biology and geography, the development of methods that also deal with spatial nonindependence in comparative data will be critical.
Analyses modeling risk as a function of intrinsic biology (i.e., not including driver information) can highlight species at lower risk than expected from their geography, ecology, and life history (56). Such species may be particularly likely to decline rapidly if drivers intensify, because their attributes are repeatedly found in rapidly declining taxa. Cardillo et al. (56) termed this “latent risk” and proposed 20 regions with largely intact, but intrinsically susceptible, mammalian faunas. These include the Nearctic boreal forests and the island arc of Southeast Asia, and are mostly not exceptionally high in numbers of total, endemic, or threatened species. Many have much less than 10% of their land within reserves, and some (especially in southeast Asia) face very rapid human population growth. As such, latent-risk hotspots might represent cost-effective options for long-term conservation. However, these analyses do not yet consider realistic scenarios of future driver patterns; rather, they implicitly assume that places with low intensity will experience an increase to more typical levels (56). The next section discusses how more policy-relevant predictions could be obtained by projecting future driver patterns based on explicit scenarios.
Modeling Future Declines
Predicting future declines is more complex than explaining present declines, because the future is not just a linear extrapolation from the past and present. Past extinctions were largely caused by invasive species and overexploitation; habitat alteration is now a more important driver (9). Changes in land use have been mapped historically (www.mnp.nl/hyde) and are tracked in the present day (http://glcf.umiacs.umd.edu/data), but analogous spatial data for other main drivers are more problematic. Wild species might be most vulnerable to overexploitation where people live at high density and have few other protein sources, suggesting that predictive models can be developed at regional scales (57, 58). The patterns and driving processes behind invasive species have varied over time (59), and, although there are clear associations with global movements from human migration and trade, identifying clear predictive methods for the intensity of invasives is a work in progress (60). The same is true for disease (61).
Given the difficulties in obtaining spatial data on the present intensity of direct drivers, let alone future projections, an alternative is to work with information on indirect drivers—in particular, human population density and growth and patterns of land conversion. Projections of these drivers are available under a range of socioeconomic scenarios (62). Intensity data alone are not enough, however; the response curves linking intensity to decline are also needed, and responses will depend on how bullet-proof the biota is. Thus, declines need to be modeled as a function of both driver intensity and relevant biological attributes. A first step (63) considered a single driver (human population density) under a single growth scenario, coupling explanatory models of carnivore extinction risk from comparative analyses with human population projections to identify species whose conservation status was likely to worsen.
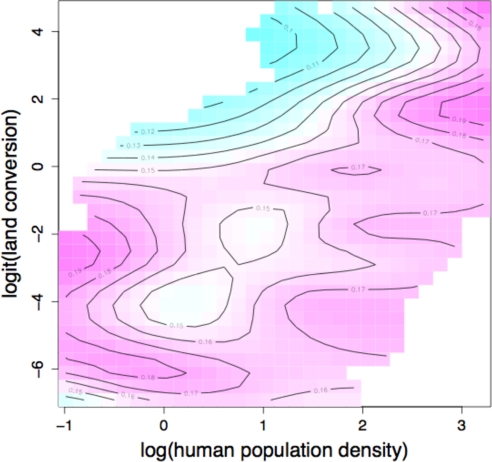
Here, we enlarge this approach in a preliminary analysis of two drivers and all mammals. Across ecoregions, the proportion of species with risk status higher than LC was modeled (as a binomial denominator) as a function of two drivers and two summaries of biological vulnerability by using generalized additive models (64) to avoid forcing any particular form on the relationship. A smooth relationship was fitted to link risk level to mean human population density (65) and the proportion of land converted to urban or cropland (66, 67). A second smooth relationship was fitted to control for two biotic variables [proportion of species weighing >3 kg, the size at which ecology and life history begin to influence risk strongly (44), and the proportion of species in the lowest quartile of global range size (K.E.J., J.B., A.P., C.D.L.O., Susanne A. Fritz, Christina Connolly, Amber Teacher, J.L.G., R.G., Elizabeth Boakes, Michael Habib, Janna Rist, Chris Carbone, Christopher A. Plaster, O.R.P.B.-E., Janine K. Foster, Elisabeth A. Rigby, Michael J. Cutts, Samantha A. Price, Wes Sechrest, Justin O'Dell, Kamran Safi, M.C., and G.M.M., unpublished data)]. Fig. 4shows the marginal effect of the drivers on extinction risk. The two drivers are strongly correlated across ecoregions and interact strongly. As expected, risk is low when both drivers are at the very lowest levels. However, risk rises rapidly as either driver increases. Medium levels of land conversion and low density are associated with high levels of risk, but risk falls as land conversion rises further. This suggests that land conversion is an extinction filter (68), removing one set of species sufficiently thoroughly that highly converted regions can again have low levels of risk, with only the more bullet-proof taxa remaining. Scenario analysis will obviously need to count projected extinctions as well as declines and may need to consider historical as well as present driver patterns. As human density reaches high levels, risk levels become uniformly higher.
Fig. 4.
The surface relating extinction-risk prevalence to urban and agricultural land use and human population density (HPD), controlling for two indices of biological susceptibility, among ecoregions. White regions in the upper left and lower right corners contain no ecoregions. Blue, low risk; purple, high risk.
A more refined model, perhaps incorporating other drivers, could be combined with projected future driver intensity to predict where a high proportion of species will decline. Such an approach uses the spatial heterogeneity in present driver intensity as a surrogate time series, with high-intensity ecoregions suggesting what will happen elsewhere as conditions deteriorate. However, incorporating climate change into this modeling approach presents major challenges. Because it has not been a major driver of present risk patterns, we have not yet seen how intensity will relate to impact and cannot use spatial heterogeneity as a guide. Bioclimatic envelope models suggest that climate change is already affecting many species including mammals (69) and may soon be the dominant driver, possibly exacerbated by interactions with invasive species and other threats (70). Climate change is likely to particularly impact species that face geographical or biological barriers to dispersal or that depend on environmental cues that may break down as climate changes (71).
Setting Priorities Among Areas
Conservation spending is not nearly enough to maintain even the current inadequate network of reserves and protected areas (72). The identification and prioritization of global conservation networks are a major focus of conservation dollars (73). However, rankings inherently depend on the currency used to evaluate regions. A simple demonstration can be provided by ranking ecoregions, in this case by applying a greedy complementarity algorithm, to maximize the capture of seven possible currencies for mammalian conservation: species richness; total numbers of species extinctions predicted in the next 100 years from current Red List status (using the probabilities of extinction in ref. 74); two measures combining evolutionary uniqueness and present extinction risk [EDGE (75) and ELEH (74), both summed across species in the ecoregion]; evolutionary dynamism (indexed as the sum of the reciprocal of the terminal branch lengths of species in the ecoregion); evolutionary history (estimated as the total branch length in the phylogeny of the ecoregion's species, adjusted for polytomies as described above); and total latent risk (56). Species-rich areas are likely to sum to higher values and, hence, rank highly across all currencies. Nonetheless, Table 1shows that rank order differs considerably even for similar measures designed to optimize the same currency (e.g., EDGE and ELEH). The biggest difference is between latent risk and numbers of extinctions. This is perhaps unsurprising, because latent risk gives high weights to species that are less threatened (although not all species were weighted for latent risk, weakening the comparison). A greedy algorithm makes choices based on maximizing the immediate gain in currency at each step and so may give globally suboptimal complementarity networks (76). In addition, ecoregions are, in any case, larger than most current conservation-management units (20). Our aim here is simply to show that surrogacy among currencies may be low. Furthermore, other more subjective or hard-to-quantify criteria might also be considered important—for example, the ecological function of a species or lineage, its cultural significance, or its charismatic appeal to humans (77)—further impeding comparisons between areas with similar numbers of species.
Table 1.
Numbers of ecoregions jointly ranked in the top 50 (of a total of 791) by each pair of currencies, according to greedy complementarity searches
| Richness | EDGE | ELEH | Extinctions | Diversification | Latent risk | Evolutionary history | |
|---|---|---|---|---|---|---|---|
| Richness | 50 | 41 | 22 | 21 | 34 | 15 | 36 |
| EDGE | 50 | 26 | 22 | 33 | 17 | 39 | |
| ELEH | 50 | 34 | 18 | 10 | 26 | ||
| Extinctions | 50 | 20 | 9 | 21 | |||
| Diversification | 50 | 16 | 30 | ||||
| Latent risk | 50 | 16 | |||||
| Evolutionary history | 50 |
Even if a currency can be agreed upon, a reserve network optimized for one clade is likely to be suboptimal for another (20). It is often tempting to use a single group as a surrogate for biodiversity as a whole, but other clades are likely to have very different—and equally complex—patterns of diversity and extinction risk. The lack of strong surrogacy among groups introduces extra uncertainty into the measured biodiversity value of the regions being considered. In addition, we have focused on conservation benefits rather than costs, but costs vary spatially by several orders of magnitude (72, 78). Cost–benefit models can suggest very different priorities from allocations based solely on perceived biodiversity value (79). So, if we assume that rational decision making must consider both benefits and costs, perhaps the most sensible investment would be in intact but susceptible regions (56, 78). Public health-care systems may provide a useful analogy: A balanced health-care strategy includes money for preventative medicine as well as for hospital wards and life support.
Beyond the Declines: The Evolutionary Future?
The primary goal for most conservation management has been to maximize preservation of current diversity. However, by altering the environment, humans also influence future evolution (80). A previous National Academy of Sciences Colloquium (81, 82) raised an important question: Should conservation goals be extended to consider the evolutionary future? A range of time scales might be considered. In the short term, species-recovery plans can address the requirements needed for continued adaptive evolution within populations (83). But what of the longer term? We can identify clades that have recently diversified and the regions in which they are found (Fig. 2b). These lineages or areas might represent engines of speciation: Are they therefore conservation priorities? The distinction between maximizing evolutionary history versus centers of diversification is nontrivial. A network of reserves designed to capture maximal evolutionary history would look very different from one designed to capture rapidly speciating lineages (Table 1), because rapid diversification results in low phylogenetic diversity per species (27, 84). However, past “success” may be a poor indicator of future performance because of the contingent nature of evolution (84, 85). The geographic pattern of mammalian diversification rates has changed markedly through time (Fig. 2 a–c), and different lineages have also radiated at different times (11, 86).
One secure prediction is that future environmental conditions will almost certainly differ from those in the past. Nonetheless, extrapolating current trends, key environmental changes are likely to include increasing habitat loss and fragmentation, drastic shifts in species abundances and distributions, and climate change (see above) (81, 87). Changes in the biotic and abiotic composition of the environment (including extinctions) may restructure niche space and moderate constraints on diversification imposed by niche saturation. Major turnovers in species composition may therefore be expected (88). Unfortunately, we lack detailed information on the past states of these attributes in geological history and so cannot easily construct quantitative empirical models that can be projected forward. Predicting the evolutionary future is hampered by large uncertainty about the magnitude and form of environmental change and by lineage-specific responses. If we wished to safeguard the evolutionary future, a sensible strategy would be to maintain a set of species that is overdispersed with respect to their ecological adaptations and (as a simple proxy) their phylogeny, maximizing the possibility of having the right set of features in an uncertain future (89).
Returning to our original question, should conservation goals consider the long-term evolutionary future? We sound two notes of caution. First, although we have restricted our focus to mammals, they are only a tiny branch on the Tree of Life, and many of the major limbs, from ciliates to Chlamydia, may be better insulated from anthropogenic disturbances (10). The evolutionary future of life on Earth is therefore unlikely to be in serious jeopardy. Second, anthropogenic environmental change and extinctions are occurring on the order of tens to hundreds of years, but times to speciation are frequently estimated in thousands to millions of years (87), and recovery times after previous mass extinction events were perhaps 5–10 million years (12). These time scales are too great for practical management. Diversity will almost certainly rebound after the current extinction event; however, it may be composed of species descended from a different, as yet unknown, subset of lineages from those that dominate now, and humans will likely not be included among them. Practical conservation should retain its focus on minimizing declines and extinctions in the present day.
Acknowledgments.
We thank Natalie Cooper, Lynsey McInnes, Jim Regetz, Gavin Thomas, and Nicola Toomey for discussion and John Avise and Doug Erwin for comments on the manuscript. This work was supported by the Natural Environment Research Council (U.K.), the National Science Foundation (U.S.), the European Union HOTSPOTS Early Stage Training Network, a Deutsche Forschungsgemeinschaft Heisenberg Scholarship, and the Leverhulme Trust.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution II: Biodiversity and Extinction,” held December 6–8, 2007, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_biodiversity.
The authors declare no conflict of interest.
References
- 1.Harvey PH, Leigh Brown AJ, Maynard Smith J, Nee S. New Uses For New Phylogenies. Oxford: Oxford Univ Press; 1996. [Google Scholar]
- 2.Bininda-Emonds ORP. The evolution of supertrees. Trends Ecol Evol. 2004;19:316–322. doi: 10.1016/j.tree.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Cracraft J, Donoghue MJ. Assembling the Tree of Life. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 4.Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis. Washington DC: World Resources Institute; 2005. [Google Scholar]
- 5.Jablonski D. Extinctions in the fossil record. Phil Trans R Soc London Ser B. 1994;344:11–16. [Google Scholar]
- 6.Purvis A, Hector A. Getting the measure of biodiversity. Nature. 2000;405:212–219. doi: 10.1038/35012221. [DOI] [PubMed] [Google Scholar]
- 7.Mace GM, et al. The Millennium Ecosystem Assessment: Current Status and Trends: Findings of the Conditions and Trends Working Group. Ecosystems and Human Well-Being. Island, Washington DC: 2005. pp. 53–98. [Google Scholar]
- 8.Wilson DE, Reeder DM. Mammal Species of the World: A Taxonomic And Geographic Reference. Third Ed. Baltimore: The Johns Hopkins Univ Press; 2005. [Google Scholar]
- 9.Baillie JEM, Hilton-Taylor C, Stuart SN. A Global Species Assessment. Gland, Switzerland: International Union for Conservation of Nature; 2004. [Google Scholar]
- 10.Nee S. In: Phylogeny and Conservation. Purvis A, Gittleman JL, Brooks T, editors. Cambridge: Cambridge Univ Press; 2005. pp. 387–399. [Google Scholar]
- 11.Bininda-Emonds ORP, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 12.Erwin DH. Lessons from the past: Biotic recoveries from mass extinctions. Proc Natl Acad Sci USA. 2001;98:5399–5403. doi: 10.1073/pnas.091092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avise JC. Three ambitious (and rather unorthodox) assignments for the field of biodiversity genetics. Proc Natl Acad Sci USA. 105(Suppl):11564–11570. doi: 10.1073/pnas.0801924105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purvis A, Katzourakis A, Agapow P-M. Evaluating phylogenetic tree shape: Two modifications to Fusco and Cronk's method. J Theor Biol. 2002;214:99–103. doi: 10.1006/jtbi.2001.2443. [DOI] [PubMed] [Google Scholar]
- 15.Mooers AØ, Heard SB. Inferring evolutionary process from phylogenetic tree shape. Q Rev Biol. 1997;72:31–54. [Google Scholar]
- 16.Purvis A. Harvey PH, Leigh Brown AJ, Maynard Smith J, Nee S, editors. Oxford Univ Press, Oxford. 1996:153–168. [Google Scholar]
- 17.Isaac NJB, Jones KE, Gittleman JL, Purvis A. Correlates of species richness in mammals: Body size, life-history and ecology. Am Nat. 2005;165:600–607. doi: 10.1086/429148. [DOI] [PubMed] [Google Scholar]
- 18.Coyne JA, Orr HA. Speciation. Sunderland MA: Sinauer; 2004. [Google Scholar]
- 19.Kerr JT, Packer L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature. 1997;385:252–254. [Google Scholar]
- 20.Grenyer R, et al. Global distribution and conservation of rare and threatened vertebrates. Nature. 2006;444:93–96. doi: 10.1038/nature05237. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins BA, et al. Energy, water and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- 22.Buckley LB, Jetz W. Environmental and historical constrains on global patterns of amphibian richness. Proc R Soc London Ser B. 2007;274:1167–1173. doi: 10.1098/rspb.2006.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme CDL, et al. Global patterns of geographic range size in birds. PLoS Biol. 2006;4:1276–1283. doi: 10.1371/journal.pbio.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stebbins GL. Flowering Plants: Evolution Above the Species Level. London: A Edwards Arnold; 1974. [Google Scholar]
- 25.Pulquério MJF, Nichols RA. Dates from the molecular clock: How wrong can we be? Trends Ecol Evol. 2007;22:180–184. doi: 10.1016/j.tree.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Clifford P, Richardson S, Hémon D. Assessing the significance of the correlation between two spatial processes. Biometrics. 1989;45:123–134. [PubMed] [Google Scholar]
- 27.Roy K, Goldberg EE. Origination, extinction, and dispersal: integrative models for understanding present-day diversity gradients. Am Nat. 2007;170:S71–S85. doi: 10.1086/519403. [DOI] [PubMed] [Google Scholar]
- 28.Mooers AØ, Atkins RA. Indonesia's threatened birds: Over 500 million years of evolutionary heritage at risk. Anim Cons. 2003;7:183–188. [Google Scholar]
- 29.Nee S. Birth–death models in macroevolution. Ann Rev Ecol Evol Syst. 2006;37:1–17. [Google Scholar]
- 30.Weir JT, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1574–1576. doi: 10.1126/science.1135590. [DOI] [PubMed] [Google Scholar]
- 31.Ricklefs RE. Global diversification rates of passerine birds. Proc R Soc London Ser B. 2003;270:2285–2292. doi: 10.1098/rspb.2003.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnosky AD. Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc Natl Acad Sci USA. 2008;105(Suppl):11543–11548. doi: 10.1073/pnas.0801918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 34.Luck GW. A review of the relationships between human population density and biodiversity. Biol Rev. 2007;82:607–645. doi: 10.1111/j.1469-185X.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- 35.Union for Conservation of Nature. 2007 IUCN Red List of Threatened Species. Gland, Switzerland: Union for Conservation of Nature; 2007. [Google Scholar]
- 36.Van Valen L. The Red Queen lives. Nature. 1976;260:575. [Google Scholar]
- 37.Raup DM, Gould SJ, Schopf TJM, Simberloff DS. Stochastic models of phylogeny and the evolution of diversity. J Geol. 1973;81:525–542. [Google Scholar]
- 38.Mace GM, Balmford A. In: Future Priorities for the Conservation of Mammalian Diversity. Entwhistle A, Dunstone N, editors. Cambridge: Cambridge Univ Press; 2000. pp. 27–52. [Google Scholar]
- 39.Russell GJ, Brooks TM, McKinney MM, Anderson CG. Present and future taxonomic selectivity in bird and mammal extinctions. Cons Biol. 1998;12:1365–1376. [Google Scholar]
- 40.Purvis A, Agapow P-M, Gittleman JL, Mace GM. Nonrandom extinction risk and the loss of evolutionary history. Science. 2000;288:328–330. doi: 10.1126/science.288.5464.328. [DOI] [PubMed] [Google Scholar]
- 41.McKinney ML. Extinction vulnerability and selectivity: Combining ecological and paleontological views. Ann Rev Ecol Syst. 1997;28:495–516. [Google Scholar]
- 42.McKinney ML. Role of human population size in raising bird and mammal threat among nations. Anim Cons. 2001;4:45–57. [Google Scholar]
- 43.Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 44.Cardillo M, et al. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030. [DOI] [PubMed] [Google Scholar]
- 45.Fisher DO, Owens IPF. The comparative method in conservation biology. Trends Ecol Evol. 2004;19:391–398. doi: 10.1016/j.tree.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Purvis A, Jones KE, Mace GM. Extinction. BioEssays. 2000;22:1123–1133. doi: 10.1002/1521-1878(200012)22:12<1123::AID-BIES10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 47.Erwin DH. Extinction: How Life Nearly Died 250 Million Years Ago. Princeton: Princeton Univ Press; 2006. [Google Scholar]
- 48.Jones KE, Sechrest W, Gittleman JL. In: Phylogeny and Conservation. Purvis A, Gittleman JL, Brooks TM, editors. Cambridge: Cambridge Univ Press; 2005. pp. 141–165. [Google Scholar]
- 49.Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 50.Böhning-Gaese K, Caprano T, van Ewijk K, Veith M. Range size: Disentangling current traits and phylogenetic and biogeographic factors. Am Nat. 2006;167:555–567. doi: 10.1086/501078. [DOI] [PubMed] [Google Scholar]
- 51.Cardillo M, et al. The predictability of extinction: Biological and external correlates of decline in mammals. Proc R Soc London Ser B. 2008;275:1441–1448. doi: 10.1098/rspb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens IPF, Bennett PM. Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. Proc Natl Acad Sci USA. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price SA, Gittleman JL. Hunting to extinction: Biology and regional economy influence extinction risk and the impact of hunting in artiodactyls. Proc R Soc London Ser B. 2007;274:1845–1851. doi: 10.1098/rspb.2007.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isaac NJB, Cowlishaw G. How species respond to multiple extinction threats. Proc R Soc London Ser B. 2004;271:1135–1141. doi: 10.1098/rspb.2004.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher DO, Blomberg SP, Owens IPF. Extrinsic versus intrinsic factors in the decline and extinction of Australian marsupials. Proc R Soc London Ser B. 2003;270:1801–1808. doi: 10.1098/rspb.2003.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardillo M, Mace GM, Gittleman JL, Purvis A. Latent risk and the future battlegrounds of mammal conservation. Proc Natl Acad Sci USA. 2006;103:4157–4161. doi: 10.1073/pnas.0510541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fa JE, Currie D, Meeuwig J. Bushmeat and food security in the Congo Basin: Linkages between wildlife and people's future. Env Cons. 2003;30:71–78. [Google Scholar]
- 58.Ling S, Milner-Gulland EJ. Assessment of the sustainability of bushmeat hunting based on dynamic bioeconomic models. Cons Biol. 2006;20:1294–1299. doi: 10.1111/j.1523-1739.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 59.Mack RN, Lonsdale WM. Humans as global plant dispersers: Getting more than we bargained for. BioScience. 2001;51:95–102. [Google Scholar]
- 60.Hastings A, et al. The spatial spread of invasions: New developments in theory and evidence. Ecol Lett. 2005;8:91–101. [Google Scholar]
- 61.Pedersen AB, Jones KE, Nunn CL, Altizer S. Infectious diseases and extinction risk in wild mammals. Cons Biol. 2007;21:1269–1279. doi: 10.1111/j.1523-1739.2007.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Millennium Ecosystem Assessment. Synthesis. Washington DC: World Resources Institute; 2005. [Google Scholar]
- 63.Cardillo M, et al. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2004;2:909–914. doi: 10.1371/journal.pbio.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 65.Center for International Earth Science Information Network (CIESIN), Centro Internacional de Agricultura Tropical (CIAT) Gridded Population of the World Version 3 (GPWv3) 2005 [Socioeconomic Data and Applications Center (SEDAC), (Columbia Univ, Palisades, NY)]. [Google Scholar]
- 66.Center for International Earth Science Information Network (CIESIN), International Food Policy Research Institute (IFPRI), The World Bank, Centro Internacional de Agricultura Tropical (CIAT) Global Rural-Urban Mapping Project (GRUMP), Alpha Version: Urban Extents. 2004 [Socioeconomic Data and Applications Center (SEDAC) (Columbia Univ, Palisades, NY)]. [Google Scholar]
- 67.European Commission, Joint Research Centre. Global Land Cover 2000 Database. Joint Research Centre: European Commission; 2003. [Google Scholar]
- 68.Balmford A. Extinction filters and current resilience; the significance of past selection pressures for conservation biology. Trends Ecol Evol. 1996;11:193–196. doi: 10.1016/0169-5347(96)10026-4. [DOI] [PubMed] [Google Scholar]
- 69.Parmesan C, Yoha G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 70.Thuiller W. Biodiversity—Climate change and the ecologist. Nature. 2007;448:550–552. doi: 10.1038/448550a. [DOI] [PubMed] [Google Scholar]
- 71.Bradshaw WE, Holzapfel CM. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- 72.James A, Gaston KJ, Balmford A. Can we afford to conserve biodiversity? BioScience. 2001;51:43–52. [Google Scholar]
- 73.Halpern BS, et al. Gaps and mismatches between global conservation priorities and spending. Cons Biol. 2006;20:56–64. doi: 10.1111/j.1523-1739.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 74.Redding DW, Mooers AO. Incorporating evolutionary measures into conservation prioritization. Cons Biol. 2006;20:1670–1678. doi: 10.1111/j.1523-1739.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- 75.Isaac NJB, Turvey ST, Collen B, Waterman C, Baillie JEM. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS One. 2007;2:e296. doi: 10.1371/journal.pone.0000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pressey RL, Possingham HP, Margules CR. Optimality in reserve selection algorithms: When does it matter and how much? Biol Cons. 1996;76:259–267. [Google Scholar]
- 77.Avise JC. In: Phylogeny and Conservation. Purvis A, Gittleman JL, Brooks TM, editors. Cambridge: Cambridge Univ Press; 2005. pp. 76–100. [Google Scholar]
- 78.Balmford A, Gaston KJ, Blyth S, James A, Kapos V. Global variation in terrestrial conservation costs, conservation benefits, and unmet conservation needs. Proc Natl Acad Sci USA. 2003;100:1046–1050. doi: 10.1073/pnas.0236945100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Possingham HP, et al. Limits to the use of threatened species lists. Trends Ecol Evol. 2002;17:503–507. [Google Scholar]
- 80.Smith TB, Bernatchez L. Evolutionary change in human-altered environments. Mol Ecol. 2008;17:1–8. doi: 10.1111/j.1365-294X.2007.03607.x. [DOI] [PubMed] [Google Scholar]
- 81.Myers N, Knoll AH. The biotic crisis and the future of evolution. Proc Natl Acad Sci USA. 2001;98:5389–5392. doi: 10.1073/pnas.091092498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cowling RM, Pressey RL. Rapid plant diversification: Planning for an evolutionary future. Proc Natl Acad Sci USA. 2001;98:5452–5457. doi: 10.1073/pnas.101093498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mace GM, Purvis A. Evolutionary biology and practical conservation: Bridging a widening gap. Mol Ecol. 2008;17:9–17. doi: 10.1111/j.1365-294X.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- 84.Mace GM, Gittleman JL, Purvis A. Preserving the Tree of Life. Science. 2003;300:1707–1709. doi: 10.1126/science.1085510. [DOI] [PubMed] [Google Scholar]
- 85.de Queiroz A. Contingent predictability in evolution: Key traits and diversification. Syst Biol. 2002;56:917–929. [PubMed] [Google Scholar]
- 86.Alroy J. New methods for quantifying macroevolutionary patterns and processes. Paleobiology. 2000;26:707–733. [Google Scholar]
- 87.Barraclough TG, Davies TJ. In: Phylogeny and Conservation. Purvis A, Gittleman JL, Brooks TM, editors. Cambridge: Cambridge Univ Press; 2005. pp. 400–418. [Google Scholar]
- 88.Tilman D, Lehman C. Human-caused environmental change: Impacts on plant diversity and evolution. Proc Natl Acad Sci USA. 2001;98:5433–5440. doi: 10.1073/pnas.091093198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crozier RH. Preserving the information content of species: Genetic diversity, phylogeny and conservation worth. Annu Rev Ecol Syst. 1997;28:243–268. [Google Scholar]