Abstract
The great mass extinctions of the fossil record were a major creative force that provided entirely new kinds of opportunities for the subsequent explosive evolution and diversification of surviving clades. Today, the synergistic effects of human impacts are laying the groundwork for a comparably great Anthropocene mass extinction in the oceans with unknown ecological and evolutionary consequences. Synergistic effects of habitat destruction, overfishing, introduced species, warming, acidification, toxins, and massive runoff of nutrients are transforming once complex ecosystems like coral reefs and kelp forests into monotonous level bottoms, transforming clear and productive coastal seas into anoxic dead zones, and transforming complex food webs topped by big animals into simplified, microbially dominated ecosystems with boom and bust cycles of toxic dinoflagellate blooms, jellyfish, and disease. Rates of change are increasingly fast and nonlinear with sudden phase shifts to novel alternative community states. We can only guess at the kinds of organisms that will benefit from this mayhem that is radically altering the selective seascape far beyond the consequences of fishing or warming alone. The prospects are especially bleak for animals and plants compared with metabolically flexible microbes and algae. Halting and ultimately reversing these trends will require rapid and fundamental changes in fisheries, agricultural practice, and the emissions of greenhouse gases on a global scale.
About 10 years ago, several of us concluded that the global ecological condition of the oceans because of overfishing was as dire as that of tropical rain forests, and that future losses would be enormous and potentially irreversible if action were not taken promptly to reverse the trajectories of decline (1–4). The scientific response was chilly, as evidenced by the statement of task for the recent National Research Council (NRC) report on the dynamics of marine ecosystems (5), which refers to our work in terms that emphasize the “high profile” of the articles (as if this were unseemly), the unconventional (and therefore suspect) nature of the data, and our assertions about the importance of shifting baselines and fishing down marine food webs, and that 90% of large predatory fish stocks are gone. In the end, the NRC report cautiously confirmed the conclusions it was convened to evaluate. But many scientists remain skeptical, apparently because (i) most conclusions are necessarily based on patterns and correlations using data gathered for many different purposes rather than experiments, (ii) the traditional emphasis in biological oceanography on bottom-up nutrient forcing rather than top-down control by predators, and (iii) the strong implication that most fisheries have been mismanaged for decades.
The focus of the NRC report (5) was on fishing, but the problems are vastly greater because of the additional effects on marine ecosystems of biological, toxic, and nutrient pollution, habitat loss, global climate change, and the synergies among all of these different drivers of ecological change (2, 6–10). There is also considerable uncertainty about the relative importance and interactions among local perturbations, such as fishing and pollution, versus global changes in climate and ocean chemistry that operate over very different and noncongruent temporal and spatial scales (11, 12). Trophic structure and biodiversity are also key components of the resistance and resilience of marine ecosystems to future perturbations (13, 14), but we are only beginning to document how these parameters change across a broad spectrum of human and natural disturbance. The problems are complex because of the huge numbers of species and different kinds of perturbations involved, the nonlinear dynamics of interactions among them, and the infancy of the emerging theoretical framework required to interpret the results (15–17).
Much new data and analysis has appeared in the last 5 years, sometimes with sharply conflicting interpretations, about the magnitude and rates of change in abundance of particular species. Regardless of these uncertainties, however, it is increasingly apparent that all of the different kinds of data and methods of analysis point in the same direction of drastic and increasingly rapid degradation of marine ecosystems. Here, I examine some of the most important of these new results since the publication of my previous synthesis (2), with emphasis on coastal seas and estuaries, continental shelves, the open ocean pelagic realm, and coral reefs, about which I am most familiar. The biology and substantial threats to the ecology of the deep sea have been recently reviewed by Koslow (18) and are not considered here. I then discuss what I believe will be the future of marine ecosystems if the drivers of change continue unabated, and address the kinds of changes in patterns of consumption and energy use that will be required to turn the situation around.
Estuaries and Coastal Seas
People have congregated along the coast from the beginnings of humanity, and the cumulative effects of exploitation, habitat destruction, and pollution are more severe in estuaries and coastal seas than anywhere else in the ocean except for coral reefs.
Bay of Fundy.
New detailed studies of the Quoddy region of the Bay of Fundy (19) and the Wadden Sea (20, 21) confirm and refine conclusions developed earlier based primarily on studies of Chesapeake Bay and Pamlico Sound (2, 22). The Bay of Fundy is particularly interesting because ecological degradation is so great despite comparatively good water quality compared with most other estuaries (20, 23). Exploitation began with whaling and hunting and fishing of other marine mammals, birds, and cod. The 16 original mammal species present before European contact were hunted to very low levels by 1900, with 3 extinct species including the sea mink Mustela macrodon, Atlantic walrus Odobenus rosmarus rosmarus, and possibly the coastal northwest Atlantic gray whale Eschrichtius robustus, as well as 7 more species that were severely reduced (19). Subsequent protection resulted in strong recovery of pinnepeds, but whales have not recovered, including the Northern Right Whale, which has declined from an estimated population of 10,000–15,000 in the northwest Atlantic to a mere 300 individuals. Among the 83 species of birds from the region, 40% have declined severely, including 3 species that were locally extirpated and another 3 hunted to extinction. Salmon were heavily exploited before records began, but even the stocks remaining in 1890–1900 were reduced by a further 99.5% by the end of the 20th century. The big three marine groundfish—cod, pollock, and haddock—were severely reduced before 1900, as elsewhere (19, 24), and catches were further reduced to just 3–37% of 1900 values by 2000. At the same time, several noncommercial species increased greatly in abundance. By the time scientific surveys began in 1970, groundfish were being rapidly replaced by shellfish and seaweeds as the major fisheries that are now also in decline (19).
Global Patterns of Exploitation.
Suggestions that we had somehow focused on only the worst-case scenarios in our article on overfishing (2) were addressed by an exhaustive review of 12 coastal seas and estuaries worldwide for which extensive archeological, historical, and early scientific data were available (23). We examined ≈80 species or species groups that were assigned to six major taxonomic groups and seven ecological guilds. Average global degradation ranged from a low of 39% for crustaceans to 91% for oysters (Table 1). Levels of overall degradation for all major taxonomic groups combined based on multivariate ordination varied roughly 2-fold among the 12 regions, from ≈43% in the bay of Fundy to 74% in the Adriatic, but the trajectories and patterns of degradation were strikingly similar for all of them (23). Most of the mammals, bird, and reptiles were severely depleted by 1900 and had declined even further by 1950. Among fish, diadromous salmon and sturgeon were depleted first, and then groundfish and large pelagics like tuna and sharks, and finally small pelagics like herring, menhaden, and sardines.
Table 1.
Percent decline (biomass, catch, percent cover) for fauna and flora from various marine environments
| Taxon | Starting date | Location | % loss | Ref. |
|---|---|---|---|---|
| Estuaries and coastal seas | ||||
| Large whales | Pristine | Global | 85% | 23 |
| Small whales | Pristine | Global | 59% | 23 |
| Pinnipeds and otters | Pristine | Global | 55% | 23 |
| Sirenia | Pristine | Global | 90% | 23 |
| Raptors | Pristine | Global | 79% | 23 |
| Seabirds | Pristine | Global | 57% | 23 |
| Shorebirds | Pristine | Global | 61% | 23 |
| Waterfowl/waders | Pristine | Global | 58% | 23 |
| Sea turtles | Pristine | Global | 87% | 23 |
| Diadromous fish | Pristine | Global | 81% | 23 |
| Groundfish | Pristine | Global | 62% | 23 |
| Large pelagics | Pristine | Global | 74% | 23 |
| Small pelagics | Pristine | Global | 45% | 23 |
| Oysters | Pristine | Global | 91% | 23 |
| Mussels | Pristine | Global | 47% | 23 |
| Crustaceans | Pristine | Global | 39% | 23 |
| Other invertebrates | Pristine | Global | 49% | 23 |
| Seagrass | Pristine | Global | 65% | 23 |
| SAV* | Pristine | Global | 48% | 23 |
| Wetlands | Pristine | Global | 67% | 23 |
| Large carnivores | Pristine | Global | 77% | 23 |
| Small carnivores | Pristine | Global | 60% | 23 |
| Large herbivores | Pristine | Global | 63% | 23 |
| Small herbivores | Pristine | Global | 54% | 23 |
| Suspension feeders | Pristine | Global | 68% | 23 |
| Shelf and pelagic fisheries | ||||
| Large predatory fishes | 1900 | N. Atlantic | 89% | 3 |
| Atlantic cod | 1852 | Scotian shelf | 96% | 24 |
| Fish 4–16 kg | Pristine | North Sea | 97% | 39 |
| Fish 16–66 kg | Pristine | North Sea | 99% | 39 |
| Large predatory fish | 1950s | Global | 90% | 4 |
| Large pelagic predators | 1950s | Tropical Pacific | 90% | 47 |
| Fishery biomass | 1959 | Bohai Sea | 95% | 38 |
| Coastal and pelagic sharks | ||||
| Hammerheads | 1986 | N.W. Atlantic | 89% | 31 |
| Scalloped hammerhead | 1972 | North Carolina | 98% | 30 |
| White | 1986 | N.W. Atlantic | 79% | 31 |
| Tiger | 1986 | N.W. Atlantic | 65% | 31 |
| Tiger | 1973 | North Carolina | 97% | 30 |
| Carcharhinus spp. | 1986 | N.W. Atlantic | 61% | 31 |
| Thresher | 1986 | N.W. Atlantic | 80% | 31 |
| Blue | 1986 | N.W. Atlantic | 60% | 31 |
| Mako | 1986 | N.W. Atlantic | 70% | 31 |
| Mako | 1950s | Gulf of Mexico | 45% | 32 |
| Oceanic whitetip | 1950s | Gulf of Mexico | 99% | 32 |
| Silky | 1950s | Gulf of Mexico | 91% | 32 |
| Dusky | 1950s | Gulf of Mexico | 79% | 32 |
| Dusky | 1972 | North Carolina | 99% | 30 |
| Blacktip shark | 1972 | North Carolina | 93% | 30 |
| Bull shark | 1973 | North Carolina | 99% | 30 |
| Sandbar shark | 1976 | North Carolina | 87% | 30 |
| Coral reefs | ||||
| Live coral cover | 1977 | Caribbean | 80% | 59 |
| Live coral cover | 1977 | Caribbean | 93% | 60 |
| Live coral cover | 1980–1982 | Indo-West Pacific | 46% | 64 |
| Commercial sponges | 1924 | Florida | 89% | 79 |
| Diadema antillarum | 1977 | Caribbean | 92% | 80 |
| Reef fish density | 1977 | Caribbean | 90% | 60 |
| Green turtle | 1700s | Caribbean | >99% | 82 |
| Hawksbill turtle | 1700s | Caribbean | >99% | 82 |
| Goliath grouper | 1956 | Florida Keys | 96% | 71 |
| Large carnivores | Pristine | Global | 85% | 8, 69 |
| Small carnivores | Pristine | Global | 61% | 8, 69 |
| Large herbivores | Pristine | Global | 87% | 8, 69 |
| Small herbivores | Pristine | Global | 66% | 8, 69 |
| Corals | Pristine | Global | 61% | 8, 69 |
| Suspension feeders | Pristine | Global | 49% | 8, 69 |
| Seagrasses | Pristine | Global | 50% | 8, 69 |
Starting dates are beginning of time series except in cases of estimated declines from the pristine, unexploited condition. See original references for details.
*Submerged aquatic vegetation.
Oysters were the first invertebrates to suffer extreme depletion, and the massive destruction of oyster reefs by dredging has permanently destroyed much of the formerly great habitat complexity of estuaries and coastal seas worldwide (2, 23, 25). Depletion of oysters moved progressively farther and farther from major markets like New York City, San Francisco, and Sydney, until eventually all of the stocks along Eastern and Western North America and Eastern Australia had collapsed (25). None of these wild stocks have substantially recovered because of eutrophication, disease, and habitat loss as described below, and oyster production now depends almost entirely on aquaculture.
Today, most fish and invertebrate stocks are severely depleted globally, and one-half to two-thirds of global wetlands and seagrass beds also have been lost (Table 1). Of the 80 species surveyed, 91% are depleted, 31% are rare, and 7% extinct (23). Nowhere are there any substantial signs of recovery, despite belated conservation efforts, except for nominal increases in some highly protected birds and mammals.
Patterns of Biological and Chemical Pollution.
Beginning in the 1950s, accelerating increases in the number of introduced species and degradation of water quality due to excess nutrients from the land have surpassed fishing as the major factors in the degradation of estuaries and coastal seas, although fishing still plays a major role (2, 22). Numbers of introduced species in the five best-studied estuaries range from 80 to 164 (average 117) species, where they have commonly displaced native animals and plants to become the dominant species. Geochemical and paleobotanical evidence of eutrophication in cores is readily apparent since at least the 19th century due to deforestation and agriculture, with consequent runoff of sediments and nutrients (23, 26, 27). These effects were aggravated by the extirpation of suspension feeders like oysters and menhaden (2, 22). The situation then largely stabilized until about 1950 when new influxes of nitrogen fertilizers began. Today, most of the estuaries experience massive nutrient inputs, extended summer eutrophication and hypoxia, and population explosions of microbes (2, 28).
Synergistic Effects.
The degrading effects of fishing, habitat destruction, introduced species, and eutrophication reinforce each other through positive feedbacks (2, 22, 23). For example, oysters were nearly eliminated by overfishing, but their recovery is now hampered by hypoxia due to eutrophication, by introduced species that compete for space and cause disease, and by the explosive rise of formerly uncommon predators that were previously kept in check by now overfished species (29, 30). Much of the overall decline of the 80 species reviewed by Lotze (23) was due to multiple suites of drivers: 45% of depletions and 42% of extinctions involved multiple impacts. Nowhere have these drivers been brought under effective regulation or control.
Continental Shelves
Ecological degradation on continental shelves is almost as severe as in estuaries and coastal seas, and the drivers are similar, albeit in somewhat different proportions.
Exploitation.
Longline fishing and trawling have removed 89% of the pristine abundance of prized large predatory fishes like cod, pollock, and haddock in the North Atlantic in the last 100 years, and cod have been depleted by 96% since 1852 (Table 1) (3, 24). The effects on sharks have also been enormous (Table 1). Large sharks most commonly caught by pelagic long lines in the northwest Atlantic were reduced by 40–89% between 1986 and 2000 (31). Likewise, in the Gulf of Mexico, longline fishing and trawling reduced the four commonest large pelagic species by 45–99% in the 40 years between the 1950s and 1990s (32). Small coastal sharks in the Gulf of Mexico have also been severely reduced, except for some species that have experienced release from predation by the overfishing of their predators (33). Of the 23 species for which adequate data were available, 16 species declined between 1972 and 2002, and the declines were statistically significant for 9 species, 3 of which were reduced to <2% of their 1972 abundance. Seven species also increased, 3 of them significantly, including the smooth dogfish, which increased 13-fold. However, these were deepwater species generally out of reach of trawling.
Release from predators can have spectacular consequences through the development of trophic cascades. For example, reductions of ≈90–99% for 11 large sharks that consume smaller elasmobranchs along the northwest Atlantic Coast of North America resulted in the increase of 12 of their 14 common prey species (30). Populations of one of these smaller species, the cownose ray Rhinoptera bonasus, exploded to some 40 million. Each ray can consume ≈210 g shell-free wet weight of bivalve mollusks per day, assuming that they are available. The rays migrate through Chesapeake Bay each year, where they stay for ≈100 days, which amounts to a potential consumption of 840,000 metric tons of mollusks/year. In contrast, the commercial harvest of bay scallops that peaked in the early 1980s had fallen by 2003 to only 300 metric tons. Thus, the rays could potentially consume 2,500 times the commercial harvest, and it is hardly surprising that the once prosperous clam fisheries have totally collapsed.
The canonical example of a trophic cascade involves the near extinction of sea otters by hunting in the Northeast Pacific that resulted in explosions of sea urchins that in turn eliminated entire kelp forests by overgrazing (34). Trophic cascades have also been documented for the formerly cod-dominated ecosystem of the northwest Atlantic (35), where removal of large groundfish resulted in large increases in pelagic shrimp and snow crabs, decreases in large zooplankton, and increases in phytoplankton. Thus, removal of top-down controls affects ecosystem structure and function of large marine ecosystems with complex food webs, as well as simpler, low-diversity systems.
The occurrence of trophic cascades is closely linked to the phenomenon of fishing down the food web (1). Analysis of Food and Agriculture Organization global fisheries data showed a decline in mean trophic level of the global predatory fish catch of ≈0.1 per decade since 1950. There has been much discussion of the quality and suitability of the data, and whether the decline in trophic level primarily reflects serial depletion of overfished species or serial additions of lower trophic level species caused by a depletion of their predators (5, 36). Regardless of the relative importance of these different mechanisms, however, there is increasingly reliable evidence for the pervasive decline in mean trophic level in heavily fished ecosystems. For example, mean trophic level fell from 4.06 to 3.41 in the Bohai Sea between1959 and 1998 (37), a decline of 0.16–0.19 per decade. This drop parallels a dramatic 95% decline in total fish catch from 190 to 10 kg per standardized haul per hour (Table 1) and a precipitous drop in the proportion of piscivorous fish in the standardized hauls from 29.3% to 0% of the catch (38). In contrast, planktivorous species increased from 4.75% to 58.0% of the catch in the same hauls.
Finally, Jennings and Blanchard (39) used the theoretical abundance–body mass relationship derived from macroecological theory to estimate the pristine biomass of fishes in the North Sea in comparison with the size and trophic structure of heavily exploited populations in 2001. The estimated total biomass of all fishes 64 g to 64 kg declined 38% while the mean turnover time of the population was estimated to have dropped from 3.5 to 1.9 years. Large fishes 4–16 kg were estimated to have declined by 97.4%, and species 16–66 kg were estimated to have declined by 99.2%. The great importance of these calculations is that they are entirely independent of all of the assumptions and controversies surrounding fisheries catch data and models, and yet lead to predictions entirely consistent with the most extreme estimates of fisheries declines.
Habitat Destruction by Trawling and Dredging.
Trawling reduces the three-dimensional structure and complexity of sea floor habitats to bare sediment; reduces the size, biological diversity, and turnover time of dominant species; and results in entirely new associations of species that may persist for decades even if trawling is halted (Fig. 1) (40–42). The magnitude of effects increases with the frequency and geographic scale of trawling. The most striking data are from New England and the Gulf of Mexico (41), although the situation is almost certainly comparable on continental shelves around the world (40). In New England, the total area fished (TAF) by trawling is 138,000 km2, and 56% of the sample areas are fished more than once a year, so that the equivalent of 115% of the TAF is fished every year. In the Gulf of Mexico, the TAF is 270,000 km2, 57% of the sample areas are fished more than once a year, and trawls sweep 255% of the TAF each year. Thus, trawling has drastically degraded most of the sea floor in these huge regions, and with multiple trawling episodes per year at favored sites, there is obviously no opportunity for ecosystem recovery.
Fig. 1.
Impact of trawling on the seafloor at ≈18 m depth in the Swan Island Conservation Area, northern Gulf of Maine (42). The straight lines are furrows made by the trawl, and the debris is polychaete worm tubes.
Eutrophication, Dead Zones, and the Rise of Slime.
Nutrient runoff is naturally greatest, and eutrophication, hypoxia, and toxic blooms are most intense, in estuaries and coastal seas like the Adriatic and Baltic seas and Chesapeake and San Francisco bays (2, 22, 23, 28). However, major river systems like the Amazon, Yangtze, and Mississippi–Missouri also discharge vast amounts of nutrients, sediments, and organic matter into relatively small areas of open coast and surrounding continental shelves. The enormous increase in the use of chemical fertilizers in the drainage basins of these great rivers over the past 50 years (43), coupled with the virtual elimination of suspension feeding oysters and wetlands along their delta margins, has resulted in the formation of vast eutrophic and hypoxic regions comparable with the worst conditions in estuaries (28).
The iconic American example is the hypoxic “dead zone” that extends some 500 km west of the Mississippi delta. The area of the hypoxic zone has doubled in the past 20 years to ≈20,000 km2, and the rate of increase in area is a linear function of nitrogen loading from the Mississippi drainage (Fig. 2) (10, 44). Analyses of the geochemistry and mineralogy of cores shows that hypoxic conditions were uncommon before the 1950s, strongly supporting the hypothesis that their formation is due to comparatively recent human impacts and is not a natural phenomenon. The dead zone expands during the summer, when hypoxia extends from shallow depths to the sea floor, and there is mass mortality of most animals that cannot swim away, including major fisheries species like shrimp. The dead zone is hardly dead, however, but supports an extraordinary biomass of diverse microbes and jellyfish that may constitute the only surviving commercial fishery. In addition, and extending beyond the dead zone, toxic blooms of dinoflagellates like Karenia brevis occur over areas as large as the entire Northwestern Gulf of Mexico.
Fig. 2.
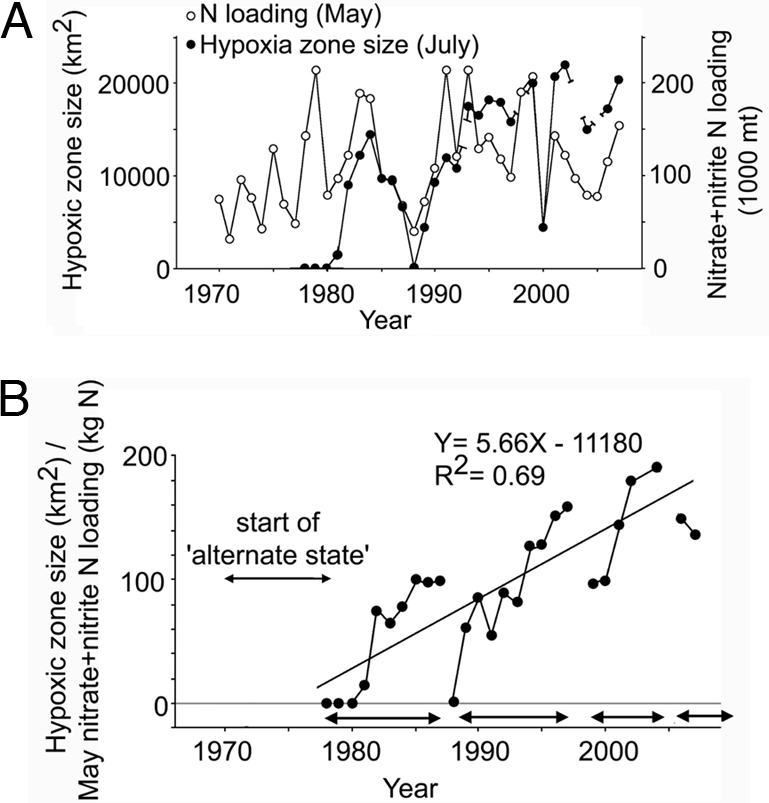
Hypoxia and nitrogen loading in the Gulf of Mexico (44). (A) Annual variations in the size of the hypoxic zone in late July and the nitrate plus nitrite nitrogen loading for the preceding May. (B) Increase in the ratio of the size of the hypoxic zone relative to nitrogen loading the preceding year. The breaks in the curve reflect hurricanes and droughts, but the overall trend is highly significant.
The Open Ocean Pelagic Realm
Myers and Worm (4) fired a shot heard around the world when they published their controversial assertion that 90% of all of the large (average approximately ≥50 kg), open ocean tuna, billfishes, and sharks in the ocean were gone. Severe depletion of coastal and shelf fisheries was widely accepted, but the open ocean was still considered one of the last great wild places on Earth.
Exploitation.
Much of the controversy revolves around the use or misuse of highly complex “state-of-the-art stock assessment methods” (45) to estimate fisheries impacts on population biomass, size, and trophic status of top-level predators that are beyond the ability of most ecologists to evaluate. There has also been a great deal of name-calling, such as referring to Myers's and Worm's calculations as “folly” and “fantasy” (46), and outrage about the publicity that their article received from the press (47). Nevertheless, the NRC report acknowledged declines in the range of 65–80% (5) that are much greater than anyone was admitting to before Myers's and Worm's article was published.
Ward and Myers (48) subsequently evaluated the status of 19 oceanic fisheries species in the Central Tropical Pacific between the 1950s and the 1990s using scientific survey and official observer data. There were 12 species of large predatory sharks, tunas, and billfishes and 7 smaller species <17 kg. All 12 large predators showed significant declines in biomass, and 11 of the 12 species decreased in average body mass by 29–73%, whereas 5 of 7 smaller species showed no significant change and skipjack tuna significantly increased (48). Moreover, the pelagic stingray Dasyatis violacea and pomfrets, which were absent in the 1950s, appeared in even greater abundance than any of the original smaller species. Overall, biomass declined 89.7% and large predators declined by 90.3%—the same as the declines originally reported by Myers and Worm (4). Perhaps more important than the actual magnitude of decline, however, is the clear shift in species composition and relative abundance in the pelagic community reminiscent of the increase in mesopredators in the northwest Atlantic and Gulf of Mexico (30, 33). Pelagic fisheries are a vast uncontrolled experiment whose ecosystem consequences are still unknown. Nevertheless, Ward's and Myers's results clearly point toward the potential for strong trophic cascades and significant declines in mean trophic level as fishing erodes top-down control.
Climate Change.
Warming and acidification of the pelagic realm due to the rise of CO2 comprise another uncontrolled experiment on a global scale (49). Sea-surface warming increases the stratification of the oceans because warmer and lighter surface waters inhibit upwelling of cooler and denser nutrient rich waters from below (9). Increased stratification may have already caused the drop in productivity in the northern Pacific that is widely described by biologists as a “regime shift” in the composition of open-ocean plankton communities (50). Moreover, climate models suggest that the oceans may move into a permanent El Niño condition (51, 52). Uncertainties abound regarding the degree to which upwelling could be permanently suppressed, but if ocean productivity declines, there will be an inevitable further decline in fisheries (50, 53).
Of even greater concern, because of the seemingly inevitable effects on all calcareous marine organisms, is ocean acidification due to the increased solution of carbon dioxide that forms carbonic acid in seawater (49). Measurements have already demonstrated a drop of 0.1 pH units in the oceans (54), and laboratory and mesocosm experiments demonstrate that calcareous planktonic coccolithophores, pteropods, and foraminifera exhibit decreased calcification and growth under even mildly acidic conditions (7, 55). The biogeochemical implications are staggering. These organisms are among the greatest producers of biogenic sediments in the ocean, are vital to particle aggregation and the production of marine snow that enhances the vertical flux of biogenic material, and are major components of the cycling of carbon and the CO2 storage capacity of the ocean (7, 55).
Coral Reefs
Coral reefs are the most diverse marine ecosystems and among the most threatened (6, 56). Just 15 years ago, many coral reef scientists still referred to coral reefs as pristine (57), yet today many scientists believe that the cumulative forces of overfishing, pollution, and climate change are so great that coral reefs may virtually disappear within a few decades (11, 12).
Demise of Reef Fauna.
Corals are dying out around the world and are being replaced by fleshy macroalgae or algal turfs that may carpet the entire reef surface (8, 58, 59). In the Caribbean, live coral cover has fallen from an average of ≈55% in 1977 to 5% in 2001 (Table 1), whereas macroalgal cover has risen from an average of ≈5% to 40% (60, 61). The demise of formerly ubiquitous and abundant elkhorn and staghorn corals (Acropora palmata and Acropora cervicornis) is particularly striking; these corals were the major rock formers on shallow Caribbean reefs for at least a million years (62–64) but are now officially listed as endangered species. The story is a little better in the Indo-West Pacific where live coral cover still averages ≈22%, which is about one-half of that in 1980 (65). However, even the Great Barrier Reef, which is arguably the best-protected coral reef system in the world, has only 23% live coral cover. The only places I know of where live coral cover still averages ≈50% or more over large areas of reef are the uninhabited and protected atolls of the Central Pacific (12).
Most Caribbean coral communities in 1977 still resembled the first detailed descriptions from the 1950s (66), as well as reconstructions of Holocene and Pleistocene assemblages in the fossil record (62–64, 67); although reefs at Barbados, had already lost their formerly dense populations of Acropora by the early 20th century (68, 69). In contrast, reef fishes throughout the entire region were only a small remnant of how they used to be (57, 70, 71), and populations declined by more than one-half again between 1977 and 2003 (61). We can piece together a clear qualitative picture of what pristine Caribbean reef fish communities were like from archeological and historical analysis (2, 57, 70). The extraordinary old photographs of fishing boats returning to Key West draped in giant sawfishes and sharks (Fig. 3) make these descriptions come alive, as do the trophy photographs of an afternoon's catch by a single charter boat of up to 16 gigantic goliath grouper, a now endangered species for which catch per unit effort (CPUE) declined 87% between 1956 and 1979 (L. McClenachan, personal communication).
Fig. 3.
A sporting day's catch of sawfish in the Florida Keys in the 1940s (courtesy of the Monroe County Public Library, Key West, Florida).
We can put numbers on these impressions by comparison of modern Caribbean fish communities on unprotected reefs versus sites inside the few long-established marine protected areas (MPAs) where fishing is prohibited and the rules are strictly enforced (Table 2) (59, 61). Unprotected reefs in the Pacific and Indian Oceans are comparably overfished, although few if any as badly as Jamaica (Table 2) (72–76). As for corals, the greatest fish biomass and largest fish occur on the uninhabited and protected atolls of the Central and North Central Pacific that may never have been severely degraded (Table 2) (12). The highest fish biomass on these isolated atolls is 1,000 g/m2, which is only double that on the best-protected Caribbean reefs. Piscivores comprise ≈50–85% of total fish biomass (12, 76), most of it large sharks. In general, apex predators are virtually absent from reefs where fish biomass is <100 g/m2 but may exceed the combined biomass of all lower trophic levels of fishes when fish biomass exceeds ≈300 g/m2. Thus, when fish biomass is high, the fish trophic pyramid is upside-down (73, 77).
Table 2.
Biomass of fishes on coral reefs in relation to human population size per km of reef or reserve status
| Location | Human population size per km reef or protection status | Fish biomass, g/m2 |
|---|---|---|
| Kingman (CP) | 0 | 1,020/530* |
| Jarvis (CP) | 0 | 800 |
| Palmyra (CP) | 0.5 | 520/260* |
| Baker (CP) | 0 | 390 |
| Cozumel (C) | Protected | 386 |
| Kiritimati (CP) | 21.1 | 310/130* |
| Cuba (C) | Protected | 275 |
| NW Hawaiian Islands (NCP) | Protected | 240 |
| Bahamas (C) | Protected | 194 |
| Tavunasica (WP) | 2.6 | 140 |
| Kenya(IO) | Protected | 115 |
| Vuaqava (WP) | 6.6 | 103 |
| Florida (C) | Unprotected | 101 |
| Totoya (WP) | 18 | 80 |
| Kabara (WP) | 43.3 | 75 |
| Main Hawaiian Islands (NCP) | Unprotected | 70 |
| Matuku (WP) | 24.4 | 67 |
| Moala (WP) | 26.2 | 60 |
| Bahamas (C) | Unprotected | 57 |
| Kenya (IO) | Unprotected | <40 |
| Jamaica | Unprotected | 39 |
See ref. 12. C, Caribbean; CP, central Pacific; IO, Indian Ocean; NCP, north central Pacific; WP, western Pacific.
*Sample dates: 1997/2005.
Reefs in Florida surveyed in 2005 (59) were also surveyed in 1880 (71). Only 30% of the 20 species that were common in 1880 were still common in 2005, and 40% of the formerly common species were absent in 2005. Most remarkably, 4 of the 20 formerly common species in 1880 are now listed as endangered or critically endangered, including the Nassau grouper (Epinephalus striatus), which was the mainstay of fish sandwiches in the Florida Keys for too many decades to the point that this once fantastically abundant species is entirely fished out.
Fishes and corals are not the only animals to have declined precipitously. Harvests of Florida commercial sponges peaked in 1924 at >3 million tons, and then crashed to nearly zero in the 1940s when stricken by disease (78). Today, the sponges have recovered to ≈11% of their abundance in the 1880s. The formerly abundant sea urchin Diadema antillarum also declined by 90–95% because of an outbreak of an unidentified disease in 1983 (79), and much of the mortality of elkhorn and staghorn corals in the 1970s and 1980s was also due to outbreaks of disease (6, 80). Green turtles have declined by well over 99% from approximately 90 million in the 18th century to perhaps 300,000 today, and hawksbill turtles declined as precipitously from approximately 11 million to 30,000 (81). The total population of the extinct Caribbean monk seal in the 18th century was ≈230,000–340,000, abundance so great that all of the remaining fish on Caribbean coral reefs would be inadequate to sustain them (82).
Indirect Effects of Exploitation.
The restriction of abundant fish biomass to protected reefs shows that MPAs do work, oceanographic factors notwithstanding, so long as they are large, well protected, and enforced (12, 59, 83). The more difficult question concerns the potential of protection from fishing to help to restore coral populations in the face of epidemic disease and global climate change (12, 56, 72, 84). As for oysters in estuaries, overfishing, increased macroalgal abundance, and degraded water quality act synergistically to decrease coral growth, recruitment, and survival. Increased abundance of fleshy macroalgae and algal turfs may kill corals directly by overgrowth or indirectly by leaking of dissolved organic matter into the surrounding water that destabilizes microbial communities on corals and promotes coral disease (11, 85–87), or by smothering the crustose coralline algae that are necessary cues for coral larvae to recruit (88). Increased abundance of fish or sea urchins is associated with a decline in macroalgae (59, 89) and increased coral recruitment (88). Coral cover has not increased, however, presumably because of the slow growth and long generation times of corals compared with fish and macroalgae (8, 59, 90).
Ocean Warming and Acidification.
Rising temperatures and falling pH are as ominous for the future of corals and coral reefs (6, 11, 56, 91) as for calcareous plankton (7). Warming has caused mass mortality of corals by coral bleaching that has increased in frequency and intensity over the past two to three decades. Reduction of pH reduces coral growth rates and skeletal density, and may eventually stop calcification entirely, so that corals lose their skeletons and resemble small colonial sea anemones (92). Regardless of whether or not the corals can survive under such circumstances, reef formation would be severely reduced or halted if acidification proceeded at current rates.
Climate change exacerbates local stress due to overfishing and decline in water quality (8, 12, 93), but the reverse is also true to the extent that the unpopulated, unfished, and unpolluted atolls of the Central Pacific still possess ≈50% coral cover while other reefs in the Pacific have less than half that amount (12, 65, 76). This is the only good news I know of for coral reefs, and there is a pressing need to study these reefs to determine why corals have so far persisted in such abundance and the degree to which coral community composition is shifting toward more physiologically resilient species or to those with shorter generation times and faster growth. Ultimately, however, it is difficult to imagine how corals will be able to survive or reefs persist if the rise in CO2 continues unabated.
The Future Ocean
The overall status of the four major categories of ocean ecosystems and the principal drivers of their degradation are summarized in Table 3. Coastal ecosystems are endangered to critically endangered on a global scale. The lesser endangerment of pelagic ecosystems reflects their remoteness from all factors except fishing and climate change, although there are no real baselines for comparison to critically evaluate changes in plankton communities. This grim assessment begs the question, What are the projected long-term consequences for the ecological condition of the ocean if we continue with business as usual?
Table 3.
Status and trends of major ocean ecosystems defined by principal symptoms and drivers of degradation in the >99% of the global ocean that is unprotected from exploitation
| Coral reefs: Critically endangered |
|---|
| Symptoms: Live coral reduced 50–93%; fish populations reduced 90%; apex predators virtually absent; other megafauna reduced by 90–100%; population explosions of seaweeds; loss of complex habitat; mass mortality of corals from disease and coral bleaching |
| Drivers: Overfishing, warming and acidification due to increasing CO2, run-off of nutrients and toxins, invasive species |
| Estuaries and coastal seas: Critically endangered |
| Symptoms: Marshlands, mangroves, seagrasses, and oyster reefs reduced 67–91%; fish and other shellfish populations reduced 50–80%; eutrophication and hypoxia, sometimes of entire estuaries, with mass mortality of fishes and invertebrates; loss of native species; toxic algal blooms; outbreaks of disease; contamination and infection of fish and shellfish; human disease |
| Drivers: Overfishing; runoff of nutrients and toxins; warming due to rise of CO2; invasive species; coastal land use |
| Continental shelves: Endangered |
| Symptoms: Loss of complex benthic habitat; fishes and sharks reduced 50–99%; eutrophication and hypoxia in ″dead zones″ near river mouths; toxic algal blooms; contamination and infection of fish and shellfish; decreased upwelling of nutrients; changes in plankton communities |
| Drivers: Overfishing; trophic cascades; trawling; runoff of nutrients and toxins; warming and acidification due to rise of CO2; introduced species; escape of aquaculture species |
| Open ocean pelagic: Threatened |
| Symptoms: Targeted fishes reduced 50–90%; increase in nontargeted fish; increased stratification; changes in plankton communities |
| Drivers: Overfishing; trophic cascades; warming and acidification due to rise of CO2 |
Predicting the future is, at best, a highly uncertain enterprise. Nevertheless, I believe we have a sufficient basic understanding of the ecological processes involved to make meaningful qualitative predictions about what will happen in the oceans if humans fail to restrain their style of exploitation and consumption. Failure to stop overfishing will push increasing numbers of species to the brink of extinction—perhaps irreversibly as for Newfoundland cod—except for small, opportunistic species. Unrestrained runoff of nutrients and toxins, coupled with rising temperatures, will increase the size and abundance of dead zones and toxic blooms that may merge all along the continents. Even farmed seafood will be increasingly toxic and unfit for human consumption unless grown in isolation from the ocean. Outbreaks of disease will increase. Failure to cap and reduce emissions of CO2 and other greenhouse gases will increase ocean temperatures and intensify acidification. Warmer and lighter surface waters will inhibit vertical mixing of the ocean, eventually leading to hypoxia or anoxia below the thermocline as in the Black Sea. Biogeochemical cycles will be perturbed in uncertain ways as they have been in the past (94). Mass extinction of multicellular life will result in profound loss of animal and plant biodiversity, and microbes will reign supreme.
These predictions will undoubtedly appear extreme, but it is difficult to imagine how such changes will not come to pass without fundamental changes in human behavior. Moreover, as we have seen, all of these trends have actually been measured to a limited degree in the past few decades. The oceans are becoming warmer and more acidic; eutrophication, hypoxia, and the numbers and sizes of dead zones are increasing in quantity and size; vertical mixing of the open ocean is measurably decreasing; and many of our most valuable fisheries have collapsed and failed to recover. Some may say that it is irresponsible to make such predictions pending further detailed study to be sure of every point. However, we will never be certain about every detail, and it would be irresponsible to remain silent in the face of what we already know.
How Can We Stop the Degradation of the Oceans?
The three major drivers of ecosystem degradation are overexploitation, nutrient and toxic pollution, and climate change. The challenges of bringing these threats under control are enormously complex and will require fundamental changes in fisheries, agricultural practices, and the ways we obtain energy for everything we do. We have to begin somewhere, however, and the following very significant actions could begin right away without further scientific research or technological innovation.
Sustainable Fisheries.
The tools for effective management of wild fisheries are well established (95, 96), and there are encouraging examples of success (97). Nevertheless, the required actions have rarely been implemented (98). In contrast, subsistence overfishing in developing nations is commonly a matter of survival, so that alternative sources of protein and livelihood are required to bring the situation under control (95, 99). More fundamentally, however, wild fisheries cannot possibly sustain increasing global demand regardless of how well they are managed. Industrial scale aquaculture of species low on the food chain is the only viable alternative. But this in turn will require strong new regulation to prevent harmful ecosystem consequences such as the destruction of mangroves for shrimp farms and the impacts on wild salmon populations caused by the explosion of parasitic copepods that infect salmon farms in British Columbia (100, 101). Despite all of these concerns, however, the only thing standing in the way of sustainable fisheries and aquaculture is the lack of political will and the greed of special interests. Simply enforcing the standards of the Magnuson–Stevens Act and the U.S. National Marine Fisheries Service would result in major improvements in United States waters within a decade (97, 98).
Coastal Pollution and Eutrophication.
Heavily subsidized overuse of chemical fertilizers and pesticides, poor soil management practices, and unregulated animal production systems are the major sources of excess nitrogen and other nutrients in the environment that fuel coastal eutrophication (2, 10, 44) and severely degrade terrestrial ecosystems (43, 102–104). Manufacture of chemical fertilizers also consumes huge amounts of energy from natural gas (105). Removal of subsidies and taxation of fertilizers would significantly reduce nutrient loading, eutrophication, and emissions of greenhouse gases with only modest decreases in food production and increased costs.
Climate Change and Ocean Acidification.
The rise in greenhouse gases and the resulting global economic, social, and environmental consequences comprise the greatest challenge to humanity today. Moderation of consumption of fossil fuels in a time of rising global aspirations and finding alternative sources of energy will require all of the ingenuity humanity can muster and will preoccupy us for the remainder of the century. The problems appear so overwhelming that many are ready to write-off coral reefs and all of the other marine life that will be drastically affected. But such defeatism belies the growing realization that local protection from overexploitation and pollution confers some as yet poorly understood level of resistance and resilience to the effects of climate change on coral reefs (12, 75, 84), and the same is very likely true for other marine ecosystems. This is an important area for new scientific research to better understand the synergies among different drivers of ecosystem change and their likely consequences. Most importantly, local conservation measures may help to buy time for marine ecosystems until we bring the rise of greenhouse gases under more effective control.
Acknowledgments.
I thank Julia Baum, Davy Kline, Nancy Knowlton, Loren McClenachan, Marah Newman, Forest Rohwer, Stuart Sandin, Enric Sala, Jennifer Smith, and Sheila Walsh for sharing their thoughts about the ecological degradation of the ocean and for many helpful suggestions. The William E. and Mary B. Ritter Chair of the Scripps Institution of Oceanography provided invaluable support.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution II: Biodiversity and Extinction,” held December 6–8, 2007, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_biodiversity.
The author declares no conflict of interest.
References
- 1.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 3.Christensen V, et al. Hundred-year decline of North Atlantic predatory fishes. Fish Fisheries. 2003;4:1–24. [Google Scholar]
- 4.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 5.NRC. Dynamic Changes in Marine Ecosystems: Fishing, Food Webs, and Future Options. Washington, DC: Natl Acad Press; 2006. [Google Scholar]
- 6.Knowlton N. The future of coral reefs. Proc Natl Acad Sci USA. 2001;98:5419–5425. doi: 10.1073/pnas.091092998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riebesell U. Effects of CO2 enrichment on marine phytoplankton. J Oceanogr. 2004;60:719–729. [Google Scholar]
- 8.Pandolfi JM, et al. Are US coral reefs on the slippery slope to slime? Science. 2005;307:1725–1726. doi: 10.1126/science.1104258. [DOI] [PubMed] [Google Scholar]
- 9.Schmittner A. Decline of the marine ecosystem caused by a reduction in the Atlantic overturning circulation. Nature. 2005;434:628–635. doi: 10.1038/nature03476. [DOI] [PubMed] [Google Scholar]
- 10.Rabalais NN, Turner RE, Sen Gupta BK, Platon E, Parsons ML. Sediments tell the history of eutrophication and hypoxia in the northern Gulf of Mexico. Ecol Appl. 2007;17:S129–S143. [Google Scholar]
- 11.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 12.Knowlton N, Jackson JBC. Shifting baselines, local impacts, and climate change on coral reefs. PLoS Biol. 2008;6:e54. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bascompte J, Melian CJ, Sala E. Interaction strength motifs and the overfishing of marine food webs. Proc Natl Acad Sci USA. 2005;102:5443–5447. doi: 10.1073/pnas.0501562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 15.Knowlton N. Thresholds and multiple stable states in coral reef community dynamics. Am Zool. 1992;32:674–682. [Google Scholar]
- 16.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh C, Glaser SM, Lucas AJ, Sugihara G. Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature. 2005;435:336–340. doi: 10.1038/nature03553. [DOI] [PubMed] [Google Scholar]
- 18.Koslow JA. The Silent Deep: The Discovery, Ecology, and Conservation of the Deep Sea. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 19.Lotze HK, Milewski I. Two centuries of multiple human impacts and successive changes in a North Atlantic food web. Ecol Appl. 2004;14:1428–1437. [Google Scholar]
- 20.Lotze HK. Radical changes in the Wadden Sea fauna and flora over the last 2,000 years. Helgol Mar Res. 2005;59:71–83. [Google Scholar]
- 21.Lotze HK, et al. Human transformations of the Wadden Sea ecosystem through time: A synthesis. Helgol Mar Res. 2005;59:84–95. [Google Scholar]
- 22.Jackson JBC. What was natural in the coastal oceans? Proc Natl Acad Sci USA. 2001;98:5411–5418. doi: 10.1073/pnas.091092898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg AA, et al. The history of ocean resources: Modeling cod biomass using historical records. Front Ecol Environ. 2005;3:84–90. [Google Scholar]
- 25.Kirby MX. Fishing down the coast: Historical expansion and collapse of oyster fisheries along continental margins. Proc Natl Acad Sci USA. 2004;101:13096–13099. doi: 10.1073/pnas.0405150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann AR, Canuel EA. Sediment geochemical records of eutrophication in the mesohaline Chesapeake Bay. Limnol Oceanogr. 2002;47:1084–1093. [Google Scholar]
- 27.Colman SM, Bratton JF. Anthropogenically induced changes in sediment and biogenic silica fluxes in Chesapeake Bay. Geology. 2003;31:71–44. [Google Scholar]
- 28.Diaz RJ. Overview of hypoxia around the world. J Environ Qual. 2001;30:275–281. doi: 10.2134/jeq2001.302275x. [DOI] [PubMed] [Google Scholar]
- 29.Lenihan HL, Peterson CH. How habitat degradation through fishery disturbance enhances impacts of hypoxia on oyster reefs. Ecol Appl. 1998;8:128. [Google Scholar]
- 30.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315:1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- 31.Baum JK, et al. Collapse and conservation of shark populations in the northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- 32.Baum JK, Myers RA. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol Lett. 2004;7:135–145. [Google Scholar]
- 33.Shepherd TD, Myers RA. Direct and indirect fishery effects on small coastal elasmobranchs in the northern Gulf of Mexico. Ecol Lett. 2005;8:1095–1104. [Google Scholar]
- 34.Estes JA, Duggins DO. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol Monogr. 1995;65:75–100. [Google Scholar]
- 35.Frank KT, Petrie B, Choi JS, Leggett WC. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 36.Essington TE, Beaudreau AH, Wiedenmann J. Fishing through marine food webs. Proc Natl Acad Sci USA. 2006;103:3171–3175. doi: 10.1073/pnas.0510964103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Tang Q, Jin X. Decadal-scale variations of trophic levels at high trophic levels in the Yellow Sea and the Bohai Sea ecosystem. J Mar Systems. 2007;67:304–311. [Google Scholar]
- 38.Tang Q, et al. Decadal-scale variations of ecosystem productivity and control mechanisms in the Bohai Sea. Fish Oceanogr. 2003;12:223–235. [Google Scholar]
- 39.Jennings S, Blanchard JL. Fish abundance with no fishing: Predictions based on macroecological theory. J Anim Ecol. 2004;73:632–642. [Google Scholar]
- 40.Dayton PK, Thrush SF, Agardy MT, Hofman RJ. Environmental effects of marine fishing. Aquat Conserv Mar Freshw Ecosyst. 1995;5:205–232. [Google Scholar]
- 41.NRC. Effects of Trawling and Dredging on Seafloor Habitat. Washington, DC: Natl Acad Press; 2002. [Google Scholar]
- 42.Auster PJ. A conceptual model of the impacts of fishing gear on the integrity of fish habitats. Conserv Biol. 1998;12:1198–1203. [Google Scholar]
- 43.Tilman D, Cassman K, Matson P, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 44.Turner RE, Rabalais NN, Justic D. Gulf of Mexico hypoxia: Alternate states and a legacy. Environ Sci Technol. 2008;42:2323–2327. doi: 10.1021/es071617k. [DOI] [PubMed] [Google Scholar]
- 45.Sibert J, Hampton J, Kleiber P, Maunder M. Biomass, size, and trophic status of top predators in the Pacific Ocean. Science. 2006;314:1773–1776. doi: 10.1126/science.1135347. [DOI] [PubMed] [Google Scholar]
- 46.Walters C. Folly and fantasy in the analysis of spatial catch raw data. Can J Fish Aquat Sci. 2003;60:1433–1436. [Google Scholar]
- 47.Polacheck T. Tuna longline catch rates in the Indian Ocean: Did industrial fishing result in a 90% rapid decline in the abundance of large predatory species? Mar Policy. 2006;30:470–482. [Google Scholar]
- 48.Ward P, Myers RA. Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology. 2005;86:835–847. [Google Scholar]
- 49.Feely RA, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305:362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- 50.McGowan JA, Bogard SJ, Lynn RJ, Miller AJ. Long term variability in the southern California Current system. Deep-Sea Res II. 2003;50:2567–2582. [Google Scholar]
- 51.Wara MW, Ravelo AC, Delaney ML. Permanent El Niño-like conditions during the Pliocene warm period. Science. 2005;309:758–761. doi: 10.1126/science.1112596. [DOI] [PubMed] [Google Scholar]
- 52.Fedorov AV, et al. The Pliocene paradox (mechanisms for a permanent El Niño. Science. 2006;312:1485–1489. doi: 10.1126/science.1122666. [DOI] [PubMed] [Google Scholar]
- 53.Field DB, Baumgartner TR, Charles CD, Ferreira-Bartrina V, Ohman MD. Planktonic Foraminifera of the California Current reflect 20th-century warming. Science. 2006;311:63–66. doi: 10.1126/science.1116220. [DOI] [PubMed] [Google Scholar]
- 54.Caldeira K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005;110:C09S04. [Google Scholar]
- 55.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:634–637. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 56.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 57.Jackson JBC. Reefs since Columbus. Coral Reefs. 1997;16:S23–S32. [Google Scholar]
- 58.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 59.Newman MJ, Paredes GA, Sala E, Jackson JBC. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol Lett. 2006;9:1216–1227. doi: 10.1111/j.1461-0248.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 60.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 61.Paredes GA. La Jolla: Univ of California at San Diego; 2007. Degradation and recovery of Caribbean coral reefs. PhD dissertation. [Google Scholar]
- 62.Jackson JBC. Pleistocene perspectives on coral reef community structure. Am Zool. 1992;32:719–731. [Google Scholar]
- 63.Aronson RB, Macintyre IG, Wapnick CM, O'Neill MW. Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology. 2004;85:1876–1891. [Google Scholar]
- 64.Pandolfi JM, Jackson JBC. Ecological persistence interrupted in Caribbean coral reefs. Ecol Lett. 2006;9:818–826. doi: 10.1111/j.1461-0248.2006.00933.x. [DOI] [PubMed] [Google Scholar]
- 65.Bruno JF, Selig ER. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS One. 2007;2:e711. doi: 10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goreau TF. The ecology of Jamaican coral reefs. I. Species composition and zonation. Ecology. 1959;40:67–90. [Google Scholar]
- 67.Pandolfi JM, Jackson JBC. Community structure of Pleistocene coral reefs of Curaçao, Netherlands Antilles. Ecol Monogr. 2001;71:49–67. [Google Scholar]
- 68.Lewis JB. The Acropora inheritance: A reinterpretation of the development of fringing reefs in Barbados, West Indies. Coral Reefs. 1984;3:117–122. [Google Scholar]
- 69.Lewis JB. Evidence from aerial photography of structural loss of coral reefs at Barbados, West Indies. Coral Reefs. 2002;21:49–56. [Google Scholar]
- 70.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 71.McClenachan L, Newman MJ, Paredes G. Florida Keys coral reef fish communities, then and now. Proc Gulf Carib Fish Inst. 2007;59:1–8. [Google Scholar]
- 72.Friedlander AM, DeMartini EE. Contrasts in density, size, and biomass of reef fishes between the Northwestern and the main Hawaiian Islands: The effects of fishing down apex predators. Mar Ecol Prog Ser. 2002;230:253–264. [Google Scholar]
- 73.Jackson JBC. When ecological pyramids were upside down. In: Estes JA, editor. Whales, Whaling, and Ocean Ecosystems. Berkeley: Univ of California Press; 2006. pp. 27–37. [Google Scholar]
- 74.McClanahan TR, Muthiga NA, Mangi S. Coral and algal changes after the 1998 coral bleaching: Interaction with reef management and herbivores on Kenyan reefs. Coral Reefs. 2001;19:380–391. [Google Scholar]
- 75.Dulvy NK, Mitchell RE, Watson D, Sweeting CJ, Polunin NVC. Scale-dependent control of motile epifaunal community structure along a coral reef fishing gradient. J Exp Mar Biol Ecol. 2002;278:1–29. [Google Scholar]
- 76.McClanahan TR, Graham NAJ, Calnan JM, MacNeil MA. Towards pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecol Appl. 2007;17:1055–1067. doi: 10.1890/06-1450. [DOI] [PubMed] [Google Scholar]
- 77.Sandin SA, et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE. 2008;3:e1548. doi: 10.1371/journal.pone.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McClenachan L. Social conflict, overfishing and disease in the Florida sponge fishery, 1849–1939. In: Starky D, editor. Oceans Past: Management Insights from the History of Marine Animal Populations. London: Earthscan; 2008. pp. 25–46. [Google Scholar]
- 79.Lessios HA, Robertson DR, Cubit JD. Spread of Diadema mass mortality through the Caribbean. Science. 1984;226:335–337. doi: 10.1126/science.226.4672.335. [DOI] [PubMed] [Google Scholar]
- 80.Aronson RB, Precht WF. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia. 2001;25:25–38. 460. [Google Scholar]
- 81.McClenachan L, Jackson JBC, Newman MJH. Conservation implications of historic sea turtle nesting beach loss. Front Ecol Environ. 2006;4:290–296. [Google Scholar]
- 82.McClenachan L, Cooper A. Extinction rate, historical population structure and ecological role of the Caribbean monk seal. Proc R Soc London Ser B. 2008;275:1351–1358. doi: 10.1098/rspb.2007.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mora C, et al. Coral reefs and the global network of marine protected areas. Science. 2006;312:1750–1751. doi: 10.1126/science.1125295. [DOI] [PubMed] [Google Scholar]
- 84.Hughes TP, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 85.Nugues MM, Smith GW, Hooidonk RJ, Seabra MI, Bak RPM. Algal contact as a trigger for coral disease. Ecol Lett. 2004;7:919–923. [Google Scholar]
- 86.Kline DI, Kuntz NM, Breitbart M, Knowlton N, Rohwer F. Role of elevated organic carbon levels and microbial activity in coral mortality. Mar Ecol Prog Ser. 2006;314:119–125. [Google Scholar]
- 87.Smith JE, et al. Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 88.Carpenter RC, Edmunds PJ. Local and regional scale recovery of Diadema promotes recruitment of scleractinian corals. Ecol Lett. 2006;9:268–277. doi: 10.1111/j.1461-0248.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 89.Mumby PJ, et al. Trophic cascade facilitates coral recruitment in a marine reserve. Proc Natl Acad Sci USA. 2007;104:8362–8367. doi: 10.1073/pnas.0702602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson JBC. Adaptation and diversity of reef corals. BioScience. 1991;41:475–482. [Google Scholar]
- 91.Kleypas JA, et al. National Science Foundation–National Oceanic and Atmospheric Administration–U.S. Geological Survey Workshop. Seattle: NOAA/Pacific Marine Environmental Laboratory; 2006. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers. A Guide for Future Research. Contr No 2897. [Google Scholar]
- 92.Fine M, Tchernov D. Scleractinian coral species survive and recover from decalcification. Science. 2007;315:1811. doi: 10.1126/science.1137094. [DOI] [PubMed] [Google Scholar]
- 93.Newton K, Cote IM, Pilling IM, Jennings S, Dulvy NK. Current and future sustainability of island coral reef fisheries. Curr Biol. 2007;17:655–658. doi: 10.1016/j.cub.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 94.Knoll AH. Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 95.Hilborn R. Moving to sustainability by learning from successful fisheries. Ambio. 2007;36:296–303. doi: 10.1579/0044-7447(2007)36[296:mtsblf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 96.Beddington JR, Agnew DJ, Clark CW. Current problems in the management of marine fisheries. Science. 2007;316:1713–1716. doi: 10.1126/science.1137362. [DOI] [PubMed] [Google Scholar]
- 97.Safina C, Rosenberg AA, Myers RA, Quinn TJ, II, Collie J. US ocean fishery recovery: Staying the course. Science. 2005;309:707–708. doi: 10.1126/science.1113725. [DOI] [PubMed] [Google Scholar]
- 98.Rosenberg AA, Swasey JH, Bowman M. Rebuilding US fisheries: Progress and problems. Front Ecol Environ. 2006;4:303–308. [Google Scholar]
- 99.McClanahan TR, Marnane MJ, Cinner JE, Kiene WE. A comparison of marine protected areas and alternative approaches to coral-reef management. Curr Biol. 2006;16:1408–1413. doi: 10.1016/j.cub.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 100.Goldberg R, Naylor R. Future seascapes, fishing, and fish farming. Front Ecol Environ. 2005;3:21–28. [Google Scholar]
- 101.Krkosek M, et al. Declining wild salmon populations in relation to parasites from farm salmon. Science. 2007;318:1772–1775. doi: 10.1126/science.1148744. [DOI] [PubMed] [Google Scholar]
- 102.Clay J. World Agriculture and the Environment: A Commodity-by-Commodity Guide to Impacts and Practices. Washington, DC: Island; 2004. [Google Scholar]
- 103.Dale VH, Polasky S. Measures of the effects of agricultural practices on ecosystem services. Ecol Econ. 2007;64:286–296. [Google Scholar]
- 104.Galloway GN, et al. Nitrogen cycles: Past, present and future. Biogeochemistry. 2004;70:153–226. [Google Scholar]
- 105.Howarth RW. Human acceleration of the nitrogen cycle: Drivers, consequences, and steps towards solutions. Water Sci Technol. 2004;49:7–13. [PubMed] [Google Scholar]




