Abstract
Type 1 diabetes is a multifactorial disease caused by a complex interaction of genetic and environmental factors. The genetic factors involved consist of multiple susceptibility genes, at least five of which, HLA, INS, CTLA4, PTPN22 and IL2RA/CD25, have been shown to be associated with type 1 diabetes in Caucasian (Western) populations, as has recently been confirmed by genome-wide association studies. It has been proposed, however, that the contribution of these genes to type 1 diabetes susceptibility may be different in Asian (Eastern) populations. HLA and INS genes are consistently associated with type 1 diabetes in both Caucasian and Asian populations, but apparent differences in disease-associated alleles and haplotypes are observed between Japanese and Caucasian subjects. The association of CTLA4 with type 1 diabetes is concentrated in a subset of patients with autoimmune thyroid disease (AITD) in both Japanese and Caucasian populations, while the association of PTPN22 with type 1 diabetes in Japanese and most Asian populations is not as clear as in Caucasians. IL2RA/CD25 genes seem to be similarly distributed in type 1 diabetes patients in the two populations, whereas genetic heterogeneity may exist regarding SUMO4, with an association of the M55V variant with type 1 diabetes observed in Asians, but not in Caucasians. Genome-wide association studies (GWA) are largely outstanding for Asian populations but they are now underway in Japan. This review reports on the discovered similarities and differences in susceptibility genes for type 1 diabetes between East and West and discusses the most recent observations made by the involved investigators.
Keywords: type 1 diabetes, genetics, autoimmune disease, genome-wide association study, HLA, insulin gene, CTLA4, PTPN22, interleukin-2 receptor alpha chain
Introduction
T ype 1 diabetes is etiologically classified as autoimmune (type 1A) and idiopathic (type 1B). The etiology and pathogenesis of the latter subtype are still unknown, but recent studies suggest that fulminant type 1 diabetes may belong to this subtype [1-3]. Type 1A diabetes is caused by autoimmune destruction of insulin-producing beta-cells in the pancreas in genetically susceptible individuals. Identification of genes predisposing to type 1 diabetes is important in establishing effective methods for disease prediction, prevention and intervention. Type 1 diabetes, however, is a multifactorial disease caused by a complex interaction of genetic and environmental factors, with the former consisting of multiple susceptibility genes, which makes identification of disease-causing variants very difficult [4].
Among the multiple susceptibility genes involved in type 1 diabetes, at least five loci, the HLA class II genes on chromosome 6p21, insulin gene (INS) on 11p15, CTLA4 on 2q33, PTPN22 on 1p13 and the interleukin-2 receptor alpha chain (IL2RA/CD25) region on 10p15, have been shown to be associated with type 1 diabetes in Caucasian populations [5-9]. Recent genome-wide association studies (GWAS) have not only confirmed these observations, but also identified several additional genes contributing to disease susceptibility [10-12]. It has been proposed, however, that the contribution of these genes to type 1 diabetes susceptibility may be different in Asian populations and a GWAS is yet to be performed. In this review, similarities and differences between East (Japanese) and West (Caucasian) are described and discussed.
Familial clustering of type 1 diabetes
In Caucasian populations, type 1 diabetes clusters in families, as is evidenced by the higher life-time risk in siblings of type 1 diabetic probands than in general populations (Table 1) [13]. In Asian populations, the incidence of type 1 diabetes is much lower than in Caucasian populations [14], making it difficult to study familial clustering of the disease. We therefore performed a nationwide survey of multiplex families with type 1 diabetes, and found a high frequency of type 1 diabetes in siblings of diabetic probands (Table 1) [15], similar to that in Caucasian populations. At least two other studies using different data sources also suggested much higher frequencies (1.3-3.3%) of type 1 diabetes in siblings of diabetic probands than in the general population in Japan (Table 1) [16], indicating that type 1 diabetes clusters in families even in low incidence countries like Japan.
Table 1. Frequencies of type 1 diabetes in siblings of diabetic probands in Caucasian and Japanese populations.
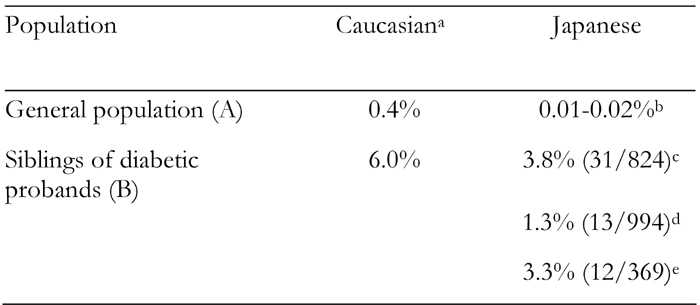
a Data from Risch [13]. b Data from Karvonen et al. [14] and Ikegami et al. [15]. c Data from Ikegami et al. [15]. d Based on a survey of family history of type 1 diabetes at summer camps for childhood diabetes in 1998 [16]. e Based on data from the Committee on Immunogenetics of Type 1 Diabetes of the Japan Diabetes Society, 1987. The frequency was calculated from the number of patients with type 1 diabetes in siblings of type 1 diabetic probands divided by the number of families studied, because of a lack of information on the number of siblings in each family. Based on the average number of children (approximately 2) in each family in Japan in 1987, the frequency may not have been overestimated as compared with the frequency calculated from the number of patients divided by the number of siblings.
Subtypes of type 1 diabetes
In addition to the etiological subtypes, type 1A and type 1B, clinical subtypes differing in the time course of beta-cell destruction have been reported. At least three subtypes of type 1 diabetes are found in Japanese populations: typical acute-onset, fulminant and slowly progressive forms. Fulminant type 1 diabetes is characterized by a markedly acute onset of diabetes and an absence of islet-related autoantibodies [1], and accounts for up to 20% of type 1 diabetes in Japan [3] and 7% in Korea [17]. In contrast to the relatively high frequencies in Asian populations, fulminant type 1 diabetes appears to be very rare in Caucasian and other non-Asian populations [3]. The reasons for the difference are still unknown, but the relatively short time since this subtype of type 1 diabetes was first reported in 2000 is one possibility [1]. The much lower incidence of type 1 diabetes compared with the Caucasian populations and the relatively high proportion of fulminant type 1 diabetes within type 1 diabetes in Japan, together with the fact that this subtype was originally found here, may have made Japanese diabetologists pay it more attention than is the case in other countries. A nationwide survey conducted by the Japan Diabetes Society, which started soon after the first report of this subtype, may have been a further factor that brought this subtype to the attention of clinicians all over Japan. Another possibility is the difference in genetic and environmental factors between Japanese and Caucasian populations, which is discussed later in this review.
Slowly progressive type 1 diabetes is characterized by positivity for islet autoantibodies, accompanied by a long non-insulin-dependent stage, usually of years, with gradual loss of beta-cells, leading to an insulin-dependent stage [18]. Slowly progressive type 1 diabetes is similar, but not identical to, latent-autoimmune diabetes in adults (LADA) in Caucasian populations [19]. Slowly progressive type 1 diabetes is defined by autoimmune etiology as reflected by positivity for islet autoantibodies, but slower progression to an insulin-dependent stage than in acute-onset type 1 diabetes [18]. LADA is defined by positivity for islet autoantibodies as in the case of slowly progressive type 1 diabetes, but progression to an insulin-dependent stage is not essential to its definition. Slowly progressive type 1 diabetes, therefore, is a more limited and better defined subtype of type 1 diabetes than LADA, with progressive beta-cell destruction leading to insulin dependency, and is more likely to be a mild form of acute-onset type 1A diabetes.
Although all three subtypes share the same clinical characteristic of insulin-dependency in the final stage, the time-course of beta-cell destruction is markedly different, which may well be based on differences in etiology, including genetic susceptibility. In the following sections of this review, type 1 diabetes refers to typical acute-onset autoimmune type 1 diabetes, unless stated otherwise.
HLA: a major susceptibility gene
Class II HLA, DRB1 and DQB1, have been consistently reported to be associated with type 1 diabetes in almost all ethnic groups [5]. However, differences in alleles and haplotypes associated with type 1 diabetes have been reported among different ethnic groups. The DR3 (DRB1*0301-DQB1*0201) and DR4 (DRB1*0401-DQB1*0302) haplotypes are positively associated with type 1 diabetes in Caucasian populations, whereas the DR4 (DRB1*0405-DQB1*0401) and DR9 (DRB1*0901-DQB1*0303) haplotypes are associated with the disease in Japanese and most East Asian populations [5, 15, 16, 20].
The differences in HLA haplotypes associated with type 1 diabetes in Japanese and Caucasian populations can be explained by the presence or absence of haplotypes in each population. The disease-associated DR3 and DR4 haplotypes in Caucasians are absent or very rare in the Japanese, which indicates that these haplotypes cannot contribute to type 1 diabetes susceptibility in the latter (Table 2). The same is true for the Asian-specific DR4 and DR9 haplotypes in that these haplotypes are almost absent in Caucasians and cannot, therefore, contribute to susceptibility to type 1 diabetes in these populations (Table 2). In contrast, the DR2 haplotype DRB1*1501-DQB1*0602, which is present in both Japanese and Caucasian populations, is negatively associated with type 1 diabetes in both populations (Table 2). The presence or absence of DR haplotypes in both populations account for the differential association with type 1 diabetes among different ethnic groups.
Table 2. Class II HLA haplotypes associated with type 1 diabetes in Japanese and Caucasian populations.
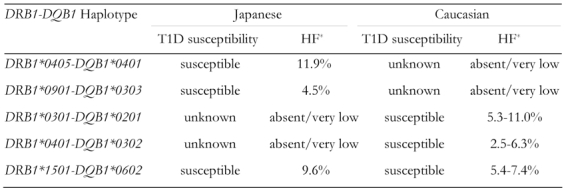
* HF: haplotype frequency based on data from the 11th International Histocompatibility Workshop.
Class II HLA has been reported to be associated not only with acute-onset type 1 diabetes, but also with fulminant and slowly progressive type 1 diabetes [2, 3, 18]. The alleles and haplotypes associated with the disease, however, appear to differ among different subtypes. Basically, the alleles and haplotypes associated with acute-onset and slowly progressive type 1 diabetes are similar, while those associated with fulminant type 1 diabetes are mostly different. In an original report on fulminant type 1 diabetes, high frequencies of class II HLA alleles known to provide resistance to type 1 diabetes were noted [1]. Subsequent studies with serological typing of class II HLA showed a higher frequency of the DR4-DQ4 as well as DR2-DQ1 haplotype than in autoimmune type 1 diabetes [21]. These data have recently been confirmed by genotyping a larger number of samples from patients with the three subtypes of type 1 diabetes (Kawabata et al. manuscript in preparation), which showed a higher frequency of DRB1*0405-DQB1*0401, in particular in the homozygous form, and a higher frequency of DRB1*1501-DQB1*0602, a haplotype well known to be resistant to autoimmune type 1 diabetes, in fulminant type 1 diabetes than in acute-onset type 1 diabetes. These differences may reflect the difference in etiology between fulminant (idiopathic) and autoimmune type 1 diabetes.
As mentioned above, there is a marked difference in the incidence of fulminant type 1 diabetes between Asian and Caucasian populations [2, 3]. The high frequency of the DRB1*0405-DQB1*0401 haplotype, in particular in the homozygous form, in fulminant type 1 diabetes may be partly responsible for the difference. Fulminant type 1 diabetes has been reported in Asian populations, in up to 20% of cases of adult-onset type 1 diabetes in Japan [2, 3] and 7% of cases of Korean type 1 diabetes [17], where DRB1*0405-DQB1*0401 is a common haplotype associated with type 1 diabetes. In contrast, fulminant type 1 diabetes appears to be absent or extremely rare in Caucasian populations, where the DRB1*0405-DQB1*0401 haplotype is also absent or very rare.
Insulin gene (INS): beta-cell specificity
Accumulating lines of evidence in both animal models and humans suggest that insulin is a primary autoantigen in type 1 diabetes [22]. The insulin gene region has been repeatedly reported to be associated with type 1 diabetes in Caucasian populations [6, 23-25]. In particular, allelic variation in the variable number of tandem repeats (VNTR) located in the 5' upstream region of INS has been suggested to be responsible for disease susceptibility [24, 25]. Shorter VNTR class I haplotypes confer susceptibility, while longer VNTR class III haplotypes provide dominant protection.
In the Japanese population, the contribution of INS to type 1 diabetes has been difficult to demonstrate because of the very high frequency (>90%) of disease-susceptible class I haplotypes in the general population [26]. In order to overcome this identification problem, large-scale studies with sufficient statistical power are necessary, as is evidenced by the recent progress made by large-scale studies in Caucasian populations in identifying susceptibility genes for type 1 diabetes [7]. In Asian populations, however, no such study has been performed because of the very low incidence (less than 1/10 of that in Caucasians) of type 1 diabetes in Asian countries [14].
Therefore, we assembled a multi-center study group, the "Japanese Study Group on Type 1 Diabetes Genetics", each member of which had experience in genetic association studies on type 1 diabetes and had previously collected a moderate number of samples (200-300) from cases and controls [27]. To date, a total of >1500 samples from type 1 diabetic patients and control subjects have been accumulated for the collaborative effort. Taking advantage of these samples, we studied the contribution of INS-VNTR to type 1 diabetes susceptibility, and confirmed that class I haplotypes are significantly associated with type 1 diabetes in Japanese subjects [28], indicating that INS-VNTR is associated with type 1 diabetes in both Japanese and Caucasian populations. The low incidence of type 1 diabetes despite the high frequency of disease-susceptible class I haplotypes in the Japanese indicate that INS-VNTR does not contribute to the low incidence of type 1 diabetes in Japan.
Recent studies showed that the insulin gene is transcribed not only in pancreatic beta-cells, but also in the thymus, and the transcription level correlates with allelic variation at the INS-VNTR [29, 30]. Disease-protective class III haplotypes are reported to be associated with 2- to 3-fold higher INS mRNA levels in the human thymus than disease-susceptible class I haplotypes [29, 30]. The higher level of INS expression in the thymus in patients with a class III haplotype is thought to facilitate immune tolerance induction through negative selection of insulin-specific T lymphocytes, providing a plausible explanation for the dominant protective effect of class III VNTR.
CTLA4: a negative regulator
The CTLA4 polymorphism was reported to be associated with susceptibility to autoimmune diseases, autoimmune thyroid disease (AITD) and type 1 diabetes [7]. The association with AITD is strong (odds ratio 1.4-1.5) and consistent, but its effect on type 1 diabetes appears to be weak (odds ratio 1.1) or even questionable, as is shown by inconsistent results among different studies.
We studied the association of CTLA4 with type 1 diabetes in Japanese subjects, using a large number of samples from the collaborative study group [27]. CTLA4 was significantly associated with AITD, but not with type 1 diabetes. The strong association of CTLA4 with AITD together with the high frequency of AITD in patients with type 1 diabetes suggests that the association of CTLA4 with type 1 diabetes in a large-scale study may be secondary to the association with concomitant AITD in patients with type 1 diabetes. We therefore divided type 1 diabetic patients into two groups, those with and without AITD, and studied the association of CTLA4 with the disease [27]. CTLA4 was significantly associated with type 1 diabetes with AITD, but not without AITD, indicating that the association of CTLA4 with type 1 diabetes is concentrated in a subset of patients with AITD [27].
This result has recently been confirmed by a UK group [31], who originally reported the association of CTLA4 with type 1 diabetes as well as AITD in a large-scale study. The association of CTLA4 with type 1 diabetes was concentrated in patients with AITD as assessed by their positivity for anti-thyroid peroxidase (TPO) antibodies [31]. These data indicate that the association of CTLA4 with type 1 diabetes is limited or concentrated in patients with AITD, suggesting that the association of CTLA4 with type 1 diabetes may generally be secondary to the association with concomitant AITD. More importantly, these data indicate how important it is to sub-group type 1 diabetes by the presence or absence of concomitant autoimmune diseases, in particular AITD, because of the high frequency of their association with type 1 diabetes, when studying susceptibility genes for type 1 diabetes [27]. This is particularly important when studying genes with a common association with multiple autoimmune diseases, such as CTLA4, PTPN22 and SUMO4.
PTPN22 encoding lymphoid tyrosine phosphatase (LYP)
A variant of PTPN22 leading to an arginine-to-tryptophan substitution at codon 620 (R620W) in the lymphoid tyrosine phospatase (LYP) molecule has been consistently reported to be associated with type 1 diabetes and other autoimmune diseases, such as rheumatoid arthritis, SLE and Graves disease, in Caucasian populations [8, 32, 33].
To clarify the contribution of the variant to type 1 diabetes in Asian populations, we studied a large number of samples from Japanese subjects from the collaborative study group as well as Korean samples [34]. Despite genotyping a large number of samples (>1500), it was found that all had arginine (R620) but none the variant (tryptophan: W620), indicating that the latter is absent in Asian populations [34]. Re-sequencing of PTPN22 in Japanese samples revealed the presence of five novel SNPs in the Japanese subjects, and one of these, the -1123 G>C SNP in the promoter region, was found to be associated with type 1 diabetes in both Japanese and Korean populations [34].
These data suggest that PTPN22 contributes to susceptibility to type 1 diabetes, but that disease-associated SNPs may differ between Asian and Caucasian populations. The absence of the R620W polymorphism in the Asian population may contribute to the low frequency of type 1 diabetes in Asia. Further studies are necessary to clarify whether the newly identified SNP in the promoter region is associated with type 1 diabetes in Caucasian populations.
Interleukin-2 receptor alpha (IL2RA/CD25) gene region
An association of the IL2RA region with type 1 diabetes in Caucasian populations has been reported by two independent groups [9, 35], and has recently been confirmed by large-scale fine mapping [36]. A genotype-phenotype association of the type 1 diabetes susceptibility genotype IL2RA with a lower concentration of soluble IL-2RA, a biomarker for immune activation, in peripheral blood has also been reported [36]. The IL2RA region has been reported to be associated not only with type 1 diabetes, but also with autoimmune thyroid diseases [37], suggesting that the IL2RA region plays a general role in autoimmunity. A similar association has been suggested in Japanese subjects by the collaborative study group described above (Kawasaki et al., manuscript in preparation).
SUMO4 on chromosome 6q25
A methionine-to-valine substitution at codon 55 (M55V) of SUMO4 was reported to be the causative variant of IDDM5 on chromosome 6q25 [38, 39], but subsequent studies yielded inconsistent results [40-42]. To clarify the contribution of SUMO4 to susceptibility to type 1 diabetes, we re-sequenced a >2000bp interval of chromosome 6q25, including the whole SUMO4 gene, in a Japanese sample and the identified polymorphisms were subjected to association studies in both Japanese and Korean populations [43]. The M55V variant was significantly associated with type 1 diabetes in the Japanese and Korean populations, and meta-analysis of published studies as well as our own data confirmed a significant association of the M55V variant with type 1 diabetes in Asian (summary odds ratio: 1.29, p = 7.0×10-6), but not Caucasian populations [43], suggesting heterogeneity in the genetic effect of SUMO4 on type 1 diabetes among diverse ethnic groups.
Genome-wide association studies
Recent advances in technology and knowledge in molecular genetics, including the availability of high-density genotyping chips, the completion of the HapMap project and a large number of well characterized samples, have made it possible to perform genome-wide association (GWA) studies with genotyping of hundreds of thousands of single nucleotide polymorphisms (SNPs) across the genome to clarify the genetic basis of common human diseases, such as type 1 diabetes.
Initially, a genome-wide non-synonymous (ns) SNP scan was performed with >6500 ns SNPs in 2029 case and 1755 control samples, and the follow-up study identified a type 1 diabetes locus in the IFIH1 (interferon induced with helicase C domain 1, also known as MDA5 for the melanoma differentiation-associated 5) region on chromosome 2q24.3 [44]. More recently, two GWA studies on type 1 diabetes with much denser SNPs have been reported with Caucasian samples (Table 3) [10, 45]. The first was performed by the Wellcome Trust Case Control Consortium (WTCCC) in the British population, which examined up to 2000 individuals for each of seven diseases, including type 1 diabetes, and up to 3000 shared controls genotyped with the GeneChip 500K Mapping Array Set, which comprises over 500,000 SNPs [10]. Follow-up studies identified several novel loci associated with type 1 diabetes on chromosomes 12q24, 12q13, 16p13 and 18p11 (Tables 3 and 4) [45].
Table 3. Genome-wide association studies in type 1 diabetes.
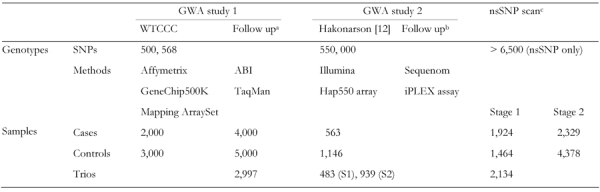
Table 4. Susceptibility loci for type 1 diabetes identified or confirmed by genome-wide association studies.
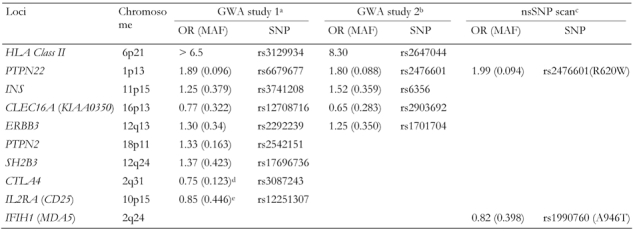
GWA: genome-wide association. OR: odds ratio. nsSNP: non-synonymous single nucleotide polymorphism. MAF: minor allele frequency (in controls). a Data from Todd et al. [11]. b Data from Hakonarson et al. [12]. c Data from Smyth et al. [44]. d,e Loci did not reach genome-wide significance level (5 x 10-7) in WTCCC results [10], but confirmed previously by candidate gene approaches by: d Ueda et al. [7] and e Lowe et al. [36].
The second study was performed with a large pediatric cohort of European descent, and suggested that a 233-kb linkage disequilibrium block on chromosome 16p13 was associated with type 1 diabetes [12]. An included replication study indicated that CLEC16A/ KIAA0350 might be involved in the pathogenesis of type 1 diabetes (Tables 3 and 4) [12]. Another replication study by the same group has recently mapped a novel susceptibility locus on chromosome 12q13 (Table 4) [46]. Significantly, several loci, such as those on chromosomes 12q13 and 16p13, were identified in both GWA studies, and genes already confirmed by a candidate gene approach, such as HLA, INS and PTPN22, were detected by GWA studies, indicating the power and accuracy of this method when applied to a sufficient number of well defined samples in identifying susceptibility genes for type 1 diabetes.
GWA studies are yet to be reported in Asian populations, but are now underway in the Japanese population. Comparative studies on GWA data between Asian and Caucasian populations will provide important information on the susceptibility genes that contribute to type 1 diabetes in each ethnic group as well as across different ethnic groups.
Conclusions
Although differences in the incidence and subtypes of type 1 diabetes are observed between Caucasian and Asian populations, the association of candidate genes with type 1 diabetes is generally similar in both populations, and there are well-defined reasons for apparent differences in the genes associated with type 1 diabetes (Table 5). HLA are consistently associated with type 1 diabetes in both populations, but apparent differences in disease-associated alleles and haplotypes are observed, because of differences in the presence and absence of disease-associated alleles in general populations.
Table 5. Similarities and differences in susceptibility haplotypes and alleles for type 1 diabetes between Japanese and Caucasian populations.
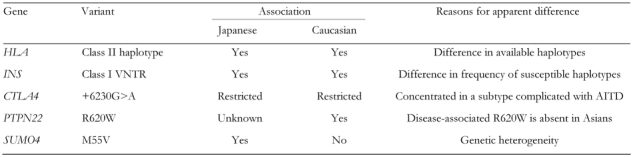
INS is consistently associated with type 1 diabetes in both populations, but there are marked differences in the frequencies of disease-associated alleles in the general population between Japanese and Caucasian subjects. The association of CTLA4 with type 1 diabetes is concentrated in a subset of patients with AITD not only in Japanese, but also in Caucasian populations. The association of PTPN22 with type 1 diabetes in Japanese and most Asian populations is not as clear as in Caucasian populations, because of the absence of a disease-associated allele (R620W) in Asian populations. The association of IL2RA/CD25 with type 1 diabetes appears to be similar in the two populations, whereas genetic heterogeneity may exist in the association of SUMO4 with type 1 diabetes, with an association of the M55V variant with type 1 diabetes observed in Asians, but not in Caucasians.
A large number of samples in each ethnic group and comparative studies in different populations, including GWA studies, will contribute to the identification of susceptibility genes for type 1 diabetes, enable better understanding of the disease mechanisms and help to establish effective methods for disease prediction, prevention and intervention.
Acknowledgments
We would like to thank the members of the Japanese Study Group of Type 1 Diabetes Genetics: Takuya Awata (Saitama Medical School), Eiji Kawasaki (Nagasaki University), Tetsuro Kobayashi (University of Yamanashi), Taro Maruyama (Saitama Social Insurance Hospital), Koji Nakanishi (Toranomon Hospital), Akira Shimada (Keio University) and Kazuma Takahashi (Iwate Medical College). This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan.
References
- 1.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 2.Imagawa A, Hanafusa T. Pathogenesis of fulminant type 1 diabetes. Rev Diabet Stud. 2006;3(4):169–177. doi: 10.1900/RDS.2006.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imagawa A, Hanafusa T. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Prac Endocrinol Metab. 2007;3:36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- 4.Biros E, Jordan MA, Baxter AG. Genes mediating environment interactions in type 1 diabetes. Rev Diabet Stud. 2005;2(4):192–120. doi: 10.1900/RDS.2005.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson G, Valdes AM, Noble JA, Kockum I, Grote MN, Najman J, Erlich HA, Cucca F, Pugliese A, Steenkiste A et al. Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: a meta-analysis. Tissue Antigens. 2007;70(2):110–127. doi: 10.1111/j.1399-0039.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 6.Julier C, Hyer RN, Davies J, Merlin F, Soularu P, Briant L, Cathelineau G, Deschamps I, Rotter JI, Froguel P et al. Insulin-IGF2 region on chromosome 11p encodes a gene implicated in HLA-DR4-dependent diabetes susceptibility. Nature. 1991;354:155–159. doi: 10.1038/354155a0. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Genova GD et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 8.Bottini N, Musumeci L, Alonso A, Rahmouni S, Konstantia N, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M et al. A functional variant of lymphoid tyrosine phosphatase is associated with type 1 diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 9.Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, Jones R, Ring SM, McArdle W, Pembrey ME et al. Localization of type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 13.Risch N. Assessing the role of HLA-linked and unlinked determinants of disease. Am J Hum Genet. 1987;40:1–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–1526. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 15.Ikegami H, Ogihara T. Genetics of insulin-dependent diabetes mellitus. Endocrine J. 1996;43:605–613. doi: 10.1507/endocrj.43.605. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami H, Kawabata Y, Noso S, Fujisawa T, Ogihara T. Genetics of type 1 diabetes in Asian and Caucasian populations. Diab Res Clin Prac. 2007;77S:S116–S121. doi: 10.1016/j.diabres.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Cho YM, Kim JT, Ko KS, Koo BK, Yang SW, Park MH, Lee HK, Park KS. Fulminant type 1 diabetes in Korea: high prevalence among patients with adult-onset type 1 diabetes. Diabetologia. 2007;50:2276–2279. doi: 10.1007/s00125-007-0812-z. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Tamemoto K, Nakanishi K, Kato N, Okubo M, Kajio H, Sugimoto T, Murase T, Kosaka K. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care. 1993;16:780–788. doi: 10.2337/diacare.16.5.780. [DOI] [PubMed] [Google Scholar]
- 19.Zimmet PZ, Tuomi T, Mackay IR, Rowley MJ, Knowles W, Cohen M, Lang DA. Latent autoimmune diabetes mellitus in adults (LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and prediction of insulin dependency. Diabet Med. 1994;11:299–303. doi: 10.1111/j.1464-5491.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawabata Y, Ikegami H, Kawaguchi Y, Fujisawa T, Shintani M, Ono M, Nishino M, Uchigata Y, Lee I, Ogihara T. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. 2002;51:545–551. doi: 10.2337/diabetes.51.2.545. [DOI] [PubMed] [Google Scholar]
- 21.Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H. Different contribution of class II HLA in fulminant and typical autoimmune type 1 diabetes mellitus. Diabetologia. 2005;48:294–300. doi: 10.1007/s00125-004-1626-x. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton J, Elliott JF et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–183. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- 24.Lucassen AM, Julier C, Beressi JP, Boitard C, Froguel P, Lathrop M, Bell JI. Susceptibility to insulin dependent diabetes mellitus maps to a 4.1kb segment of DNA spanning the insulin gene and associated with VNTR. Nat Genet. 1993;4:305–310. doi: 10.1038/ng0793-305. [DOI] [PubMed] [Google Scholar]
- 25.Bennett ST, Lucassen AM, Gough SC, Powell EE, Undlien DE, Pritchard LE, Merriman ME, Kawaguchi Y, Dronsfield MJ, Pociot F et al. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet. 1995;9:284–292. doi: 10.1038/ng0395-284. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Ikegami H, Shen GQ, Nakagawa Y, Fujisawa T, Hamada Y, Ueda H, Fu J, Uchigata Y, Kitagawa Y et al. Insulin gene region contributes to genetic susceptibility to, but may not to low incidence of, insulin-dependent diabetes mellitus in Japanese. Biochem Biophys Res Commun. 1997;233:283–287. doi: 10.1006/bbrc.1997.6440. [DOI] [PubMed] [Google Scholar]
- 27.Ikegami H, Awata T, Kawasaki E, Kobayashi T, Maruyama T, Nakanishi K, Shimada A, Amemiya S, Kawabata Y, Kurihara S et al. The association of CTLA4 polymorphism with type 1 diabetes is concentrated in patients complicated with autoimmune thyroid disease: a multi-center collaborative study in Japan. J Clin Endocrinol Metab. 2006;91:1087–1092. doi: 10.1210/jc.2005-1407. [DOI] [PubMed] [Google Scholar]
- 28.Awata T, Kawasaki E, Ikegami H, Kobayashi T, Maruyama T, Nakanishi K, Shimada A, Iizuka H, Kurihara S, Osaki M et al. Insulin gene/IDDM2 locus in Japanese type 1 diabetes: contribution of class I alleles and influence of class I subdivision in susceptibility to type 1 diabetes. J Clin Endocrinol Metab. 2007;92:1791–1795. doi: 10.1210/jc.2006-2242. [DOI] [PubMed] [Google Scholar]
- 29.Vafiadia P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 30.Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bertlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–296. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 31.Howson JM, Dunger DB, Nutland S, Stevens H, Wicker LS, Todd JA. A type 1 diabetes subgroup with a female bias is characterised by failure in tolerance to thyroid peroxidase at an early age and a strong CTLA-4 gene association. Diabetologia. 2007;50:741–746. doi: 10.1007/s00125-007-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siminovitch KA. PTPN22 and autoimmune disease. Nat Genet. 2004;36:1248–1249. doi: 10.1038/ng1204-1248. [DOI] [PubMed] [Google Scholar]
- 33.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki E, Awata T, Ikegami H, Kobayashi T, Maruyama T, Nakanishi K, Shimada A, Uga M, Kurihara S, Kawabata Y et al. Systematic search for single nucleotide polymorphisms in a lymphoid tyrosine phosphatase (PTPN22) gene: association between promoter polymorphism and type 1 diabetes in Asian populations. Am J Med Genet. 2006;140:586–593. doi: 10.1002/ajmg.a.31124. [DOI] [PubMed] [Google Scholar]
- 35.Qu HQ, Montpetit A, Ge B, Hudson TJ, Polychronakos C. Toward further mapping of the association between the IL2RA locus and type 1 diabetes. Diabetes. 2007;56:1174–1176. doi: 10.2337/db06-1555. [DOI] [PubMed] [Google Scholar]
- 36.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet. 2007;39:1074–1078. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 37.Brand OJ, Lowe CE, Heward JM, Franklyn JA, Cooper JD, Todd JA, Gough SC. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol. 2007;66:508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 38.Guo D, Li M, Zhang Y, Yang P, Eckenrode S, Hopkins S, Zheng W, Purohit S, Podolsky RH, Muir A et al. A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat Genet. 2004;36:837–841. doi: 10.1038/ng1391. [DOI] [PubMed] [Google Scholar]
- 39.Owerbach D, Pina L, Gabbay KH. A 212-kb region on chromosome 6q25 containing the TAB2 gene is associated with susceptibility to type 1 diabetes. Diabetes. 2004;53:1890–1893. doi: 10.2337/diabetes.53.7.1890. [DOI] [PubMed] [Google Scholar]
- 40.Smyth DJ, Howson JM, Lowe CE, Walker NM, Lam AC, Nutland S, Hutchings J, Tuomilehto-Wolf E, Tuomilehto J, Guja C et al. Assessing the validity of the association between the SUMO4 M55V variant and risk of type 1 diabetes. Nat Genet. 2005;37:110–111. doi: 10.1038/ng0205-110. [DOI] [PubMed] [Google Scholar]
- 41.Qu H, Bharaj B, Liu XQ, Curtis JA, Newhook LA, Paterson AD, Hudson TJ, Polychronakos C. Assessing the validity of the association between the SUMO4 M55V variant and risk of type 1 diabetes. Nat Genet. 2005;37:111–112. doi: 10.1038/ng0205-111. [DOI] [PubMed] [Google Scholar]
- 42.Park Y, Park S, Kang J, Yang S, Kim D. Assessing the validity of the association between the SUMO4 M55V variant and risk of type 1 diabetes. Nat Genet. 2005;37:112. doi: 10.1038/ng0205-112a. [DOI] [PubMed] [Google Scholar]
- 43.Noso S, Ikegami H, Fujisawa T, Kawabata Y, Asano K, Hiromine Y, Tsurumaru M, Sugihara S, Lee I, Kawasaki E et al. Genetic heterogeneity in association of SUMO4 M55V variant with susceptibility to type 1 diabetes. Diabetes. 2005;54:3582–3586. doi: 10.2337/diabetes.54.12.3582. [DOI] [PubMed] [Google Scholar]
- 44.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38(6):617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 45.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakonarson H, Qu HQ, Bradfield JP, Marchan L, Ki CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC et al. A novel susceptibility locus for type 1 diabetes on Chr12q13 identified by a genome-wide association study. Diabetes. 2008;57:1143–1146. doi: 10.2337/db07-1305. [DOI] [PubMed] [Google Scholar]


