Abstract
Until recently, the pathogenesis of type 2 diabetes mellitus (T2DM) has been conceptualized in terms of the predominant defects in insulin secretion and insulin action. It is now recognized that abnormalities in other hormones also contribute to the development of hyperglycemia. For example, the incretin effect, mediated by glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), is attenuated in T2DM. Intravenous administration of GLP-1 ameliorates hyperglycemia in patients with T2DM, but an extremely short half-life limits its utility as a therapeutic agent. Strategies to leverage the beneficial effects of GLP-1 include GLP-1 receptor agonists or analogs or dipeptidyl peptidase-4 (DPP-4) inhibitors—agents that act by slowing the inactivation of endogenous GLP-1 and GIP. The GLP-1 agonist exenatide has been shown to improve HbA1c and decrease body weight. However, exenatide is limited by its relatively short pharmacologic half-life, various gastrointestinal (GI) side effects, and the development of antibodies. Studies of a long-acting exenatide formulation suggest that it has improved efficacy and also promotes weight loss. Another prospect is liraglutide, a once-daily human GLP-1 analog. In phase 2 studies, liraglutide lowered HbA1c by up to 1.7% and weight by approximately 3 kg, with apparently fewer GI side effects than exenatide. DPP-4 inhibitors such as sitagliptin and vildagliptin result in clinically significant reductions in HbA1c, and are weight neutral with few GI side effects. This review will provide an overview of current and emerging agents that augment the incretin system with a focus on the role of GLP-1 receptor agonists and DPP-4 inhibitors.
Keywords: type 2 diabetes, GLP-1 analog, incretin, exenatide, DPP-4, liraglutide, sitagliptin, vildagliptin
Introduction
Metabolic abnormalities evident in individuals with type 2 diabetes mellitus (T2DM) include obesity, insulin resistance, qualitative and quantitative abnormalities in insulin secretion, dysregulated secretion of other islet hormones, such as amylin and glucagons, and increased endogenous glucose production. Another important abnormality, which came to the forefront of diabetes research, is the decreased incretin effect due to impairments in secretion and action of the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). It is increasingly recognized that glucose homeostasis is governed by a complex interaction among various mediators, including insulin, glucagon, amylin, and incretin hormones. Deficits in any one of these components may contribute to the pathophysiology of T2DM (Table 1) [1]. Many pharmacologic studies in T2DM have been directed at increasing insulin secretion or sensitivity using GLP-1 agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors and evaluating the safety of drugs based on incretins. Good surveys on these studies are available [2-4]. The present review examines the pathophysiology of T2DM, with a focus on our evolving understanding of incretin dysregulation and the role of pharmacologic treatment based on GLP-1 agonists and DPP-4 inhibitors.
Table 1. Effects of primary glucoregulatory hormones.
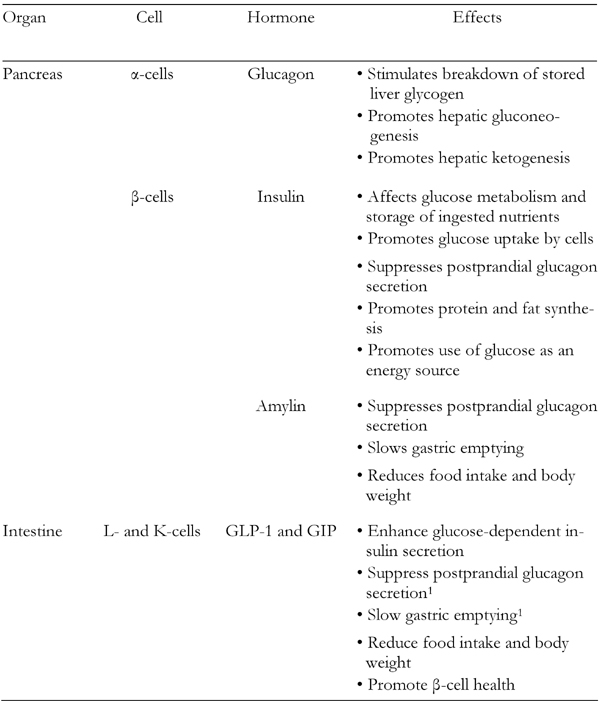
1 Not a significant effect of GIP.
Hormonal and metabolic abnormalities in the pathogenesis of T2DM
Due to the dominant effect of insulin on glucose metabolism, most studies of the pathogenesis of T2DM have focused on defining abnormalities in insulin secretion and action. In response to a glucose challenge, the secretion of insulin occurs in phases with distinct physiological functions [5]. Early-phase insulin secretion serves to efficiently switch metabolism from the fasting state—in which endogenous glucose production and non-insulin mediated glucose disposal predominate—to the postprandial state, in which endogenous glucose production is suppressed and insulin-mediated glucose disposal predominates. Late-phase insulin secretion enhances insulin-mediated glucose disposal in skeletal muscle and adipose tissue. Under normal circumstances, insulin secretion is tightly coupled to insulin action. Thus, normal glucose tolerance is maintained by a compensatory increase in insulin secretion in individuals with insulin resistance.
In T2DM, both qualitative and quantitative abnormalities in insulin secretion are present. Early-phase insulin secretion is almost always blunted or absent. Late-phase insulin secretion, on the other hand, may appear quantitatively normal, but is nevertheless inadequate relative to the degree of hyperglycemia. In response to an oral glucose tolerance test, the secretion of insulin in patients with T2DM tends to be slower in onset and prolonged compared to responses in individuals with normal glucose tolerance. Insulin resistance in skeletal muscle, adipose tissue, and liver can also be demonstrated in the vast majority of individuals with T2DM.
Abnormalities in insulin secretion and action can be demonstrated in individuals prior to the onset of hyperglycemia. Impaired insulin secretion is a predictor of progression to diabetes and, longitudinally, insulin secretion deteriorates with the progression from normal glucose tolerance to diabetes. In a cross-sectional study, individuals with impaired fasting glucose and impaired glucose tolerance were more insulin resistant and had a significantly lower acute insulin response to intravenous glucose relative to individuals with normal glucose tolerance [6]. In a prospective study, insulin resistance and a low acute insulin response predicted the development of diabetes independently of obesity and independently of one another [7]. In a longitudinal study sequentially measuring insulin action and secretion, patients who developed T2DM showed a 14% decline in insulin action during the transition from normal glucose tolerance to diabetes, whilst the controls, who did not progress to diabetes, showed a similar 11% decline in insulin action [8]. In contrast, the acute insulin response in progressors declined 27% during the transition from normal to impaired glucose tolerance, followed by an additional 51% during the transition from impaired glucose tolerance to diabetes. Whereas in non-progressors, it actually increased by 30% over the ~5 years of observation. These results demonstrate the primary role of impairments in insulin secretion and action in the pathogenesis of T2DM.
Progressive declines in insulin secretory function are also thought to contribute to worsening glycemic control after the diagnosis of T2DM. The UK Prospective Diabetes Study (UKPDS) showed that among patients with newly diagnosed T2DM, insulin resistance did not change, but there was a linear decline in β-cell function over several years of observation [9]. This decline in β-cell function may represent the natural progression of the disease, and treatment with sulfonylureas (SU), metformin (MET), and insulin did not considerably alter the slope of the decline and impairment in β-cell function [10].
Human autopsy studies indicate that individuals with diabetes, and even those with pre-diabetes, manifest up to a 60% reduction in β-cell mass relative to individuals with normal glucose tolerance [11]. Whether reduced β-cell mass is a primary defect contributing to the development of diabetes in humans or whether there is a progressive loss secondary to hyperglycemia and an abnormal metabolic milieu is not known.
Insulin resistance and insulin secretory dysfunction in the pathogenesis of T2DM have been well studied, but less is known about the development of the other metabolic abnormalities that typify T2DM. Excessive endogenous glucose production in patients results from both inadequate insulin signaling, due to defective secretion and hepatic insulin resistance, and dysregulated glucagon secretion from pancreatic α-cells. A growing number of studies indicate that glucagon secretion is high and inadequately suppressed in response to an oral nutrient challenge in individuals with T2DM relative to those with normal glucose tolerance [12]. Glucagon secretion may also be abnormal in subjects with impaired glucose tolerance [13].
Amylin, which is co-secreted with insulin from pancreatic β-cells, is also dysregulated in T2DM. While the precise physiological role of amylin is not known, it appears to suppress glucagon secretion, and also has effects to delay gastric emptying and increase satiety. In response to an oral glucose challenge, amylin secretion is delayed and blunted in patients with T2DM relative to those with normal glucose tolerance [14].
The physiologic role of incretins
The incretin concept, as it is currently used, dates back to early observations that ingested glucose results in a considerably larger and more sustained insulin response than glucose administered intravenously. This suggests the presence of substances within the gastrointestinal (GI) tract that stimulate insulin release [15, 16]. Two incretins have been identified: GIP, secreted by enteroendocrine K-cells in the proximal gut and GLP-1 secreted by L-cells in the distal gut. Both GIP and GLP-1 are secreted into the circulation as active hormones within minutes in response to food consumption and are rapidly inactivated by the enzyme DPP-4, an ubiquitous serine protease. Both GIP and GLP-1 bind to specific G-protein coupled receptors present on β-cells and other target tissues. Activation of the incretin receptors on β-cells acutely enhances glucose-dependent exocytosis of insulin. In addition, activation of the incretin receptors results in other longer term effects, including stimulation of insulin synthesis [17], enhancement of β-cell proliferation, and promotion of resistance to apoptosis [3, 10, 18]. GLP-1 also lowers glucose through inhibition of glucagon secretion, deceleration of gastric emptying, and inhibition of food intake, and GLP-1 may promote enhanced glucose disposal in peripheral tissues (Figure 1) [19-22].
Figure 1. Physiology of GLP-1 secretion and action on GLP-1 receptors in different organs and tissues.
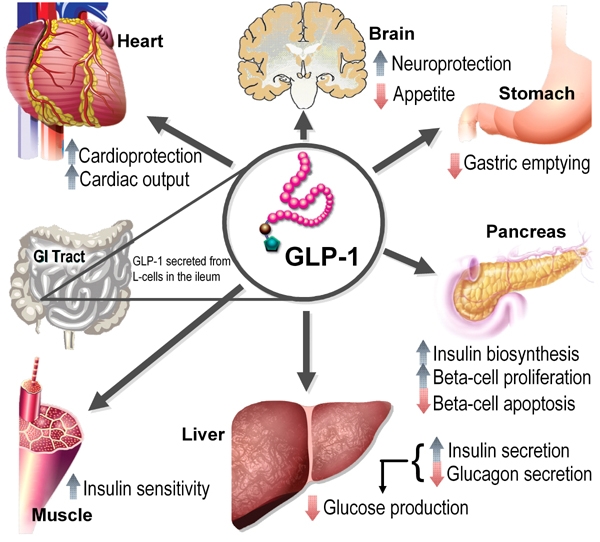
GLP-1 is produced postprandially by intestinal L-cells. Through activation of insulin receptors on beta-cells GLP-1 (like GIP) stimulates insulin biosynthesis and secretion and inhibits glucagon secretion in the pancreas, which in turn reduces hepatic gluconeogenesis. GLP-1 release also exerts protective effects on heart and brain. Insulin sensitivity in the periphery is increased by improved insulin signaling and reduced gluconeogenesis. Figure modified with permission from Cell Metabolism [3].
Other studies suggest that GLP-1 has effects on target tissues not directly involved in glucose metabolism, including a protective effect against ischemia/reperfusion injury [23, 24] and endothelial dysfunction [25]. GLP-1 also promotes endothelium-independent artery relaxation [26], and may increase diuresis and natriuresis, suggesting a possible renal protective effect [27, 28]. Furthermore, there is evidence that the synthetic GLP-1 receptor agonists may reduce systolic blood pressure and triglyceride levels and have beneficial effects on markers of cardiovascular risk, such as plasminogen activator inhibitor (PAI-1) and brain natriuretic peptide (BNP) [29].
A diminished incretin effect in T2DM
Using carefully matched oral and intravenous glucose challenges, Nauck et al. demonstrated that the incretin effect accounted for ~2/3 of the insulin secretory response in subjects with normal glucose tolerance, whereas in patients with T2DM it accounted for less than 20% (Figure 2) [16, 30]. Thus, impairments in the incretin response may contribute to dysregulation of insulin and glucagon secretion, particularly during the postprandial period, leading to hyperglycemia.
Figure 2. Diminished incretin effect in type 2 diabetes.
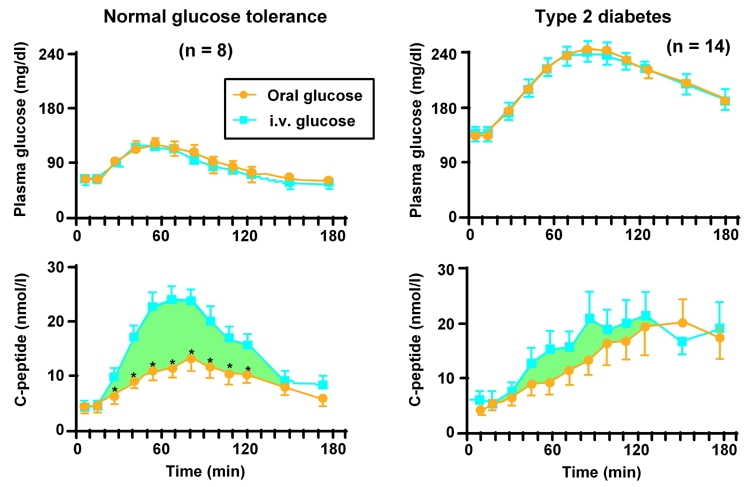
Differences in C-peptide responses between oral and i.v. glucose challenges are attributed to the incretin effect that takes place in the intestine. This effect is not present in parenteral glucose administration (turquoise curves). In healthy individuals (left panel) the difference is significant (* p ≤ 0.05). In type 2 diabetes patients (right panel) the incretin effect is smaller or almost diminished compared with normal healthy individuals. Figure reproduced with permission from Journal of Clinical Endocrinology and Metabolism [16].
Evidence suggests that the impaired incretin response in patients with T2DM may be due to decreased GLP-1 levels following food ingestion [31], which cannot be attributed to enhanced clearance. In studies comparing the kinetics of GLP-1 degradation in healthy subjects and in those with T2DM, the clearance of GLP-1 is unaffected by the presence or absence of T2DM [32]. These data suggest that the impaired incretin effect seen after a meal in patients with T2DM is caused, at least in part, by decreased secretion of GLP-1. In contrast, secretion of GIP appears to be largely unaffected by the presence of T2DM. In addition to decreased secretion of GLP-1, impairment of the incretin response may be related to downregulation of both the GLP-1 and GIP receptor in pancreatic islets, as a result of chronic hyperglycemia [33].
Effects of exogenous GLP-1 in patients with T2DM
Despite evidence that responsiveness to GLP-1 is impaired, intravenous infusion of GLP-1 normalizes β-cell responsiveness to glucose [34] and restores both first- and second-phase insulin responses in patients with T2DM [35]. In one study, fasting subjects inadequately controlled on diet and SU therapy received continuous intravenous saline or native intact GLP-1 [36]. After less than 4 hours of GLP-1 treatment, plasma glucose decreased to normal fasting levels (p < 0.05) and glucagon levels were also reduced (p < 0.05). Thus, GLP-1 infusion is able to restore a more physiologic balance between insulin and glucagon secretion in patients with T2DM. In addition to its acute effects, continuous infusion of GLP-1 has been observed to reduce diurnal plasma glucose levels to near-normal levels [37].
While GLP-1 has marked benefits in patients with T2DM, native GLP-1 administration, as a treatment strategy is severely limited by a short half-life in vivo (T½ = 1-2 minutes) due to inactivation by DPP-4 and the impracticality of continuous infusion. Thus, pharmacologic strategies have evolved to overcome these limitations, either directly by modifying native GLP-1 to make it resistant to the effects of DPP-4 (GLP-1 mimetics and analogs), or indirectly (by inhibiting the action of DPP-4).
GLP-1 receptor agonists in T2DM
Insulin secretagogues, insulin sensitizers, and insulin itself are effective antidiabetic agents, but may be associated with either weight gain or hypoglycemia, or both. Moreover, the efficacy of most oral agents diminishes with progression of T2DM. Therapies that could amplify insulin secretion without causing hypoglycemia and weight gain are desirable [38].
Exenatide, a first-generation, twice-daily injectable drug that mimics the physiologic actions of GLP-1, is currently the only GLP-1 receptor agonist approved for the treatment of T2DM. The second-generation, once-daily human GLP-1 analog, liraglutide, is under regulatory review. Several additional GLP-1 receptor agonists, including albiglutide (GLP-1 conjugated to albumin) and PC-DAC:Exendin-4 (exendin-4 conjugated to human albumin), are under active investigation, but limited data are available on these agents.
Exenatide: an exendin-4 analog
Exenatide was the first approved therapeutic agent in the incretin class of hypoglycemic/glucoregulatory agents. It is currently available in the United States as adjunctive therapy to improve glycemic control in patients with T2DM who have inadequate glycemic control despite receiving either MET, SU, a thiazolidinedione (TZD), a combination of MET + SU, or a combination of MET + TZD [39].
Exenatide is a synthetic 39-amino acid peptide with 53% homology to human GLP-1 [40]. Exenatide is directly derived from exendin-4, which was originally isolated from the salivary secretions of the reptile Heloderma suspectum (Gila monster). Exendin-4 reproduces many of the glucoregulatory functions of GLP-1 in mammals, with a substantially longer plasma half-life than native human GLP-1 [41]. These effects are mediated by binding to the pancreatic GLP-1 receptor. Since the second N-terminal amino acid alanine is replaced by serine in exendin-4, the duration of effect with exendin-4 is substantially longer than that of native GLP-1 [42].
Combination therapy of exenatide with oral antidiabetic agents
A series of randomized, double-blind, phase 3 trials, the "3 AMIGOs", evaluated the efficacy of exenatide 10 μg twice daily in patients with T2DM (n = 1446) receiving either SU [43] (n = 377); MET (n = 336) [44]; or combination SU + MET (n = 733) [45]. Although the initial treatment phase was 30 weeks, data from up to 2 years of exenatide exposure have been reported from open-label, uncontrolled extension phases [46-48].
Decreases in HbA1c, fasting plasma glucose (FPG), and weight were obtained when exenatide (5 μg or 10 μg) was given in combination with SU, MET, or SU + MET (Table 2). In contrast, FPG increased in groups treated with an injectable placebo. Mean decreases in HbA1c of 1.22% [43] and 1.35% [45] were achieved in patients with a baseline HbA1c ≥ 9% with 10 μg exenatide. In meal tolerance tests conducted at weeks 0, 4, and 30 in a subset of patients, postprandial glucose area under curve (AUC)15-180min values were 34% lower than baseline in the exenatide groups compared with 9% in the MET group [44], and 59% and 87% lower in the 5-μg and 10-μg exenatide + SU + MET groups [45].
Table 2. Combined summary data for AMIGO trials with exenatide.
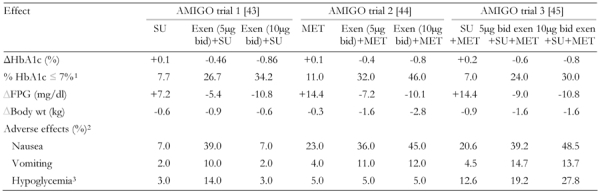
Baseline HbA1c was 8.6% in the first AMIGO trial [43], 8.2% in AMIGO 2 [44] and 8.5% in AMIGO 3 [45]. SU: sulfonylurea. Exen: exenatide. MET: metformin. FPG: fasting plasma glucose. BID: twice daily. 1 Fraction of patients (in %) with HbA1c ≤ 7% at the end of the study. 2 Fraction of patients with adverse effects (in %). 3 Mild to moderate hypoglycemia.
An open-label extension analysis, which included overweight (BMI 27-45 kg/m2) patients (n = 314) enrolled in the AMIGO trials, who received 10 μg exenatide for an additional 52 weeks, found sustained reductions in mean HbA1c of 0.9% and 1.1% and progressive mean reductions of body weight from 2.1 kg - 4.4 kg after 30 and 82 weeks of treatment, respectively (Table 3) [46]. Improvements in lipid profiles and blood pressure were also noted after 82 weeks [46, 47].
Table 3. Combined summary data for additional clinical trials with exenatide.
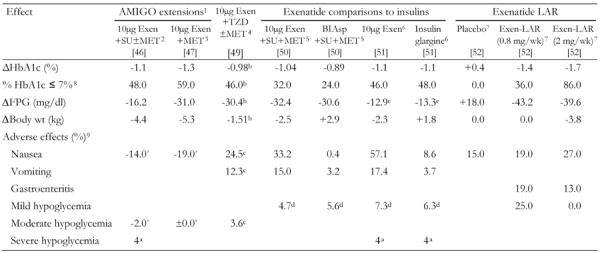
BID administration of exenetide in all studies with exenatide. Exen: exenatide. SU: sulfonylurea. MET: metformin. LAR: long-acting release. FPG: fasting plasma glucose. BIAsp: biphasic insulin aspart. BID: twice daily. 1 82 weeks; only included patients treated with exenatide [46, 47]. 2 Overweight patients in all groups. 39% of patients who entered the extended study withdrew. No antibody results reported. Nausea reported from weeks 30-40 to weeks 70-82 [46]. 3 Overweight patients. 43% of patients who entered the extended study withdrew. No antibody results reported. Adverse effects reported from weeks 30-40 to weeks 70-82 [47]. 4 16% of exenatide patients withdrew because of adverse events. Of the 115 (from 121 total) who were assessed, 40% were positive for anti-exenatide antibodies [49]. 5 21.3% of patients withdrew from the exenatide arm vs. 10.1% from the insulin BIAsp 70/30 arm. 45% were positive for anti-exenatide antibodies [50]. 6 19.4% of patients withdrew from the exenatide arm vs. 9.7% from the insulin glargine arm. 43% were positive for anti-exenatide antibodies [51]. 7 67% were positive for anti-exenatide antibodies [52]. 8 Fraction of patients (in %) with HbA1c ≤ 7% at the end of the study. 9 Fraction of patients with adverse effects (in %). In some cases, where marked with an asterisk (*), change of fraction of patients with adverse effects (in %) within the extended study (i.e. after completion of the first 30 wk study). a Cases (not percent). b Placebo-subtracted change. c Change treated patients vs. placebo. d Events per patient-year. e FPG reported as a 4 hour AUC in units of mmol/hr/l.
A pooled analysis of the 3 AMIGO trials that included patients who had completed 2 years of open-label, uncontrolled exenatide treatment (n = 283) found sustained reductions in mean HbA1c (1.1%), FPG (25.2 mg/dl), and weight (4.7 kg). An HbA1c ≤ 7% was achieved by 50% of subjects. Patients entering trials with a baseline HbA1c ≥ 9% achieved a mean 2% reduction in HbA1c [48]. In a subgroup of patients (n = 112), small improvements in β-cell function were found along with significant reductions in the hepatic-injury biomarkers alanine aminotransferase and aspartate aminotransferase in those with elevated levels at entry [48]. It should be noted that open-label extension trials may have overstated the efficacy of exenatide, as only responders were likely to have continued treatment.
In a trial conducted by Zinman and colleagues, the effects of 16 weeks of therapy with exenatide were compared with placebo among patients who were inadequately controlled on TZD therapy alone, or in combination with MET [49]. Exenatide significantly reduced HbA1c values by 0.98%, serum fasting glucose values by 1.69 mmol/l (30.5 mg/dl), and body weight by 1.51 kg (all measured as mean differences compared with placebo) [49]. Adverse effects (AEs) and antibody generation were similar to levels reported in other trials (Table 3) [43, 46, 49-52]. No significant changes were found in homeostasis model assessment of insulin sensitivity (HOMA-S), blood pressure, or lipids.
Comparison of exenatide with insulin
Exenatide was compared with the insulin analogs biphasic insulin aspart 70/30 (BIAsp 30) [50] and insulin glargine (Table 3) [51]. Results from these trials suggest that exenatide was at least noninferior to insulin therapy in terms of HbA1c reduction, and may provide better postprandial glycemic control and decrease body weight [50].
Dosing of insulin in these studies may not have been optimal [53]. In the study comparing exenatide to BIAsp 30, the mean total daily dose was 24.4 units per day, which achieved a 1.0% reduction in HbA1c. A dose of 78.5 units per day was used in the INITIATE study, which resulted in a 2.8% reduction in HbA1c [54]. In the study comparing exenatide to insulin glargine, the total daily dose was 25 U/day compared with 47 U/day in the Treat-to-Target Trial [55]. It is not known how exenatide compares with properly optimized insulin regimens, given that insulin has a glucose-lowering capacity that is limited only by the potential for hypoglycemia [56].
Exenatide: safety and adverse effects
The most common AEs associated with exenatide are dose-dependent nausea, vomiting, and diarrhea that may decline in severity and frequency with extended treatment [39]. In trials lasting 16-30 weeks, nausea was reported in 51% of subjects receiving exenatide + SU [43], 45% of subjects receiving exenatide + MET [44], 5% of subjects given exenatide + SU + MET [45], and 40% of those receiving exenatide + TZDs [49] (Table 2). In the 2-year pooled analysis, the incidence of nausea was 39% during wk 0-10 in the eligible intent-to-treat population (n = 521), and 8% by wk 100-104 among the 283 patients in the intent-to-treat population who completed 2 years of treatment [48].
There were substantially higher levels of nausea (57.1% vs. 8.6%), vomiting (17.4% vs. 3.7%), and diarrhea (8.5% vs. 3.0%) in trials comparing exenatide to insulin glargine (Table 3) [51]. These AEs were associated with a higher withdrawal rate in the exenatide group (19.4%) compared with the insulin glargine group (9.7%), and 9% in the exenatide + TZD trial [49]. Dizziness was reported in 15% and 16% in patients given exenatide + SU or exenatide + MET, compared with 7% and 8% among patients who received SU or MET only.
Exenatide is associated with increased risk for hypoglycemia when used in combination with SU but not with MET. In the AMIGO trials, mild hypoglycemia occurred in 3% of subjects treated with SUs only, in 36% of exenatide + SU subjects, in 5.3% of exenatide + MET subjects, and in 27.8% of exenatide + SU + MET subjects [43-45]. Mild-to-moderate hypoglycemic events occurred in up to 12% of subjects in the second extension study [46], with 4 cases of severe hypoglycemia (i.e., requiring medical attention) reported for patients taking SU [46]. No cases of severe hypoglycemia were observed during the MET extension trial [47]. Only a low incidence of hypoglycemia occurred when exenatide was added to TZD therapy [51], and in the comparison trial against insulin glargine [43].
Pooled data indicate that 38% of patients exhibit low titer antibodies with no apparent adverse clinical consequences. Higher titer antibodies were seen in 6% of patients in the 30-week clinical trials of exenatide added to MET or SU, and in 9% of patients who received exenatide in combination with TZD [39]. On average, approximately half the patients with higher titer antibodies in the SU + MET trials, and a greater proportion of those from the TZD study, experienced a potentially clinically significant attenuation in glycemic response to exenatide [39]. Antibody titers were not reported in the extension trials. These data suggest that glycemic response to exenatide should be monitored frequently during early therapy, and alternative treatments instituted if there is worsening glycemic control or failure to achieve targeted glycemic control.
Finally, exenatide is not recommended in patients with severe renal insufficiency or end-stage renal disease [38].
Long-acting exenatide
A long-acting release formulation of exenatide (exenatide LAR), administered once weekly, is currently in phase 3 clinical development. In a phase 2 study, exenatide LAR (0.8 or 2.0 mg) was administered subcutaneously for 15 weeks to patients with T2DM who were suboptimally controlled with MET and/or diet and exercise (n = 45) [52].
Exenatide LAR reduced mean HbA1c levels by 1.4-1.7% in the 0.8- and 2.0-mg groups, respectively (p < 0.0001 vs. placebo). Mean blood glucose decreased by 39.6 mg/dl (0.8 mg) and 45 mg/dl (2.0 mg) compared with an increase in the placebo group of 16.2 mg/dl. An HbA1c of ≤7% was achieved by 36% and 86% of subjects receiving 0.8 and 2.0 mg exenatide LAR, respectively, compared with 0% of subjects receiving placebo. Only the 2.0 mg/week dosage of exenatide LAR was associated with a mean body weight reduction (3.8 kg, p < 0.05) [52].
Nausea was reported in 19%, 27%, and 15% of patients in the exenatide LAR 0.8-mg and 2.0-mg groups and placebo group, respectively; gastroenteritis (19% and 13%) was reported in both LAR groups. Hypoglycemia was reported in 25% of patients who received the 0.8-mg dosage, and none who received the 2.0-mg dosage. At week 15, 67% of subjects in the exenatide LAR treatment groups were positive for anti-exenatide antibodies. However, there was no clear association with either efficacy or safety. Injection-site bruising was observed only in the exenatide LAR-treated patients (13% and 7% in the 0.8-mg and 2.0-mg groups, respectively) [52]. The clinical significance of higher anti-exenatide antibodies (67% with exenatide LAR [52] vs. approximately 38% with exenatide [39]) warrants investigation.
Exenatide LAR may find its primary clinical application in patients who have already demonstrated that they can tolerate the short-acting form of the drug. The extended half-life of this agent, and the long time to reach steady state, pose formidable problems when considering GLP-1-agonist-naïve patients for therapy, because the potential AEs of treatment may be greatly extended.
Exenatide: effects on β-cell function and insulin secretion
Exenatide improves insulin secretion in subjects with T2DM who previously did not display a normal first-phase response [57]. As suggested by studies of native GLP-1, exenatide inhibits β-cell apoptosis in vitro, preserves endogenous β-cell mass in animal studies, and improves β-cell function in patients with T2DM [10, 35, 49, 58]. A study conducted by Mari and colleagues evaluated postprandial β-cell function by mathematical modeling in a group of patients with T2DM (baseline HbA1c = 8.1%) inadequately controlled on MET, with or without SU. Subjects (n = 13) were randomized initially to exenatide 5 μg twice daily, or placebo. After 4 weeks, one arm of the exenatide group was uptitrated to 10 μg twice daily. After 30 weeks of therapy, insulin secretion rates increased 40% and 72% in the 5- and 10-μg groups, respectively, compared with a 21% reduction in placebo patients; moreover, exenatide at both dosages enhanced potentiation of insulin secretion [59].
Results from Zinman et al. (discussed earlier) support an effect of exenatide on β-cell function. Homeostasis model assessment of β-cell function (HOMA-B) after 16 weeks of treatment increased by 19% among patients who received exenatide, but decreased 6% among patients who received placebo [49]. HOMA-S increased to a greater extent in the exenatide group (23%) compared with a 10% increase in the placebo group. Similarly, a pooled analysis of 2-year results from the AMIGO studies found significant improvements in HOMA-B, and modest statistically significant improvements in HOMA-S, among patients who received exenatide [48].
Recent developments in exenatide research
In data presented at the American Diabetes Association (ADA) 2007 Scientific Sessions, Buse et al. reported mean reductions in HbA1c (1.0%), FPG (24 mg/dl), and weight (5.3 kg) in 217 patients after 3 years of exenatide treatment, with 46% of patients reaching an HbA1c ≤ 7%. A 17% increase in HOMA-B was also determined in a subgroup analysis [60].
Klonoff and colleagues [61] have analyzed efficacy parameters in 217 patients with T2DM who had completed ≥3 years of twice-daily exenatide as adjunctive therapy to MET and/or SU. Mean reductions in HbA1c (-1.0%), and weight (-5.3 kg) were found at 3 years. In a subset of 151 patients with 3.5 years of exenatide exposure, significant improvements in mean values of triglycerides (-12%), total cholesterol (-5%), low-density lipoprotein cholesterol (-6%), systolic blood pressure (-2%), and diastolic blood pressure (-4%) were observed. A substantial statistically significant 24% increase in HDL-C was seen.
A 24-week study presented as an abstract at the ADA 2008 Scientific Sessions evaluated exenatide monotherapy in 233 drug-naïve patients with T2DM. Reductions in HbA1c from baseline were significantly greater for subjects who received exenatide 5 μg twice daily (-0.7%) and 10 μg twice daily (-0.9%), compared to placebo (-0.2%). Although the proportion of patients who reached target HbA1c of ≤7% did not differ significantly between exenatide (60-61%) and placebo (48%) [62]. In a 30-week, open-label study, also presented at the ADA 2008 Scientific Sessions, 295 intention-to-treat patients treated with diet and exercise only, 1 oral antidiabetic drug (OAD), or 2 OADs, were randomized to additional therapy with exenatide 10 μg twice daily, or exenatide LAR 2 mg once weekly. Compared to twice-daily exenatide, the once-weekly formulation was associated with a significantly superior HbA1c reduction (-1.9% vs. -1.5%), FPG level (-42 mg/dl vs. -25 mg/dl), and proportion of patients who achieved HbA1c ≤ 7% (77% vs. 61%) [63].
Several recent studies have evaluated exenatide use in the "real world". In the first, a retrospective cohort study conducted in 67 patients with T2DM, exenatide therapy resulted in a reduction in HbA1c from 7.7-7.1% at 6 months (0.6% decrease) and a 3.4-kg reduction in weight at 6 months [64]. These results stand in contrast to a report by Loh and Clement that suggests that the effects of exenatide in the real world may not be as robust as initially suggested by phase 3 and long-term efficacy studies [65]. Real-world data also suggest that weight loss with exenatide is rapid, but reaches a plateau after about 6 months [66]. This outcome may be explained by patients’ decreasing adherence to exenatide, or by a progressive diminution of the effect of exenatide on anorexia and satiety over time [67].
Liraglutide: A once-daily human GLP-1 analog
Liraglutide is a once-daily, human GLP-1 analog with 97% amino acid homology to native human GLP-1. Modifications include a substitution of the lysine at position 34 with an arginine, and the attachment of a C16 acyl chain via a glutamyl spacer to lysine at position 26 [68]. These substitutions slow the absorption and degradation of liraglutide in comparison with native GLP-1, potentially through interaction with albumin and a capability to form aggregates in the subcutaneous tissue, yielding a time to maximum concentration of 9-14 hours, and half-life of up to 13 hours after subcutaneous administration [69, 70]. Therefore, liraglutide is distinguishable from the twice-daily exenatide formulation because it is a human derivative and the pharmacokinetics of the drug permit once-daily injection and 24-hour coverage.
Clinical studies with liraglutide
In all trials to date, liraglutide was dosed once daily. Liraglutide was associated with improvements in glycemic control and weight reduction and had a relatively low potential for AEs, including hypoglycemia and GI AEs. Five phase 3 clinical trials have been completed, and 4 of these were reported at the ADA 2008 Scientific Sessions.
Three early, double-blind, randomized, active-controlled (SU) and placebo-controlled, dose-ranging, 8- and 12-week phase 2 studies have been conducted. The doses used in these trials [71-73] (0.045-0.75 mg/d) were substantially lower than those used in later phase 2 trials (up to 2.0 mg/d) (Table 4) [29, 74] and in the subsequent phase 3 program.
Table 4. Combined summary data for clinical trials with liraglutide.
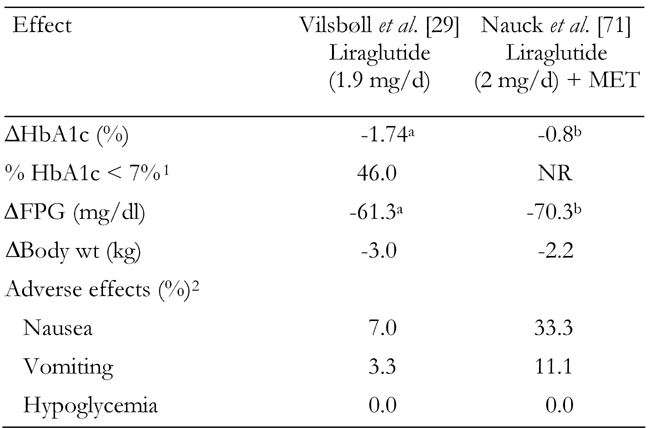
Only highest doses from these trials are shown. MET: metformin. FPG: fasting plasma glucose. NR: not reported. 1 Fraction of patients (in %) with HbA1c ≤ 7% at the end of the study. 2 Fraction of patients with adverse effects (in %). a Placebo-subtracted change. b Change vs. MET monotherapy.
In two phase 2 trials, liraglutide was administered once daily in clinically relevant doses. In the first trial, Vilsbøll and colleagues reported the effects of 14 weeks of liraglutide monotherapy on glycemic control and body weight in 165 patients with T2DM following treatment with an oral agent (primarily MET or SU). At the highest dosage (1.9 mg), liraglutide monotherapy reduced the estimated mean HbA1c by 1.74% from an average baseline of 8.5% (Table 4). When treated with either 1.25 or 1.9 mg liraglutide, almost half of patients reached the ADA target for postprandial control (<10 mmol/l or 180 mg/dl). A dose-dependent decrease in body weight was observed, with a mean weight loss of 3.0 kg in the 1.9-mg liraglutide group [29]. Similar results were observed in a study by Nauck and colleagues, in which liraglutide was added to MET (Table 4) [74].
Liraglutide may also have beneficial effects on cardiovascular risk in patients T2DM, having demonstrated a significant mean reduction in systolic blood pressure of 7.9 mmHg (p = 0.039), and a mean reduction in triglycerides of 22% at a dosage of 1.9 mg [29].
Liraglutide: safety and adverse effects
The incidence of GI AEs with liraglutide appeared to be low [72, 74] and transient [71], with few patient withdrawals from liraglutide groups due to AEs. Diarrhea was seen in most trials [29, 71, 72, 74] (ranging from 5.6-21.1%), but resolved within a few days. The incidence of injection-site reactions (5-6%) was also low [72].
Liraglutide: effects on β-cell function
Clinical and preclinical data indicate that liraglutide has a positive effect on β-cell function. In preclinical studies, liraglutide increased β-cell mass in rodent models of T2DM [75-77], decreased β-cell apoptosis [78], and increased β-cell differentiation in immature human pancreatic cell preparations in vitro, with a 1.95-fold increase in insulin-positive cells (p < 0.001) [79].
In human studies, one week of daily liraglutide improved β-cell function, as measured by significant improvements in both first- and second-phase glucose-induced insulin secretion [80]. In a longer-term study [81], patients with T2DM (n = 39) were randomized to treatment with 0.65, 1.25, or 1.9 mg/d liraglutide or placebo for 14 weeks and compared with a nondiabetic control group. In this study, liraglutide 1.25 and 1.9 mg treatment significantly increased maximal β-cell insulin secretory capacity compared with placebo by 114% and 93%, respectively (p < 0.05), and first-phase insulin secretion by 118% and 103%, respectively (p < 0.05). Both doses of liraglutide improved first-phase insulin secretion to 31% and 34% of controls, and maximal insulin secretory capacity to 48% and 40% of controls. Second-phase insulin secretion increased to levels similar to those of nondiabetic controls among patients in the 1.25-mg (p = 0.005 vs. control) and 1.90-mg liraglutide groups (p = NS vs. control) [81]. Mean β-cell function assessed by HOMA was increased in a 12-week study of liraglutide 0.75 mg [71], and treatment with liraglutide 6 μg/kg body weight significantly enhanced β-cell function during conditions of normal living [82]. In this last study, which used a validated β-cell model to evaluate 24-hour insulin secretion profiles, results in the liraglutide group demonstrated an increase in insulin secretion at 9 mmol/l glucose from 189-322 pmol/min/m2 (p < 0.005) [82].
New developments in liraglutide research
Horowitz and colleagues have presented patient-reported ratings of GI symptoms in patients with T2DM receiving liraglutide monotherapy for 14 weeks. Results supported the transient nature of GI side effects following liraglutide administration, with substantial symptoms occurring only in the first 2 weeks after treatment initiation. Symptom scores subsequently returned to baseline levels [83].
Two recent reports evaluated the use of liraglutide in Japanese subjects. These studies did not find significant concerns surrounding safety or tolerability. Once-daily liraglutide resulted in significant mean HbA1c reductions of 1.6% and 1.8% at dosages of 0.6 and 0.9 mg/d, compared with placebo over 14 weeks [84, 85].
Phase 3 studies of liraglutide were reported at the ADA 2008 Scientific Sessions. The studies assessed liraglutide as monotherapy or in combination with either MET, SU, or MET + SU in patients with T2DM. Results of the 52-week monotherapy study of liraglutide 1.2 and 1.8 mg once daily, indicated that liraglutide was associated with significantly greater reduction in HbA1c (-0.84% and -1.14%, respectively) than glimepiride 8 mg once daily (-0.51%). Weight loss with liraglutide (-2.05 to -2.45 kg) was also significantly superior to the weight gain seen with glimepiride (+1.12 kg). GI adverse events occurred in 28-29% of patients in the liraglutide group vs. 9% in the glimepiride group, but the symptoms in the liraglutide groups were transient, and there was a significantly lower rate of hypoglycemic events with liraglutide relative to glimepiride [86]. In 26-week studies of liraglutide added to MET, SU, or MET + SU, reductions were as large as -1.33% for HbA1c and -2.8 kg for weight, with concomitant improvements in β-cell function [87-90]. Liraglutide also decreased systolic blood pressure by as much as -4.5 mmHg vs. comparator treatments. The blood pressure reduction, which occurred rapidly (i.e., after 2 weeks), could not be explained by the decrease in weight [91]. Among liraglutide-treated patients, liraglutide antibodies were found in 0-4% of those in the MET add-on study, 9-13% of those in the SU add-on study, and 10% of those in the MET + SU add-on study [87-89], rates that appear lower than those observed in pooled studies of exenatide [39].
Other GLP-1 agonists in development
Additional GLP-1 agonists in early clinical development include a recombinant GLP-1/albumin conjugate (albiglutide), a recombinant exendin-4/albumin conjugate, and a once-weekly GLP-1 analog (taspoglutide). These agents may have increased half-lives compared with exenatide or liraglutide [92]. CJC-1131, a synthetic GLP-1 analog covalently bound to albumin, with a half-life of approximately 10 days, has demonstrated an effect on fasting and postprandial glucose similar to that of liraglutide [93]. Phase 2 data presented at the 2008 ADA Scientific Sessions demonstrated that once-weekly taspoglutide (R1583) significantly reduced glucose levels and weight in patients with T2DM, with a favorable safety and tolerability profile [94, 95].
Summary: efficacy and tolerability of GLP-1 receptor agonists
The efficacy, AEs, effects on body weight and incidence of hypoglycemia of exenatide and liraglutide are summarized in Tables 2-4 and compared in Figure 3 [19, 43-45, 49, 51, 71, 74, 96-106]. As illustrated, GLP-1 receptor agonists with a longer duration of action such as exenatide LAR and liraglutide appear to have superior efficacy than the marketed formulation of exenatide, reducing HbA1c levels in excess of 1.7%, while still promoting significant weight loss and without provoking significant hypoglycemia.
Figure 3.
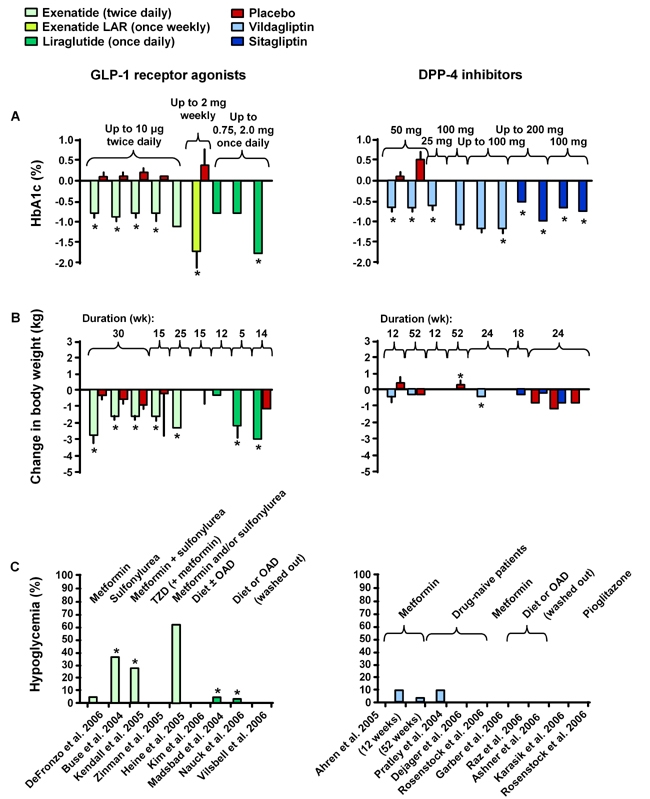
Clinical effects of GLP-1 receptor agonists (exenatide, exenatide LAR, liraglutide) and DPP-4 inhibitors (sitagliptin, vildagliptin) on HBA1c (A), change in body weight (B) and hypoglycemia (C) [19, 43-45, 49, 51, 71, 74, 96-106]. DPP-4: dipeptidyl peptidase-4. TZD: thiazolidinedione. OAD: oral antidiabetic drug. * p < 0.05. Reproduced with permission from Lancet [19].
DPP-4 inhibitors in T2DM
While GLP-1 receptor agonists directly affect the incretin system by mimicking the effects of endogenous GLP-1, DPP-4 inhibitors act as incretin enhancers by preventing the inactivation of endogenous incretins by DPP-4, thereby elevating active incretin levels [104]. Thus, efficacy of DPP-4 inhibitors is dependent upon endogenous incretin secretory capacity that, at least for GLP-1, appears to be diminished in patients with T2DM. In one major meta-analysis of sitagliptin and vildagliptin trials, DPP-4 inhibitors lowered HbA1c an average of 0.74% with no weight change or hypoglycemia, and were noninferior to other agents (including glipizide and TZDs) [35].
The DPP-4 inhibitors are orally bioavailable, small molecular weight drugs that act via competitive, reversible inhibition of DPP-4, providing up to 90% inhibition of plasma DPP-4 activity over 24 hours in vivo [105]. These agents have been shown to provide 2-3 fold enhancements in the levels of active GIP and GLP-1, improving impaired insulin secretion and reducing glucagon levels in patients with T2DM. Because these agents rely on endogenous incretin secretion, they may best be employed in early disease [29, 30, 106].
Sitagliptin
Sitagliptin, a potent and specific inhibitor of DPP-4, is the only DPP-4 inhibitor currently approved for use in the United States; a combination product (sitagliptin + MET) is also available. In trials lasting from 18-52 weeks, various doses of sitagliptin were utilized with once- or twice-daily dosing. Results from a number of clinical trials are summarized in Table 5 [103, 107, 109-113].
Table 5. Combined summary data for clinical trials with DPP-4 inhibitor sitagliptin only and in combination with other anti-diabetic drugs.
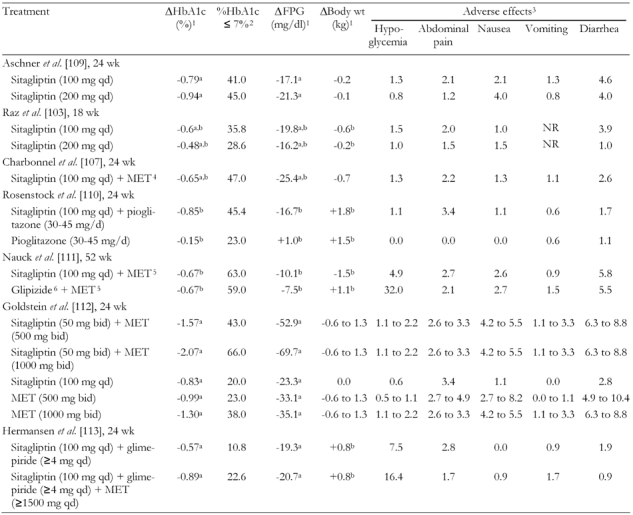
MET: metformin. FPG: fasting plasma glucose. BID: twice daily. QD: once daily. NR: not reported. 1 From baseline. 2 Fraction of patients (in %) with HbA1c ≤ 7% at the end of the study. 3 Fraction of patients with adverse effects (in %). 4 MET < 1500 mg/d. 5 MET titrated to 1500 mg/d. 6 Glipizide titrated to 20 mg/d. a Placebo-subtracted mean change. b Least squares mean change.
In one double-blind, placebo-controlled, 24-week study, sitagliptin 100-200 mg once daily provided significant placebo-subtracted reductions in HbA1c and FPG in patients naive to OADs [109]. In contrast to the results seen with exenatide and liraglutide, there were no meaningful changes in mean body weight with sitagliptin (-0.1 to -0.2 kg) [109]. Similar results were observed in an 18-week study of sitagliptin 100-200 mg once daily in patients inadequately controlled on exercise and diet [103]. Hypoglycemia rates were similar across groups. Although there was a slightly higher incidence of constipation, nasopharyngitis, pharyngitis, pharyngolaryngeal pain, urinary tract infection, myalgia, arthralgia, hypertension, and dizziness in the sitagliptin group [103, 109].
In patients inadequately controlled by MET monotherapy (> 1500 mg/d), the addition of sitagliptin 100 mg once daily provided significant mean reductions compared with placebo in HbA1c, FPG, 2-hour postmeal glucose, and other glycemic parameters (Table 5). Also, it substantially improved the percentage of patients who achieved an HbA1c of < 7% (47.0% for sitagliptin patients vs. 18.3% with placebo) [107]. Notably, unlike MET—known to elicit GI AEs—there was no increased risk for GI events compared with placebo, and there was no effect on body weight [107]. Similarly, 100 mg of sitagliptin administered once daily provided incremental reductions in HbA1c levels when added to ongoing pioglitazone therapy (30 to 45 mg once daily) of approximately 0.70% (Table 5) [110].
In a head-to-head comparison, sitagliptin 100 mg once daily was non-inferior to glipizide 20 mg once daily as an adjunct to MET ≥ 1500 mg once daily. 52-week mean reductions in HbA1c of 0.67% were achieved both in patients receiving sitagliptin and those receiving glipizide, with the added benefit of small weight losses with sitagliptin (-1.5 kg) compared to small weight gains with glipizide (+1.1 kg) [111].
Simultaneous initiation of sitagliptin and MET provided significant reductions in HbA1c over either agent alone [112]. Sitagliptin administered twice daily at 50 mg + MET 500 mg or 1000 mg administered twice daily were associated with placebo-subtracted mean HbA1c reductions of 1.57% and 2.07%, respectively. In comparison, all single agents produced placebo-subtracted mean HbA1c reductions of 1.30% or less. A similar pattern was seen for FPG [112]. These effects were achieved without substantial increases in the rate or severity of AEs. Sitagliptin has also been shown to reduce HbA1c in patients inadequately controlled on the combination of glimepiride and MET [113].
Most trials of sitagliptin in combination with other OADs indicated that DPP-4 inhibitors produce incremental, or sometimes additive, improvements in glycemic parameters. Together, extant clinical data suggest that sitagliptin is a moderately effective antidiabetic agent, providing HbA1c reductions of up to 0.94% when used as monotherapy [109] and additional reductions in HbA1c when used as part of combination therapy [112]. Unlike the majority of OADs, these incremental reductions in HbA1c are not associated with significant weight increases.
Vildagliptin
Vildagliptin, another potent and specific DPP-4 inhibitor, is marketed in several countries and has been approved in the European Union. Approval in the United States is pending additional trials to examine the safety and efficacy in patients with renal impairment. In phase 3 trials as monotherapy and in combination with a variety of oral agents, vildagliptin provided HbA1c reductions of 0.8-1.0%. Table 6 summarizes results from various major studies on vildagliptin only and in combination with other anti-diabetic drugs [98, 114-119].
Table 6. Combined summary data for clinical trials with DPP-4 inhibitor vildagliptin only and in combination with other anti-diabetic drugs.
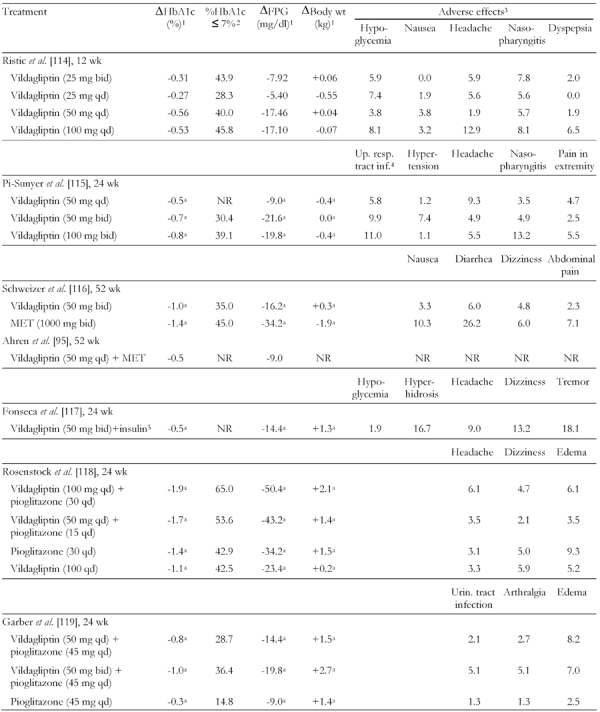
MET: metformin. FPG: fasting plasma glucose. BID: twice daily. QD: once daily. NR: not reported. 1 From baseline. 2 Fraction of patients (in %) with HbA1c ≤ 7% at the end of the study. 3 Fraction of patients with adverse effects (in %). 4 Upper respiratory tract infection. 5 Additional adverse effect: asthenia (16.7). a Adjusted mean change.
In a study comparing twice-daily dosages of vildagliptin (50 mg) and MET (1000 mg), both drugs reduced HbA1c levels. At 1 year, significant mean HbA1c reductions from baseline of 1.0% for vildagliptin and 1.4% for MET were seen, with neutral effects on body weight for vildagliptin (+0.3 kg) and reductions in body weight among patients who received MET (-1.9 kg) [116]. In this trial, vildagliptin was associated with a significantly more favorable GI AE profile than was MET (Table 6).
In a 24-week trial, vildagliptin 50-100 mg once daily provided significant incremental mean reductions in HbA1c levels when added to once-daily pioglitazone (15-30 mg). Reductions of 1.9% and 1.7% were seen in patients receiving vildagliptin/pioglitazone combinations of 100/30 mg and 50/15 mg, respectively, compared with reductions of 1.4% and 1.1% in patients receiving pioglitazone and vildagliptin monotherapy [117]. Vildagliptin was weight neutral in this trial. Incremental improvements in mean HbA1c and FPG were reported for another trial that tested vildagliptin (50 or 100 mg/day) as add-on therapy for patients poorly controlled by pioglitazone (45 mg/day). Although the FPG changes after vildagliptin add-on were not significantly different from pioglitazone continuation, small weight gains occurred [118] (Table 6).
Safety and adverse effects of DPP-4 inhibitors
Hypoglycemia and GI AEs are infrequent in patients who receive DPP-4 inhibitors. The DPP-4 inhibitor sitagliptin is primarily (87%) secreted via renal elimination; therefore, the dosage must be adjusted in patients with moderate-to-severe renal impairment [119]. However, in a meta-analysis these agents were associated with a 1.2-fold increased risk of infection of nasopharyngitis, a 1.5-fold increased risk for urinary tract infection, and a 1.4-fold increased frequency of headache [38]. Long-term safety remains to be established.
Effect of DPP-4 inhibitors on β-cell function and insulin secretion
Data suggest that—like GLP-1 agonists—the DPP-4 inhibitors have the potential to improve β-cell function and insulin sensitivity. A 12-week study conducted by Ahren and colleagues examined the effects of vildagliptin on meal-related β-cell function and insulin sensitivity over 52 weeks in MET-treated patients with T2DM [98]. Insulin sensitivity assessed during a glucose tolerance test increased among patients who received vildagliptin + MET but not in those who received MET alone. Insulin secretion related to insulin sensitivity (adaptation index) increased with vildagliptin + MET but decreased among patients who received MET alone. The change in adaptation index correlated to the change in HbA1c (r = -0.39, p = 0.004).
New developments in DPP-4 research
A clinical trial presented at ADA 2007 indicates that sitagliptin added to MET is effective in reducing HbA1c and is associated with a small but significant decrease in body weight [120]. Sitagliptin also provides significant glycemic control (with HbA1c reductions of 0.7%, FPG reductions of 20 mg/dl, and 2-hour postprandial glucose reductions of 36 mg/dl) when added to either SU or combination SU + MET. However, body weight increased in patients receiving sitagliptin (0.8 kg) compared with those receiving either SU or combination SU + MET (-0.4 kg) [121].
A pooled analysis of 5 monotherapy studies supports the efficacy of vildagliptin in T2DM, with mean reductions of approximately 1.0% in HbA1c and 20 mg/dl in FPG, over 24 weeks at dosages of 100 mg daily. Modest but significant body weight reductions and low rates of hypoglycemia were also noted [122]. It has also recently been reported that DPP-4 inhibitors may have GLP-1 agonist-like effects on gastric emptying [123]. Furthermore, vildagliptin significantly reduced systolic and diastolic blood pressure in hypertensive patients with T2DM compared with MET, improved insulin sensitivity and β-cell function in patients with impaired fasting glucose, and demonstrated significant reductions in postprandial triglyceride responses [124-126].
Three studies presented at the ADA 2008 Scientific Sessions demonstrated the glucose-lowering efficacy and tolerability of sitagliptin in Japanese patients with T2DM, when added to ongoing treatment with MET, SU, or pioglitazone [127-129]. In a pre-specified analysis of data from a study that compared sitagliptin with glipizide (each added to MET), the risk of physician-confirmed, symptomatic hypoglycemia was assessed. Compared to glipizide, sitagliptin showed a lower risk of hypoglycemia of 11-fold in patients aged <65 years and 29-fold in those aged ≥65 years [130]. Another study found improvement in indices of β-cell function in patients with T2DM who received initial therapy with sitagliptin and MET in combination [131]. A monotherapy study demonstrated the glucose-lowering efficacy of vildagliptin in a Japanese population [132]. Data were also presented indicating that vildagliptin enhances glucagon release in hypoglycemic conditions, but reduces glucagon secretion during hyperglycemia, suggesting an ability to enhance α-cell glucose sensing [133].
New DPP-4 inhibitors in development
Data from the phase 3 alogliptin development program were released at the 2008 ADA Scientific Sessions. In 5 different studies, alogliptin 12.5 and 25 mg/day were evaluated as T2DM monotherapy and in combination with MET, SU, pioglitazone (with or without additional MET or SU), or insulin. Both doses of alogliptin produced significantly greater mean changes from baseline in HbA1c (up to -0.59% as monotherapy and -0.80% as combination therapy) than placebo in all studies. Alogliptin was not associated with an elevated incidence of hypoglycemia or GI symptoms, and did not induce weight gain [134-138].
A phase 3 study presented at the ADA 2007 Scientific Sessions assessed saxagliptin, at dosages of 2.5-10 mg, added to MET. Saxagliptin reduced HbA1c by up to 0.83% and FPG by up to 24 mg/dl, with no change in weight or risk for hypoglycemia [139]. A subsequent study of saxagliptin monotherapy (2.5 mg/day and 10 mg/day) demonstrated significant placebo-subtracted decreases in HbA1c at week 24 (up to -0.73%), in addition to significantly higher rates of HbA1c target achievement compared to placebo. Saxagliptin was weight neutral, and no cases of hypoglycemia were observed. The most common adverse effects were upper respiratory tract infection, headache, urinary tract infection, nasopharyngitis, and sinusitis [140].
Summary: efficacy and tolerability of DPP-4 inhibitors
The efficacy, AEs, effects on body weight, and incidence of hypoglycemia of sitagliptin and vildagliptin are summarized in Table 5 and compared in Figure 3. The efficacy of vildagliptin appears somewhat better than that of sitagliptin. This is possibly due to differences in trial design and HbA1c entry criteria, as both drugs are potent and selective inhibitors of DPP-4. Both sitagliptin and vildagliptin are weight neutral and associated with very low rates of hypoglycemia.
The evolving role of GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of T2DM
Both GLP-1 receptor agonists and DPP-4 inhibitors provide unique benefits that complement and extend the present therapeutic armory for the treatment of T2DM. Some of the benefits of incretin-based therapies, such as enhancement of glucose-dependent insulin secretion and suppression of glucagon, are shared by GLP-1 receptor agonists and DPP-4 inhibitors, while promoting satiety and weight loss are unique to GLP-1 receptor agonists.
It is evident that DPP-4 inhibitors are effective, well-tolerated, weight-neutral oral agents. Type 2 diabetes is characterized by a progressive loss of β-cell function in the setting of insulin resistance. These core defects are accompanied by a substantial impairment in the incretin effect, potentially due to impairments of GLP-1 secretion and action [16, 33]. Since DPP-4 inhibitors are dependent on endogenous incretin secretion, they might be utilized most effectively early in the course of the disease. Moreover, DPP-4 inhibitors produce only minor increases in fasting active GLP-1 levels. Their predominant effects are seen postprandially. Thus, DPP-4 inhibitors lower HbA1c levels by only a modest degree. Nevertheless, their ease of use for both patients and providers suggests that they will play an increasingly important role in the treatment of T2DM, particularly in primary care settings. They may be appropriate as monotherapy for patients who cannot tolerate MET or in those with renal insufficiency who should not receive MET because of the risk of lactic acidosis.
As DPP-4 inhibitors are weight-neutral and associated with a low risk for hypoglycemia, they may be appropriate as monotherapy in place of SU in patients for whom weight gain and hypoglycemia are undesirable. Since the mechanism of action of DPP-4 inhibitors complements that of MET and TZDs, DPP-4 inhibitors are ideal in combination with these agents. Such combinations are particularly effective at reducing hyperglycemia without provoking hypoglycemia. Finally, DPP-4 inhibitors are well-suited for treating elderly patients with T2DM, due to their favorable tolerability profile, low risk for hypoglycemia, and lack of significant drug-drug interactions [79].
In contrast to DPP-4 inhibitors, GLP-1 agonists do not rely on endogenous incretin secretion. Also, pharmacologic levels of GLP-1 activity can be achieved by injection of agonists, rather than the high-physiological levels attained following DPP-4 treatment. Although the half-life of the current formulation of exenatide is substantially longer than native GLP-1, its relatively short half-life does not provide 24-hour coverage. Therefore, the efficacy of exenatide is limited, particularly with respect to fasting glucose levels.
Longer-acting GLP-1 receptor agonists in development, such as liraglutide, exenatide-LAR and other molecules have more robust effects on fasting glucose levels. As a result they may offer superior efficacy to exenatide and most OADs. An important feature of GLP-1 receptor agonists is their ability to promote significant mean reductions in body weight.
In contrast to the DPP-4 inhibitors, all GLP-1 receptor agonists must be given as injections. Furthermore, the incidence of nausea and other GI AEs is higher with these agents than with DPP-4 inhibitors or other OADs. The currently available GLP-1 receptor agonist, exenatide, is indicated for patients with T2DM in combination with oral agents such as MET, SU, and TZDs. Obese patients with T2DM in particular may benefit from this class, due to the weight loss effects—which can be significant in some individuals—and other nonglycemic benefits such as lower blood pressure and improved lipid profiles. If these initial indications of superior efficacy are confirmed in additional trials, long-acting GLP-1 receptor agonists such as liraglutide, exenatide LAR, albiglutide, and taspoglutide could achieve widespread use as second, or even first-line treatments to bring more patients with T2DM to goal.
Acknowledgments
We would like to thank John Ferguson for providing medical writing services and Adelphi Inc. for providing editorial services paid for by Novo Nordisk.
References
- 1.Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectrum. 2004;17:183–190. [Google Scholar]
- 2.Gallwitz B. New therapeutic strategies for the treatment of type 2 diabetes mellitus based on incretins. Rev Diabet Stud. 2005;2(2):61–69. doi: 10.1900/RDS.2005.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.de Valk HW. DPP-4 Inhibitors and Combined Treatment in Type 2 Diabetes: Re-evaluation of Clinical Success and Safety. Rev Diabet Stud. 2007;4(3):126–133. doi: 10.1900/RDS.2007.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenisis of type II diabetes mellitus. Diabetologia. 2001;44:929–945. doi: 10.1007/s001250100580. [DOI] [PubMed] [Google Scholar]
- 6.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Weyer C, Bogardus C, Mott DM, Pratley R. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16: overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 10.Gallwitz B. The fate of beta-cells in type 2 diabetes and the possible role of pharmacological interventions. Rev Diabet Stud. 2006;3(4):208–216. doi: 10.1900/RDS.2006.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 12.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283:109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- 13.Henkel E, Menschikowski M, Koehler C, Leonhardt W, Hanefeld M. Impact of glucagon response on postprandial hyperglycemia in men with impaired glucose tolerance and type 2 diabetes mellitus. Metabolism. 2005;54:1168–1173. doi: 10.1016/j.metabol.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Kahn SE, Verchere CB, Andrikopoulos S, Asberry PJ, Leonetti DL, Wahl PW, Boyko EJ, Schwartz RS, Newell-Morris L, Fujimoto WY. Reduced amylin release is a characteristic of impaired glucose tolerance and type 2 diabetes in Japanese Americans. Diabetes. 1998;47:640–645. doi: 10.2337/diabetes.47.4.640. [DOI] [PubMed] [Google Scholar]
- 15.Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 16.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 17.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 19.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonist and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 20.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 21.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273(5 pt 1):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 22.Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rössner S, Hellström PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–311. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 23.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 24.Thrainsdottir I, Malmberg K, Olsson A, Gutniak M, Ryden L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–43. doi: 10.3132/dvdr.2004.005. [DOI] [PubMed] [Google Scholar]
- 25.Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 26.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–3061. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 29.Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges JP, Verhoeven R, Buganova I, Madsbad S. Liraglutide, a long-acting human GLP-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes mellitus. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 30.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in Type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 31.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 32.Vilsboll T, Agerso H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88:220–224. doi: 10.1210/jc.2002-021053. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 34.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effect on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 35.Vilsboll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide - regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–4903. doi: 10.1210/jc.2003-030738. [DOI] [PubMed] [Google Scholar]
- 36.Nauck MA, Kleine N, Orskov C, Holst JJ, Williams B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 37.Rachman J, Barrow BA, Levy JC, Turner RC. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 38.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 39.Amylin Pharmaceuticals; San Diego, California: 2007. Byetta (exenatide injection) Prescribing Information. [Google Scholar]
- 40.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 42.Ritzel U, Leonhardt U, Ottleben M, Rühmann A, Eckart K, Spiess J, Ramadori G. A synthetic glucagon-like peptide-1 analog with improved plasma stability. J Endocrinol. 1998;159:93–102. doi: 10.1677/joe.0.1590093. [DOI] [PubMed] [Google Scholar]
- 43.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 45.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 46.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 47.Ratner RE, Maggs D, Nielsen LL, Stonehouse AH, Poon T, Zhang B, Bicsak TA, Brodows RG, Kim DD. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419–428. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 48.Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29:139–153. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146(7):477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 50.Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 51.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 52.Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 53.Home PD. Comment on: Nauck MA, Duran S, Kim D, et al (2007) A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(7):1561–1562. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 54.Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, Bode B, Garber A INITIATE Study Group. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 55.Riddle MC, Rosenstock J, Gerich J for the Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 56.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 57.Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–5997. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2006;346:1067–1074. doi: 10.1016/j.bbrc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 59.Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res. 2006;38:838–844. doi: 10.1055/s-2006-956505. [DOI] [PubMed] [Google Scholar]
- 60.Buse J, MacConell L, Stonehouse A, Guan XS, Malone J, Okerson T, Maggs D, Kim D. Exenatide maintained glycemic control with associated weight reduction over three years in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 0283-OR. [Google Scholar]
- 61.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 62.Brodows R, Milton D, Ridge TD, MacConell L, Okerson T, Wolka AM, Moretto TJ. Exenatide monotherapy improves glycemic control and is well tolerated over 24 weeks in drug-naive patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 485-P. [Google Scholar]
- 63.Drucker DJ, Buse JB, Taylor K, Kendall D, Trautmann M, Zhuang D, Porter L. Exenatide once weekly results in significantly greater improvements in glycemic control compared to exenatide twice daily in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 107-OR. [Google Scholar]
- 64.Briceno RM, Larhari-Libhaber VS, Meneghini LF. Clinical observation study of the safety, effectiveness, and tolerability of exenatide in a real-world setting; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 2147-PO. [Google Scholar]
- 65.Loh J, Clement SC. Efficacy of exenatide therapy over two years in a "real world" setting; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 105-OR. [Google Scholar]
- 66.Wolfe G, King A. Lack of continued weight loss beyond six months despite continued exenatide treatment; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 2161-PO. [Google Scholar]
- 67.Wolfe G, King A. The change in the anorexia and satiety of exenatide (EX) treatment over 12 months; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 2162-PO. [Google Scholar]
- 68.Vilsboll T. Liraglutide: a once-daily GLP-1 analogue for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs. 2007;16:231–237. doi: 10.1517/13543784.16.2.231. [DOI] [PubMed] [Google Scholar]
- 69.Elbrond B, Jakobsen G, Larsen S, Agerso H, Jensen LB, Rolan P, Sturis J, Hatorp V, Zdravkovic M. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25:1398–1404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 70.Agerso H, Jensen LB, Elbron B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 71.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 72.Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with type 2 diabetes. Diabet Med. 2005;22:1016–1023. doi: 10.1111/j.1464-5491.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- 73.Harder H, Nielsen L, Thi TD, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27:1915–1921. doi: 10.2337/diacare.27.8.1915. [DOI] [PubMed] [Google Scholar]
- 74.Nauck MA, Hompesch M, Filipczak R, Le TD, Zdravkovic M, Gumprecht J. Five weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114:417–423. doi: 10.1055/s-2006-924230. [DOI] [PubMed] [Google Scholar]
- 75.Sturis J, Gotfredsen CF, Romper J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, Knudsen LB. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol. 2003;140:123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–E752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 77.Bock T, Pakkenberg B, Buschard K. The endocrine pancreas in non-diabetic rats after short-term and long-term treatment with the long-acting GLP-1 derivative NN2211. Apmis. 2003;111:1117–1124. doi: 10.1111/j.1600-0463.2003.apm1111207.x. [DOI] [PubMed] [Google Scholar]
- 78.Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–584. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Piper K, Dijkstra I, Dunleavey L, Hanley NA. The long-acting GLP-1 analog, liraglutide, increases beta cell numbers during early human development. Diabetes 2005. 54:A394. Abstract 1636-P. [Google Scholar]
- 80.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 81.Vilsboll T, Brock B, Perrild H, Levin K, Lervang HH, Kolendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T et al. Liraglutide, a once-daily human GLP-1 analogue improves beta-cell function and arginine-stimulated insulin secretion at hyperglycaemia in patients with type 2 mellitus. Diabet Med. 2008;25:152–156. doi: 10.1111/j.1464-5491.2007.02333.x. [DOI] [PubMed] [Google Scholar]
- 82.Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care. 2007;30:2032–2033. doi: 10.2337/dc07-0310. [DOI] [PubMed] [Google Scholar]
- 83.Horowitz M, Vilsboll T, Zdravkovic M, Hammer M, Madsbad S. Patient-reported rating of gastrointestinal adverse effects during treatment of type 2 diabetes with the once-daily human GLP-1 analogue, liraglutide. Diabetes Obes Metab. 2008;10:593–596. doi: 10.1111/j.1463-1326.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- 84.Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;81:161–168. doi: 10.1016/j.diabres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Irie S, Matsumura Y, Zdravkovic M, Jacobsen LV, Kageyama S. Tolerability, pharmacokinetics and pharmacodynamics of the once-daily human GLP-1 analog liraglutide in Japanese healthy subjects: a randomized, double-blind, placebo-controlled dose-escalation study. Int J Clin Pharmacol Ther. 2008;46:273–279. doi: 10.5414/cpp46273. [DOI] [PubMed] [Google Scholar]
- 86.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez Pattzi HM, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. Significantly better glycemic control and weight reduction with liraglutide, a once-daily human GLP-1 analog, compared with glimepiride: all as monotherapy in type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 07-LB. [Google Scholar]
- 87.Nauck MA, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Düring M, Zdravkovic M, Matthews D. Liraglutide, a once-daily human GLP-1 analog, in type 2 diabetes provides similar glycemic control with reduced body weight compared with glimepiride when added to metformin; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 504-P. [Google Scholar]
- 88.Marre M, Shaw J, Brandle M, Wan Mohamad Wan Bebakar, Kamarrudin NA, Strand J, Zdravkovic M, Duyen Le-Thi T, Colagiuri S. Liraglutide, a once-daily human GLP-1 analog, added to a sulfonylurea (SU) offers significantly better glycemic control and favorable weight change compared with rosiglitazone and SU combination therapy in subjects with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 13-OR. [Google Scholar]
- 89.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic SS, Zdravkovic M, Ravn GM, Simo R. Significantly better glycemic control and weight reduction with liraglutide, a once-daily human GLP-1 analog, compared with insulin glargine: all as add-on to metformin and a sulfonylurea in type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 536-P. [Google Scholar]
- 90.Matthews D, Marre M, Duyen Le-Thi T, Zdravkovic M, Simo R. Liraglutide, a once-daily human GLP-1 analog, significantly improves beta-cell function in subjects with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 505-P. [Google Scholar]
- 91.Colagiuri S, Frid A, Zdravkovic M, Duyen Le-Thi T, Vaag A. The once-daily human GLP-1 analog liraglutide reduces systolic blood pressure in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 554-P. [Google Scholar]
- 92.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 93.Guivarch PH, Castaigne JP, Gagnon L, Peslherbe L, Dreyfus JH, Drucker DJ. The once-daily human GLP-1 analog liraglutide reduces systolic blood pressure in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 554-P. [Google Scholar]
- 94.Balena R, Ratner R, Berria R, Asnaghi V, Grant R, Snaith J, Boldrin M, Nauck M. Eight weeks of treatment with the long acting, human GLP-1 analogue R1583 improves glycemic control and lowers body weight in subjects with type 2 diabetes mellitus (T2DM) treated with metformin: a double-blind placebo-controlled phase 2 study; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 108-OR. [Google Scholar]
- 95.Ratner R, Nauck M, Asnaghi V, Berria R, Cressier F, Boldrin M, Balena R. Safety and tolerability of high doses of the long acting, human GLP-1 analogue R1583 in diabetic subjects treated with metformin: a double-blind, placebo-controlled phase 2 study; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 108-OR. [Google Scholar]
- 96.Kim D, Macconell L, Zhuang D, Schnabel C, Taylor K, Li WI, Trautmann M. Safety and efficacy of a once-weekly, long-acting release formulation of exenatide over 15 weeks in patients with type 2 diabetes. Diabetes. 2006;55 (Suppl 1):116. [Google Scholar]
- 97.Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges J, Verhoeven T, Buganova I, Madsbad S. Liraglutide significantly improves glycemic control, and lowers body weight without risk of either major or minor hypoglycemic episodes in subjects with type 2 diabetes. Diabetes. 2006;55 (Suppl 1):27–28. [Google Scholar]
- 98.Ahren B, Pacini G, Foley JE, Schweitzer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005;28:1936–1940. doi: 10.2337/diacare.28.8.1936. [DOI] [PubMed] [Google Scholar]
- 99.Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–428. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- 100.Dejager S, Lebeaut A, Couturier A, Schweizer A. Sustained reduction in HbA1c during one-year treatment with vildagliptin in patients with type 2 diabetes (T2DM) Diabetes. 2006;55(Suppl 1):29. [Google Scholar]
- 101.Rosenstock J, Baron MA, Schweizer A, Mills D, Dejager S. Vildagliptin is as effective as rosiglitazone in lowering HbA1c but without weight gain in drug-naive patients with type 2 diabetes (T2DM) Diabetes. 2006;55(Suppl 1):133. [Google Scholar]
- 102.Garber A, Camisasca RP, Ehrsam E, Collober-Maugeais C, Rochotte E, Lebeaut A. Vildagliptin added to metformin improves glycemic control and may mitigate metformin-induced GI side effects in patients with type 2 diabetes (T2DM) Diabetes. 2006;55(Suppl 1):29. [Google Scholar]
- 103.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 104.Aschner P, Kipnes M, Lunceford J, Mickel C, Davies M, Williams-Herman D. Sitagliptin monotherapy improved glycemic control in the fasting and postprandial states and beta-cell function after 24 weeks in patients with type 2 diabetes (T2DM) Diabetes. 2006;55(Suppl 1):462. [Google Scholar]
- 105.Karasik A, Charbonnell B, Liu J, Wu M, Meehan A, Meininger G. Sitagliptin added to ongoing metformin therapy enhanced glycemic control and beta-cell function in patients with type 2 diabetes. Diabetes. 2006;55(Suppl 1):119–120. [Google Scholar]
- 106.Rosenstock J, Brazg R, Andryuk PJ, McCrary Sisk C, Lu K, Stein P. Addition of sitagliptin to pioglitazone improved glycemic control with neutral weight effect over 24 weeks in inadequately controlled type 2 diabetes (T2DM) Diabetes. 2006;55(Suppl 1):132–133. [Google Scholar]
- 107.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 108.Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibitors: a promising new therapeutic approach for the management of type 2 diabetes. Int J Biochem Cell Biol. 2006;38:831–844. doi: 10.1016/j.biocel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 109.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 110.Rosenstock J, Brazg R, Andryuk PJ, Kaifeng L, Stein P for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Therap. 2006;28:1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 111.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 112.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–1987. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- 113.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 114.Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7:692–698. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 115.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 116.Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naive patients with Type 2 diabetes. Diabet Med. 2007;24:955–961. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 117.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 118.Rosenstock J, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175–185. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 119.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–174. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 120.Karasik A, Wu M, Williams-Herman D, Meininger G. Sitagliptin added to ongoing metformin therapy provides sustained glycemic control over 54 weeks, with a low incidence of hypoglycemia and with weight loss; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 0523-P. [Google Scholar]
- 121.Migoya E, Miller M, Larson P, Tanen M, Hilliard D, Deacon C, Gutierrez M, Stoch A, Herman G, Stein P Sitagliptin, a selective DPP-4 inhibitor, and metformin have complementary effects to increase active GLP-1 concentrations; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 0286-OR. [Google Scholar]
- 122.Pratley RE, Schweizer A, Rosenstock J, Foley JE, Banerji MA, Pi-Sunyer FX, Mills D, Dejager S. Robust improvements in fasting and prandial measures of beta-cell function with vildagliptin in drug-naive patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab. 2007 doi: 10.1111/j.1463-1326.2007.00835.x. In Press. [DOI] [PubMed] [Google Scholar]
- 123.Woerle H, Lindenberger T, Linke R, Foley J, Ligueros-Saylan M, Zhang Y, He HL, Beglinger C, Goeke B, Schirra J. A single dose of vildagliptin (VILDA) decelerates gastric emptying (GE) in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 0500-P. [Google Scholar]
- 124.Bose E, Byiers SR, Cohen SE. Vildagliptin significantly decreases blood pressure in hypertensive patients with type 2 diabetes compared with metformin; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 0521-P. [Google Scholar]
- 125.Utzschneider KM, Tong J, Montgomery B, Udayasankar J, Gerchman F, Marcovina SM, Watson CE, Ligueros-Saylan MA, Foley JE, Holst JJ et al. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care. 2008;31:108–113. doi: 10.2337/dc07-1441. [DOI] [PubMed] [Google Scholar]
- 126.Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, Dunning BE, Foley JE, Taskinen MR. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–2057. doi: 10.1007/s00125-006-0340-2. [DOI] [PubMed] [Google Scholar]
- 127.Kadowaki T, Tajima N, Odawara M, Nishii M, Nonaka K, Stein PP. Sitagliptin added to ongoing treatment with metformin improved glycemic control and was well tolerated in Japanese patients with type 2 diabetes (T2DM); Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 2135-PO. [Google Scholar]
- 128.Tajima N, Kadowaki T, Odawara M, Nishii M, Nonaka K, Stein PP. Sitaglitptin added to ongoing treatment with glimepiride improved glycemic control and was well tolerated in Japanese patients with type 2 diabetes (T2DM); Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 2134-PO. [Google Scholar]
- 129.Kashiwagi A, Tajima N, Kadowaki T, Nonaka K, Okamoto T, Okuyama K, Nishii M, Stein PP. Sitagliptin added to ongoing treatment with pioglitazone improved glycemic control and was well tolerated in Japanese patients with type 2 diabetes (T2DM); Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 2136-PO. [Google Scholar]
- 130.Krobot KJ, Stein PP, Allen S, Davies MJ, Meininger G. Lower risk of hypoglycemia with sitagliptin compared to glipizide when added to ongoing metformin therapy: an analysis based on A1C-adjusted event rates; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 2195-PO. [Google Scholar]
- 131.Williams-Herman D, Xu L, Davies MJ, Stein PP. Substantial improvement in beta-cell function with initial combination therapy of sitagliptin and metformin in patients with type 2 diabetes after 1 year of treatment; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 543-P. [Google Scholar]
- 132.Iwamoto Y, Kashiwagi A, Yamada N, Terao S, Suzuki M. Vildagliptin versus voglibose monotherapy in patients with type 2 diabetes (T2DM): superior efficacy and tolerability with vildagliptin; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 561-P. [Google Scholar]
- 133.Ahren B, Schweizer A, Dejager S, Dunning BE, Persson M, Foley JE. Vildagliptin suppresses prandial glucagon but enhances glucagon response to hypoglycemia; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 560-P. [Google Scholar]
- 134.DeFronzo R, Fleck P, Wilson C, Mekki Q. Alogliptin monotherapy improves glycemic control in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 446-P. [Google Scholar]
- 135.Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin monotherapy in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 477-P. [Google Scholar]
- 136.Pratley R, Kipnes M, Fleck P, Wilson C, Mekki Q. Alogliptin added to sulfonylurea therapy in patients with type 2 diabetes reduces HbA1c without increasing hypoglycemia; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 445-P. [Google Scholar]
- 137.Pratley R, Reusch J, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to pioglitazone therapy in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 478-P. [Google Scholar]
- 138.Rosenstock J, Rendell M, Gross J, Fleck P, Wilson C, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA1c without increasing weight gain or hypoglycemia; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 444-P. [Google Scholar]
- 139.DeFronzo RA, Hissa M, Blauwet MB, Chen RS. Saxagliptin added to metformin improves glycemic control in patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 67th Sessions; June 22-26, 2007; Chicago, Illinois. 2007. Abstract 0285-OR. [Google Scholar]
- 140.Rosenstock J, Aguilar-Salinas CA, Klein E, List J, Blauwet MB, Chen R. Once-daily saxagliptin monotherapy improves glycemic control in drug-naive patients with type 2 diabetes; Program and abstracts of the American Diabetes Association (ADA) 68th Sessions; June 6-10, 2008; San Francisco, California. 2008. Abstract 517-P. [Google Scholar]


