Abstract
Rationale: Virus-induced wheezing episodes in infancy often precede the development of asthma. Whether infections with specific viral pathogens confer differential future asthma risk is incompletely understood.
Objectives: To define the relationship between specific viral illnesses and early childhood asthma development.
Methods: A total of 259 children were followed prospectively from birth to 6 years of age. The etiology and timing of specific viral wheezing respiratory illnesses during early childhood were assessed using nasal lavage, culture, and multiplex reverse transcriptase–polymerase chain reaction. The relationships of these virus-specific wheezing illnesses and other risk factors to the development of asthma were analyzed.
Measurements and Main Results: Viral etiologies were identified in 90% of wheezing illnesses. From birth to age 3 years, wheezing with respiratory syncytial virus (RSV) (odds ratio [OR], 2.6), rhinovirus (RV) (OR, 9.8), or both RV and RSV (OR , 10) was associated with increased asthma risk at age 6 years. In Year 1, both RV wheezing (OR, 2.8) and aeroallergen sensitization (OR, 3.6) independently increased asthma risk at age 6 years. By age 3 years, wheezing with RV (OR, 25.6) was more strongly associated with asthma at age 6 years than aeroallergen sensitization (OR, 3.4). Nearly 90% (26 of 30) of children who wheezed with RV in Year 3 had asthma at 6 years of age.
Conclusions: Among outpatient viral wheezing illnesses in infancy and early childhood, those caused by RV infections are the most significant predictors of the subsequent development of asthma at age 6 years in a high-risk birth cohort.
Keywords: rhinovirus, respiratory syncytial virus, wheezing, asthma, allergic sensitization
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Viral respiratory wheezing illnesses are common in early childhood, and many children who wheeze subsequently develop asthma. However, the impact on asthma risk of the specific viral etiology of these illnesses is not known.
What This Study Adds to the Field
Early rhinovirus wheezing illnesses are the most robust predictor of subsequent asthma development in high-risk children.
Wheezing viral respiratory illnesses during infancy are often the first clinical manifestation of the most common chronic disease of childhood, asthma. However, many children who wheeze with viral infections during infancy will not go on to develop asthma, suggesting a role for virus- and/or host-specific factors in asthma pathogenesis.
Previous work has focused on the potential association of early respiratory syncytial virus (RSV) infections and the subsequent risk for asthma and allergic disease (1, 2). With the availability of more sensitive molecular techniques (3), rhinovirus (RV) has been implicated as an important pathogen in early childhood wheezing. In fact, Kotaniemi-Syrjanen and colleagues implicated RV infections leading to hospitalization during infancy as an early predictor of the subsequent development of asthma (4). More recently, we reported that outpatient RV wheezing illnesses during infancy were the most significant predictors of wheezing through 3 years of age in the Childhood Origins of ASThma (COAST) cohort (5). Similarly, Kusel and associates demonstrated an association between wheezing outpatient RV and/or RSV illnesses in infancy and persistent wheezing at 5 years of age; however, this finding was most significant in children with early allergic sensitization (6). Taken together, these data indicate that illness severity, viral etiology, and allergic sensitization may all contribute to the development of persistent wheezing and/or asthma. However, additional data are needed to more precisely define the relationships of specific viral wheezing episodes in early life on asthma development during childhood. Therefore, in our high-risk birth cohort, we prospectively assessed the relationship between the timing and etiology of wheezing viral respiratory infections in early life and the subsequent development of childhood asthma at 6 years of age. Some of the results of this study have previously been reported as abstracts (7, 8).
METHODS
Study Subjects
A total of 289 newborns were enrolled from November 1998 through May 2000 in the COAST study as previously described (9–11). Of these children, 285, 275, and 259 were followed prospectively for 1, 3, and 6 years, respectively. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) and/or a history of physician-diagnosed asthma. The Human Subjects Committee of the University of Wisconsin approved the study, and informed consent was obtained from the parents. There were no differences in demographic characteristics between those children followed to at least 1 year of age compared with those followed up to 6 years of age (Table E1 of the online supplement).
Nasal Lavage Samples
Nasopharyngeal mucus samples were collected during scheduled clinic visits (2, 4, 6, 9, and 12 mo) and during times of acute respiratory illnesses (5). Parents notified a study coordinator when their child developed a respiratory tract illness and a symptom scorecard was completed (10). If the symptom score was 5 or greater, classified as a moderate to severe respiratory illness, a nasal lavage was performed and processed as described (10).
Viral Diagnostics
Nasal specimens were analyzed for respiratory viruses including RSV, RV, influenza types A and B (flu), parainfluenza virus types 1–4 (PIV), adenovirus (AdV), and enteroviruses (EnV) using standard techniques (10). In addition, samples were also evaluated for RV RNA by seminested reverse transcriptase–polymerase chain reaction (RT-PCR) (3, 10), and for the viruses listed above plus coronaviruses (OC143, NL63, and 0229) (CV) and metapneumoviruses (MPV) by multiplex PCR (Respiratory MultiCode PLx Assay; EraGen Biosciences, Madison WI) (12).
Allergen-specific IgE
Allergen-specific IgE was measured at 1 and 3 years of age for dust mite, Alternaria alternata, dog, cat, peanut, milk, egg, and soy, as previously described (11). Allergen-specific IgE values of 0.35 kU/L (class I) or greater were considered positive. The presence of allergic sensitization at age 1 and 3 years was defined by having one or more positive values for allergen-specific IgE.
Aeroallergen Skin Testing
Skin prick testing was performed to 12 common standardized aeroallergens at age 5 years: Dermatophagoides pteronyssinus, D. farinae, grass (7-grass mix), cat (standardized), dog (mixed breeds), cockroach (American and German mix), A. alternata, Aspergillus fumigatus, Cladosporium herbarum, ragweed (giant and short mix), late fall pollen mix, and tree (Eastern 6-tree mix) (Greer Laboratories, Lenoir, NC), using a protocol previously used and described by the Childhood Asthma Research and Education (CARE) Network (13).
Clinical Definitions
Daycare attendance (14) and atopic dermatitis (11) were defined as previously described. The term “viral infection” describes virus detection in nasal secretions during symptomatic or asymptomatic time periods. If symptoms were present, this episode was considered a viral illness. A wheezing respiratory illness during the first 3 years of life was defined as meeting one or more of the following criteria: (1) physician-diagnosed wheezing at an office visit; (2) an illness for which the child was prescribed short- or long-acting β-agonists and/or controller medications; or (3) an illness given the following specific diagnoses: bronchiolitis, wheezing illness, reactive airway disease, asthma, or asthma exacerbation. Current asthma was diagnosed at the end of the sixth year of life based on the documented presence of one or more of the following characteristics in the previous year: (1) physician diagnosis of asthma, (2) use of albuterol for coughing or wheezing episodes (prescribed by physician), (3) use of a daily controller medication, (4) step-up plan including use of albuterol or short-term use of inhaled corticosteroids during illness, and (5) use of prednisone for asthma exacerbation. Four separate investigators, blinded to any antecedent histories concerning viral illnesses or patterns of aeroallergen sensitization, independently evaluated each subject for the presence or absence of asthma based on the above criteria.
Statistical Methods
Relationships between asthma and the etiology of wheezing illnesses in the first 3 years of life were assessed using logistic regression analyses, both in univariate models and in multivariable models including the risk factors given above. Relationships among asthma, wheezing illnesses, and allergic sensitization were assessed using logistic regression models. Other risk factors (maternal and paternal asthma, birth month and weight, dog and cat exposure, presence of older siblings, passive smoke exposure, daycare [any in first year], exclusive breastfeeding [first 6 mo], active atopic dermatitis [1 yr of age], and allergic sensitization) for asthma were assessed individually and jointly using logistic regression analyses. Covariates included in multivariate models were chosen using backward selection based on Akaike's information criterion (15). The Wilcoxon signed rank test was used to compare numbers of wheezing illnesses by year within children with and without asthma at age 6 years. Rates of viral recovery from scheduled clinic visits were compared using a Wilcoxon rank sum test.
RESULTS
Viral Isolates
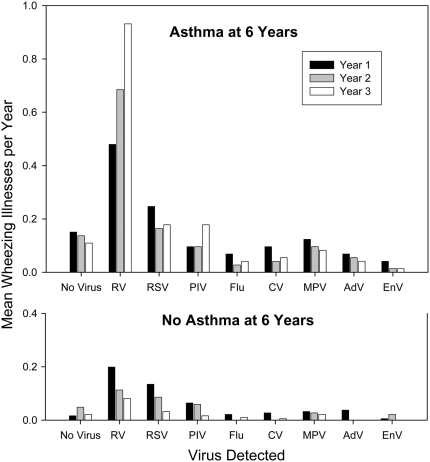
A total of 454 wheezing respiratory illnesses were documented during the first 3 years of life: 153 illnesses in 76 children ages 0–1 year, 155 illnesses in 72 children ages 1–2 years, and 146 illnesses in 63 children in the third year of life. Nasopharyngeal wash specimens were obtained during 442 (97%) of these wheezing illnesses. A viral etiology was identified in 398 (90%) of these specimens. The types of viruses detected during the first 3 years of life included RV (212; 48%), RSV (93; 21%), PIV (51; 12%), MPV (33; 7%), CV (20; 5%), AdV (17; 4%), flu (16; 4%), and EnV (10; 2%). Multiple viruses were identified for 48 wheezing illnesses. The following viruses were recovered from these multiple-virus infections: RV (29; 60%), RSV (20; 42%), MPV (15; 31%), AdV (10; 21%), flu (9; 19%), PIV (8; 17%), CV (7; 15%), and EnV (5; 10%). The pattern of early-life infections was distinct in children diagnosed with asthma at age 6 years (Figure 1). Notably, the frequency of RV-induced wheezing episodes increased over the first 3 years of life for children diagnosed with asthma at age 6 years (P = 0.05), and decreased during the same time period in children without asthma (P = 0.0004) (Figure 1).
Figure 1.
Viral etiology of wheezing illnesses in the first 3 years of life in children with and without asthma at age 6 years. Frequency of rhinovirus (RV) wheezing illnesses during the first 3 years of life increased in children with asthma (n = 73) and decreased in children without asthma (n = 186) at age 6 years. AdV = adenovirus; CV = coronaviruses (OC143, NL63, and 0229); EnV = enteroviruses; Flu = influenza types A and B; MPV = metapneumoviruses; PIV = parainfluenza virus types 1–4; RSV = respiratory syncytial virus.
Viral Illnesses in Early Childhood and Asthma in the Sixth Year of Life
In the sixth year of life, 73 of 259 (28%) children had asthma based on predefined clinical criteria. On the basis of National Asthma Education and Prevention Program (NAEPP) guideline criteria, 35 (48%) of these children had intermittent asthma, 25 (34%) had mild persistent asthma, and 13 (18%) had moderate persistent asthma. Of these 73 children with asthma, 46 (63%) were boys and 27 (37%) were girls. Skin prick testing was performed at age 5 years on 64 of 73 children with asthma; 37 (58%) were positive to at least one allergen. This compares to a sensitization rate of 46% (69 of 149) for the children without asthma at age 6 years (P = 0.12).
The relationship between the viral etiology of wheezing illnesses in each of the first 3 years of life and the diagnosis of asthma at age 6 years was analyzed. Because RV and RSV were most frequently identified as the cause of wheezing illnesses, the analysis focused on these two viruses. Children who wheezed with RV during infancy were at greater risk of asthma at age 6 than children who did not wheeze with RV or RSV, regardless of whether they wheezed only with RV (odds ratio [OR], 2.9; 95% confidence interval [CI], 1.1, 7.5) or had additional wheezing illnesses with RSV (OR, 2.7; 95% CI, 1.2, 6.3) during this same time period (Table 1). In contrast, children who wheezed only with RSV during infancy developed asthma at age 6 at a rate similar to those who did not wheeze with RV or RSV (OR, 1.2; 95% CI, 0.4, 3.2) (Table 1).
TABLE 1.
RHINOVIRUS AND RESPIRATORY SYNCYTIAL VIRUS WHEEZING ILLNESSES IN YEARS 1, 2, AND 3, AND RISK OF ASTHMA AT AGE 6 YEARS
| Wheezing Illness | n | Asthma Age 6 Years (n) | Asthma Age 6 Years (%) | OR | 95% CI | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First year of life | ||||||||||||||
| RSV | No | 211 | 55 | 26 | 1.0 | |||||||||
| Yes | 48 | 18 | 38 | 1.7 | (0.9, 3.3) | 0.11 | ||||||||
| RV | No | 214 | 52 | 24 | 1.0 | |||||||||
| Yes | 45 | 21 | 47 | 2.7 | (1.4, 5.3) | 0.003 | ||||||||
| RV and RSV | Neither | 192 | 46 | 24 | 1.0 | |||||||||
| RSV only | 22 | 6 | 27 | 1.2 | (0.4, 3.2) | 0.73 | ||||||||
| RV only | 19 | 9 | 47 | 2.9 | (1.1, 7.5) | 0.03 | ||||||||
| Both | 26 | 12 | 46 | 2.7 | (1.2, 6.3) | 0.02 | ||||||||
| Second year of life | ||||||||||||||
| RSV | No | 231 | 61 | 26 | 1.0 | |||||||||
| Yes | 28 | 12 | 43 | 2.1 | (0.9, 4.7) | 0.07 | ||||||||
| RV | No | 222 | 49 | 22 | 1.0 | |||||||||
| Yes | 37 | 24 | 65 | 6.5 | (3.1, 13.7) | <0.0001 | ||||||||
| RV and RSV | Neither | 203 | 44 | 22 | 1.0 | |||||||||
| RSV only | 19 | 5 | 26 | 1.3 | (0.4, 3.8) | 0.64 | ||||||||
| RV only | 28 | 17 | 61 | 5.6 | (2.4, 12.8) | <0.0001 | ||||||||
| Both | 9 | 7 | 78 | 12.6 | (2.5, 63.1) | 0.002 | ||||||||
| Third year of life | ||||||||||||||
| RSV | No | 242 | 60 | 25 | 1.0 | |||||||||
| Yes | 17 | 13 | 76 | 9.9 | (3.1, 31.4) | 0.0001 | ||||||||
| RV | No | 225 | 43 | 19 | 1.0 | |||||||||
| Yes | 34 | 30 | 88 | 31.7 | (10.6, 94.9) | <0.0001 | ||||||||
| RV and RSV | Neither | 214 | 35 | 16 | 1.0 | |||||||||
| RSV only | 11 | 8 | 73 | 13.6 | (3.4, 54.0) | 0.0002 | ||||||||
| RV only | 28 | 25 | 89 | 42.6 | (12.2, 148.9) | <0.0001 | ||||||||
| Both | 6 | 5 | 83 | 25.6 | (2.9, 225.6) | 0.004 | ||||||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio; RSV = respiratory syncytial virus; RV = rhinovirus.
In the second year of life, RV wheezing illnesses continued to be strongly associated with asthma at age 6 years whether children also wheezed with RSV (OR, 12.7; 95% CI, 2.5, 63.1) or did not (OR, 5.6; 95% CI, 2.4, 12.8) (Table 1). Children who wheezed only with RSV during Year 2 were not at increased risk of asthma at age 6 years (OR, 1.3; 95% CI, 0.4, 3.8) (Table 1). In contrast to the first 2 years of life, children who wheezed only with RSV in Year 3 had a significantly increased risk of asthma at age 6 years (OR, 13.6; 95% CI, 3.4, 54.0) (Table 1). Year 3 RV wheezing illnesses were associated with a dramatically increased risk of Year 6 asthma (OR, 31.7; 95% CI, 10.6, 94.9). Among children with one to two wheezing illnesses, RV wheezing was associated with significantly greater asthma risk than non-RV wheezing (89% [16/18] vs. 55% [16/29], P = 0.02). All children who wheezed three or more times in Year 3 wheezed at least once with RV.
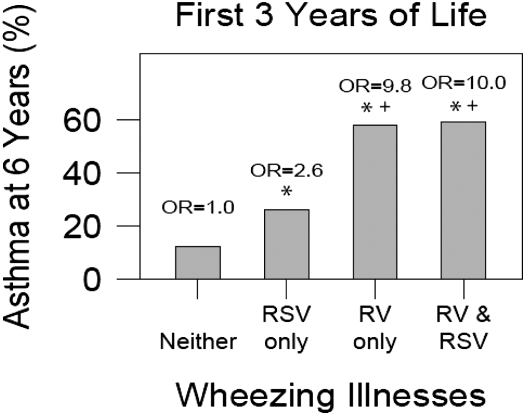
When wheezing history during the entire first 3 years of life was considered, wheezing with RSV alone was associated with an increased risk of asthma at age 6 years compared with children who did not wheeze with RV or RSV (OR, 2.6; 95% CI, 1.0, 6.3) (Figure 2). Wheezing with RV, regardless of RSV wheezing history, was associated with a substantially increased risk of asthma at age 6 years compared with children who did not wheeze with either RV or RSV (RV only: OR, 9.8; 95% CI, 4.3, 22.0; and RV and RSV: OR. 10.0; 95% CI. 4.5, 22.2) (Figure 2) Furthermore, wheezing with RV alone or in addition to RSV was associated with significantly greater asthma risk compared with wheezing with RSV alone (RV only: OR, 3.8; 95% CI, 1.4, 10.4; and RV and RSV: OR, 3.9; 95% CI, 1.5, 10.5).
Figure 2.
Risk of asthma at age 6 years in children who wheezed during the first 3 years of life with rhinovirus (RV), respiratory syncytial virus (RSV), or both (*P < 0.05 vs. Neither; +P < 0.05 vs. RSV only). OR = odds ratio.
We next compared rates of asthma for children who wheezed during the first 3 years of life with RV with those who wheezed with any other viruses (including RSV). Rates of Year 6 asthma were 9% for nonwheezing children and 31% for children who wheezed only with viruses other than RV (OR, 4.2; 95% CI, 1.8, 9.9). Wheezing with RV, either alone or in addition to other viruses, was associated with a significantly greater asthma risk (58%) compared with children who did not wheeze (OR, 13.1; 95% CI, 6.3, 27). Asthma risk was similar for children who wheezed only with RV (53%) and children who wheezed with RV and other viruses (60%) (P = 0.57).
There were no differences between rates of infection as determined by viral recovery from nasal lavage samples performed during scheduled clinic visits during Year 1 for children with and without asthma at age 6 years (asthma, 1.2 ± 1.1, and no asthma, 1.2 ± 1.0 infections/y; P = 0.91). Moreover, RV moderate to severe illnesses (MSI) without wheeze were not associated with increased asthma risk at age 6 (no RV MSI Years 1–3, 12% asthma risk; RV MSI without wheeze Years 1–3, 16% asthma risk; RV MSI with wheeze Years 1–3, 59% asthma risk).
Nonviral Risk Factors and Asthma
The associations between environmental factors and the development of asthma by age 6 years were analyzed. In univariate and stepwise multivariate analyses, allergic sensitization to either aeroallergen or food at age 1 year (aeroallergen: OR, 2.7; 95% CI, 1.2, 6.2; food: OR, 2.0; 95% CI, 1.0, 3.9) and older siblings in the home during infancy (OR, 1.9; 95% CI, 1.0, 3.5) were associated with increased risk for asthma at age 6 years. The presence of a dog in the home at the time of birth was associated with reduced asthma risk by univariate analyses (OR, 0.5; 95% CI, 0.3, 0.9), but this did not reach statistical significance in a stepwise multivariate model (OR, 0.6; 95% CI, 0.3, 1.1) (Table 2).
TABLE 2.
RISK FACTORS FOR ASTHMA AT AGE 6 YEARS
| Univariate
|
Multivariate
|
Multivariate (Stepwise)
|
|||||
|---|---|---|---|---|---|---|---|
| Risk Factors | With Risk Factor, n (%) | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Maternal asthma | 106/249 (43) | 1.3 (0.7, 2.3) | 0.36 | 1.7 (0.9, 3.5) | 0.12 | ||
| Paternal asthma | 74/242 (31) | 1.2 (0.7, 2.2) | 0.57 | 1.0 (0.5, 2.3) | 0.95 | ||
| Birth month | — | — | 0.42 | — | 0.38 | ||
| Birth weight, lb | — | 1.1 (0.8, 1.3) | 0.67 | 0.9 (0.7, 1.3) | 0.64 | ||
| Cat in household at birth | 76/259 (29) | 1.0 (0.5, 1.7) | 0.90 | 0.9 (0.4, 2.0) | 0.86 | ||
| Dog in household at birth | 92/259 (36) | 0.5 (0.3, 0.9) | 0.02 | 0.6 (0.3, 1.2) | 0.16 | 0.6 (0.3, 1.1) | 0.08 |
| Older siblings | 145/259 (56) | 1.8 (1.0, 3.1) | 0.05 | 1.5 (0.7, 3.1) | 0.27 | 1.9 (1.0, 3.5) | 0.03 |
| Passive smoke exposure (1st yr) | 64/259 (25) | 1.1 (0.6, 2.1) | 0.76 | 0.7 (0.3, 1.5) | 0.33 | ||
| Daycare (1st yr) | 123/259 (47) | 0.7 (0.4, 1.2) | 0.20 | 0.6 (0.3, 1.4) | 0.24 | ||
| Exclusive breastfeeding (1st 6 mo) | 84/259 (32) | 1.1 (0.6, 2.0) | 0.70 | 0.9 (0.4, 1.9) | 0.73 | ||
| Atopic dermatitis (1st yr) | 65/253 (26) | 1.7 (0.9, 3.1) | 0.08 | 1.2 (0.5, 2.5) | 0.70 | ||
| Aeroallergen sensitization (1st yr) | 34/255 (13) | 3.6 (1.7, 7.5) | 0.0008 | 4.3 (1.4, 13.0) | 0.01 | 2.7 (1.2, 6.2) | 0.02 |
| Food sensitization (1st yr) | 65/255 (25) | 2.8 (1.6, 5.2) | 0.0006 | 1.7 (0.7, 3.9) | 0.23 | 2.0 (1.0, 3.9) | 0.04 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Risk factors included in the multivariate (stepwise) model were chosen using backward elimination based on Akaike's information criterion. Values in boldface are statistically significant (P < 0.05).
Allergic Sensitization, RV Wheezing, and Asthma
Aeroallergen sensitization without RV wheezing in Year 1 and RV wheezing without concomitant aeroallergen sensitization were each associated with increased rates of asthma at age 6 years (45 and 39%, respectively; Table 3). Infants with both RV wheezing and aeroallergen sensitization by age 1 year had the highest incidence (86%) of subsequent asthma. Sensitization to aeroallergens did not alter the impact of RV wheezing on subsequent asthma development (interaction P = 0.61).
TABLE 3.
RELATIVE CONTRIBUTION OF RHINOVIRUS WHEEZING ILLNESSES AND AEROALLERGEN SENSITIZATION TO RISK OF ASTHMA AT AGE 6 YEARS
| OR (95% CI)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RV Wheezing | Aeroallergen Sensitization | Asthma
|
RV Wheezing | Aeroallergen Sensitization | ||||||||
| n | n | % | ||||||||||
| First year of life | ||||||||||||
| No | No | 183 | 38 | 21 | 2.8 (1.4, 5.6) | 3.6 (1.7, 7.7) | ||||||
| No | Yes | 27 | 12 | 45 | ||||||||
| Yes | No | 38 | 15 | 39 | ||||||||
| Yes | Yes | 7 | 6 | 86 | ||||||||
| Third year of life | ||||||||||||
| No | No | 150 | 21 | 14 | 25.6 (8.2, 79.6) | 3.4 (1.7, 6.9) | ||||||
| No | Yes | 50 | 18 | 36 | ||||||||
| Yes | No | 16 | 13 | 81 | ||||||||
| Yes | Yes | 14 | 13 | 93 | ||||||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio; RV = rhinovirus.
Aeroallergen sensitization was determined by RAST in the first and third year of life. ORs for Year 6 asthma were determined by logistic regression analyses. Aeroallergen sensitization did not modify the effects of RV wheezing on subsequent asthma risk (interaction P value, 0.61 and 0.99, in the 1st and 3rd yr of life, respectively).
In the third year of life, RV wheezing illnesses had a more prominent effect on asthma risk (OR, 25.6), whereas aeroallergen sensitization was associated with a smaller, but still significant increase in asthma risk (OR, 3.4) (Table 3). Again, aeroallergen sensitization did not modify the relationship between RV wheezing illnesses and asthma development (interaction P = 0.99).
DISCUSSION
Wheezing RV illnesses occurring at any time during the first 3 years of life were associated with a nearly 10-fold increase in asthma risk at age 6 years, making them the most significant predictor of asthma development in the high-risk COAST cohort. Interestingly, RSV wheezing illnesses during the first 3 years of life were associated with a smaller increase in asthma risk at age 6 years. In Years 1 and 2, the relationship between viral wheezing and subsequent asthma development was specific to RV. By age 3 years, wheezing with either virus was strongly associated with asthma at age 6; however, RV wheezing remained the strongest predictor. This extends our previous findings by demonstrating that outpatient wheezing illnesses with RV during infancy predict not only persistent wheezing at 3 years of age (5) but also asthma at 6 years of age.
A significant advance of this study is the comprehensive identification of the etiology and timing of early childhood respiratory wheezing illnesses during the first 3 years of life, and the prospective link to asthma. Using a combination of conventional and molecular viral diagnostics, we were able to identify a viral etiology for 90% of the wheezing illnesses in the first 3 years of life. This recovery rate compares favorably with other studies (4, 6, 16).
Previous results generated by the Tucson Children's Respiratory Study group and others linked RSV lower respiratory tract illnesses in the first 3 years of life with a persistent wheezing phenotype at age 6 years (1, 2). We have confirmed this association; however, with the assistance of more comprehensive molecular techniques, we have clearly demonstrated that RV wheezing illnesses in early childhood confer the greatest risk of asthma at age 6 years. Moreover, the risk of developing asthma after outpatient RSV wheezing illnesses during the first 2 years of life is increased only in those children who also wheeze with RV.
In the COAST cohort, RV wheezing and aeroallergen sensitization during Year 1 independently conferred increased risk of asthma at age 6. By Year 3, RV wheezing was a far more robust predictor of asthma development at age 6 than was aeroallergen sensitization. Indeed, nearly 90% of children wheezing with RV at age 3 subsequently developed asthma regardless of the presence or absence of aeroallergen sensitization. Kusel and colleagues also recently demonstrated an association between infantile wheezing RSV and RV illnesses and current wheezing at age 5 years; however, their finding was limited to children with early allergic sensitization (6). They also associated RV wheezing in infancy and asthma at age 5 years, but this association was no longer significant when the results were controlled for other risk factors (6). In contrast, in the COAST cohort, RV wheezing illnesses in infancy and early childhood alone were a significant risk factor for asthma at age 6 years, and this relationship was enhanced after controlling for other risk factors. One potential explanation for the differences in study results is the improved viral detection rate in COAST compared with other cohorts, particularly with respect to RVs. The PCR primers used in this system have proven to detect all 101 known strains of RV (12). Furthermore sequencing of PCR products from a subset of specimens in this study has led to the discovery of 26 additional strains of RV, including 9 whose 5′ sequences cluster into a group that is distinct from group A and B RVs (17).
Recent observations by Devulapalli and colleagues suggested that the number and/or persistence of bronchial obstructive episodes in the first 2 years of life are important predictors of subsequent asthma risk at age 10 years (18). Although viral diagnostics were not performed, these findings suggest that respiratory illness burden in early life is closely associated with asthma risk. We evaluated a number of outcomes that provide further insight into these relationships. First, numbers of documented infections during routine scheduled visits were not related to subsequent asthma. Second, illnesses had to be accompanied by wheezing in order for a lower respiratory illness to be a significant determinant of asthma risk at age 6 years. A novel aspect of our findings is that wheezing with specific viral pathogens (i.e., RV vs. other viruses) conferred differential rates of asthma risk. Importantly, the burden of RV wheezing illnesses increased during the first 3 years of life for children who went on to develop asthma (Figure 1). By Year 3, children who wheezed three or more times had invariably wheezed with RV.
Our data clearly link RV wheezing illnesses during early life and increased asthma risk in later childhood. These findings therefore raise the question: do early RV illnesses actually cause asthma? Alternately, RV wheezing episodes might instead serve to reveal children who are already predisposed to this disease on the basis of abnormal lung physiology and/or immune responses. In support of the latter hypothesis, it has been demonstrated that host-related factors related to low lung function and immunoregulation can enhance susceptibility to virus-induced wheezing (19, 20). For example, low interferon responses in stimulated blood mononuclear cells increase the risk of viral illnesses and wheezing in infancy (14, 21, 22). These two hypotheses are not mutually exclusive, and we propose that, in the genetically susceptible host, RV wheezing illnesses could cause pathologic effects in the airways that promote asthma. Additional studies are needed to resolve this important question.
In conclusion, it has long been recognized that infants who wheeze with respiratory viruses, and particularly those few infants who are hospitalized, are at increased risk of subsequent asthma. Our results expand this paradigm by focusing on outpatient illnesses and demonstrate that the etiology and severity of viral respiratory infections significantly predict asthma development in a high-risk cohort. It is noteworthy that, among outpatient illnesses, which are much more common than those requiring hospitalization, RV-associated wheezing is a better indicator of asthma risk than RSV-associated wheezing. A major goal for future research efforts should therefore be to define host and viral factors that promote wheezing RV illnesses in early childhood. Furthermore, additional studies are needed to determine whether RV wheezing illnesses in early childhood predict specific phenotypes of asthma (atopic vs. nonatopic) throughout childhood and beyond. These studies could provide novel therapeutic targets related to the initiation of and/or progression from early viral wheezing episodes to the subsequent development of childhood asthma.
Supplementary Material
Supported by National Institutes of Health grants R01 HL61879, P01 HL70831, and M01 RR03186.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200802-309OC on June 19, 2008
Conflict of Interest Statement: D.J.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.E.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.A.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.L.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.E.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.-M.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.T.C.-D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.P.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.F.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.J.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.G. serves on the Scientific Advisory Board for Eragen Biosciences and has stock options in the company. He received $1,200 in consulting fees in 2007. R.F.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501–1507. [DOI] [PubMed] [Google Scholar]
- 2.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. [DOI] [PubMed] [Google Scholar]
- 3.Ireland DC, Kent J, Nicholson KG. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J Med Virol 1993;40:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy: the first sign of childhood asthma? J Allergy Clin Immunol 2003;111:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemanske RF Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005;116:571–577. [DOI] [PubMed] [Google Scholar]
- 6.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007;119:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson DJ, Evans MD, Anderson EL, Salazar L, DaSilva DF, Pappas TE, Roberg KA, Tisler CJ, Gangnon R, Gern JE, et al. Wheezing rhinovirus illnesses during early childhood and the subsequent development of asthma. J Allergy Clin Immunol 2008;121:S62. [Google Scholar]
- 8.Pappas TE, Roberg KA, Evans MD, Gangnon R, Lee WM, Gern JE, Lemanske RF. Etiology of viral wheezing illnesses in children from birth to age 6 years. J Allergy Clin Immunol 2008;121:S22. [Google Scholar]
- 9.Lemanske RF Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol 2002;13:38–43. [DOI] [PubMed] [Google Scholar]
- 10.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, Adler K, Gilbertson-White S, Hamilton R, Shult PA, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol 2002;13:386–393. [DOI] [PubMed] [Google Scholar]
- 11.Neaville WA, Tisler C, Bhattacharya A, Anklam K, Gilbertson-White S, Hamilton R, Adler K, Dasilva DF, Roberg KA, Carlson-Dakes KT, et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J Allergy Clin Immunol 2003;112:740–746. [DOI] [PubMed] [Google Scholar]
- 12.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol 2007;45:2626–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, Hodgdon K, Morgan W, Sorkness CA, Lemanske RF Jr. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol 2003;112:883–892. [DOI] [PubMed] [Google Scholar]
- 14.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, Kirk CJ, Roberg KA, Anderson EL, Tisler CJ, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med 2004;170:175–180. [DOI] [PubMed] [Google Scholar]
- 15.Akaike H. A new look at statistical model identification. IEEE Trans Automat Contr 1974;19:716–723. [Google Scholar]
- 16.Papadopoulos NG, Moustaki M, Tsolia M, Bossios A, Astra E, Prezerakou A, Gourgiotis D, Kafetzis D. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med 2002;165:1285–1289. [DOI] [PubMed] [Google Scholar]
- 17.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE 2007;2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devulapalli CS, Carlsen KC, Haland G, Munthe-Kaas MC, Pettersen M, Mowinckel P, Carlsen KH. Severity of obstructive airways disease by age 2 years predicts asthma at 10 years of age. Thorax 2008;63:8–13. [DOI] [PubMed] [Google Scholar]
- 19.Singh AM, Moore PE, Gern JE, Lemanske RF Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene–virus interactions in asthma causation. Am J Respir Crit Care Med 2007;175:108–119. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med 1995;332:133–138. [DOI] [PubMed] [Google Scholar]
- 21.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, Tisler C, Dasilva D, Roberg KA, Mikus LD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol 2006;117:72–78. [DOI] [PubMed] [Google Scholar]
- 22.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol 2007;120:835–841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.