Abstract
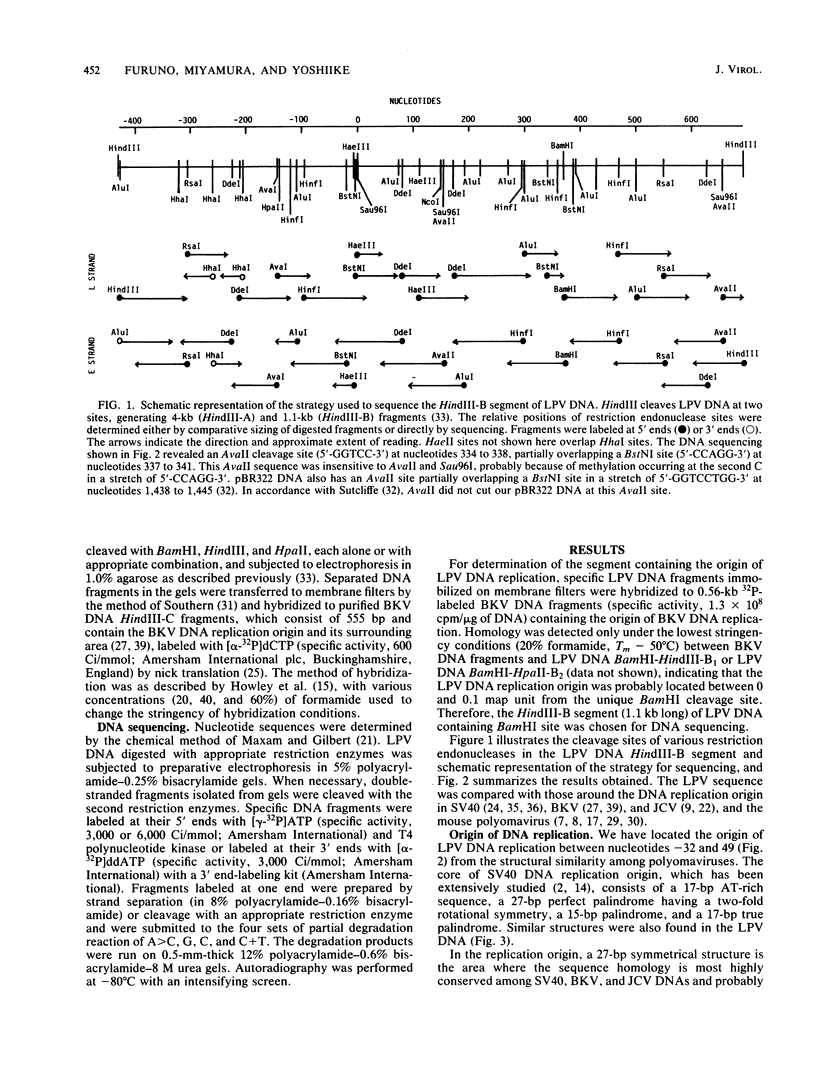
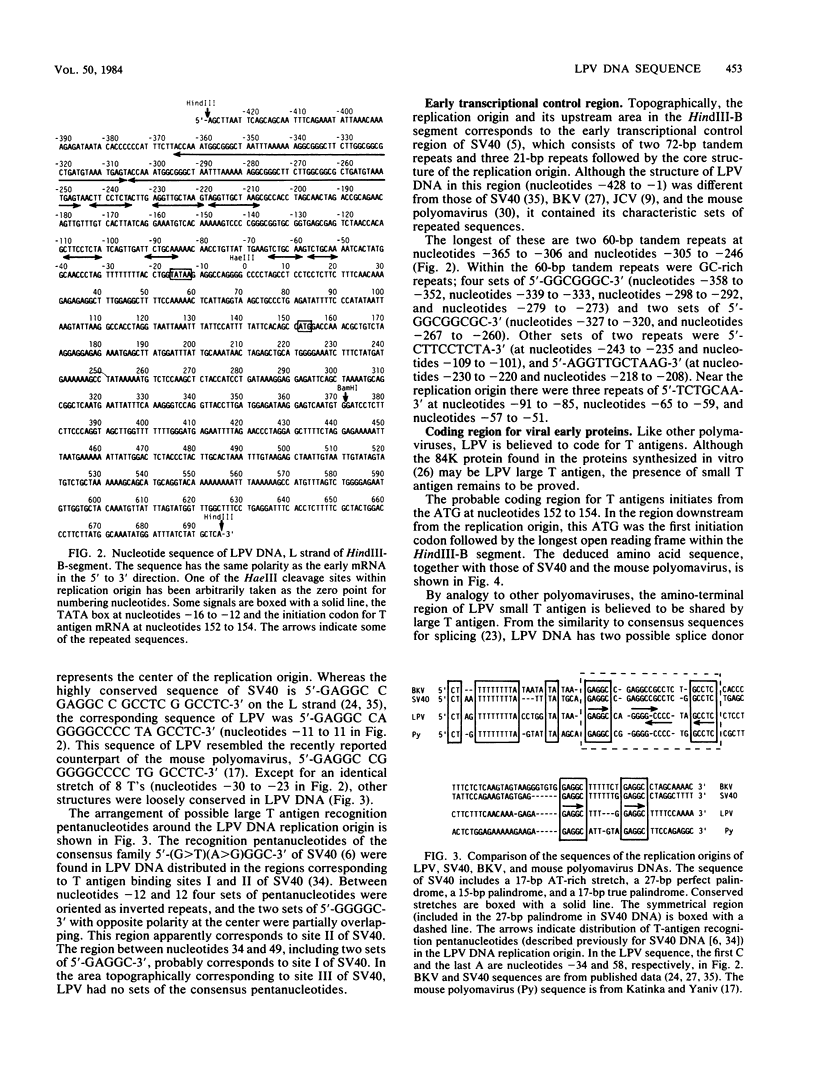
We have determined the nucleotide sequence of the HindIII-B DNA segment of African green monkey B-lymphotropic papovavirus (LPV), which shows a highly restricted host range and whose genome is a 5.1-kilobase-long circular DNA. The segment, consisting of 1,123 base pairs, contained the origin of DNA replication, the putative control region for early transcription, and the region probably coding for the amino-terminal portion of T antigens. The symmetrical region at the center of replication origin, 5'-GAGGC CA GGGGCCCC TA GCCTC-3' (on the L strand), has diverged in the central portion from the corresponding regions of primate polyomaviruses simian virus 40, BK virus, and JC virus, but resembles that of mouse polyomavirus. The structure of the control region upstream of the replication origin was unique to LPV and contained several repeated sequences, the longest of which were two 60-base-pair tandem repeats. The amino-terminal region common to LPV small T and large T antigens showed some homology (41%) in the deduced amino acid sequence to that of both simian virus 40 and the mouse polyomavirus. Like other polyomaviruses, the probable carboxyl-terminal region unique to LPV small T antigen contained two sets of the Cys-x-Cys-x-x-Cys structure. These data show that, despite the unique structures in the control region, LPV is evolutionally related to the mouse polyomavirus and to simian virus 40.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Olive D. M., Hartzell S. W., Subramanian K. N. Territorial limits and functional anatomy of the simian virus 40 replication origin. Proc Natl Acad Sci U S A. 1982 Jan;79(2):381–385. doi: 10.1073/pnas.79.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Müller-Lantzsch N., zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6(4):301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- Brade L., Vogl W., Gissman L., zur Hausen H. Propagation of B-lymphotropic papovavirus (LPV) in human B-lymphoma cells and characterization of its DNA. Virology. 1981 Oct 15;114(1):228–235. doi: 10.1016/0042-6822(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Byrne B. J., Davis M. S., Yamaguchi J., Bergsma D. J., Subramanian K. N. Definition of the simian virus 40 early promoter region and demonstration of a host range bias in the enhancement effect of the simian virus 40 72-base-pair repeat. Proc Natl Acad Sci U S A. 1983 Feb;80(3):721–725. doi: 10.1073/pnas.80.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Doolittle R. F., Walter G. Amino acid sequence homology between polyoma and SV40 tumour antigens deduced from nucleotide sequences. Nature. 1978 Jul 20;274(5668):291–293. doi: 10.1038/274291a0. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Frisque R. J. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983 Apr;46(1):170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Gheysen D., van de Voorde A., Contreras R., Vanderheyden J., Duerinck F., Fiers W. Simian virus 40 mutants carrying extensive deletions in the 72-base-pair repeat region. J Virol. 1983 Jul;47(1):1–14. doi: 10.1128/jvi.47.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P. Simian virus 40 early mRNA's contain multiple 5' termini upstream and downstream from a Hogness-Goldberg sequence; a shift in 5' termini during the lytic cycle is mediated by large T antigen. J Virol. 1981 Oct;40(1):224–240. doi: 10.1128/jvi.40.1.224-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Kanda T., Yoshiike K., Takemoto K. K. Alignment of the genome of monkey B-lymphotropic papovavirus to the genomes of simian virus 40 and BK virus. J Virol. 1983 Apr;46(1):333–336. doi: 10.1128/jvi.46.1.333-336.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Yaniv M. DNA replication origin of polyoma virus: early proximal boundary. J Virol. 1983 Jul;47(1):244–248. doi: 10.1128/jvi.47.1.244-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Chambon P. The SV40 early region TATA box is required for accurate in vitro initiation of transcription. Nature. 1981 Mar 26;290(5804):310–315. doi: 10.1038/290310a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyamura T., Jikuya H., Soeda E., Yoshiike K. Genomic structure of human polyoma virus JC: nucleotide sequence of the region containing replication origin and small-T-antigen gene. J Virol. 1983 Jan;45(1):73–79. doi: 10.1128/jvi.45.1.73-79.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Segawa K., Takemoto K. K. Identification of B-lymphotropic papovavirus-coded proteins. J Virol. 1983 Feb;45(2):872–875. doi: 10.1128/jvi.45.2.872-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Furuno A., Kato K., Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J Virol. 1982 May;42(2):502–509. doi: 10.1128/jvi.42.2.502-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. doi: 10.1111/j.1432-1033.1979.tb02032.x. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Van de Voorde A., Fiers W. Nucleotide sequence of the simian virus 40 small-t gene. Proc Natl Acad Sci U S A. 1978 May;75(5):2160–2164. doi: 10.1073/pnas.75.5.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982 Jun;42(3):978–985. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]
- Yoshiike K., Miyamura T., Chan H. W., Takemoto K. K. Two defective DNAs of human polyomavirus JC adapted to growth in human embryonic kidney cells. J Virol. 1982 May;42(2):395–401. doi: 10.1128/jvi.42.2.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]


