Abstract
Rationale: Chronic exposure to air pollution has been associated with adverse effects on children's lung growth.
Objectives: We analyzed the effects of chronic exposure to urban levels of particulate matter (PM) on selected phases of mouse lung development.
Methods: The exposure occurred in two open-top chambers (filtered and nonfiltered) placed 20 m from a street with heavy traffic in São Paulo, 24 hours/day for 8 months. There was a significant reduction of the levels of PM2.5 inside the filtered chamber (filtered = 2.9 ± 3.0 μg/m3, nonfiltered = 16.8 ± 8.3 μg/m3; P = 0.001). At this exposure site, vehicular sources are the major components of PM2.5 (PM ≤ 2.5μm). Exposure of the parental generation in the two chambers occurred from the 10th to the 120th days of life. After mating and birth of offspring, a crossover of mothers and pups occurred within the chambers, resulting in four groups of pups: nonexposed, prenatal, postnatal, and pre+postnatal. Offspring were killed at the age of 15 (n = 42) and 90 (n = 35) days; lungs were analyzed by morphometry for surface to volume ratio (as an estimator of alveolization). Pressure–volume curves were performed in the older groups, using a 20-ml plethysmograph.
Measurements and Main Results: Mice exposed to PM2.5 pre+postnatally presented a smaller surface to volume ratio when compared with nonexposed animals (P = 0.036). The pre+postnatal group presented reduced inspiratory and expiratory volumes at higher levels of transpulmonary pressure (P = 0.001). There were no differences among prenatal and postnatal exposure and nonexposed animals.
Conclusions: Our data provide anatomical and functional support to the concept that chronic exposure to urban PM affects lung growth.
Keywords: particulate matter, lung development, alveolization, pressure–volume curves, mouse
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Epidemiologic studies have shown adverse effects of traffic-related particulate matter on children's lung growth.
What This Study Adds to the Field
Mice exposed to traffic-related particulate matter in the pre- and postnatal period develop significant alterations of alveolar structure and lung elastic properties, reinforcing the hypothesis that traffic-related pollution adversely impacts lung growth.
Several epidemiologic studies have associated ambient levels of air pollution with adverse respiratory health effects in children, especially in children with chronic respiratory disorders such as asthma. Therefore, increasing attention has been given to the effects of chronic exposure of ambient pollutants on children's respiratory health, especially effects on lung growth (1–7).
The developing lung is an extremely plastic, complex, and highly ordered structure, with more than 40 cell types involved in multiple functions. Lung development may therefore be highly susceptible to damage caused by exposure to environmental toxicants. Exposure to toxicants in the developing lung may alter mechanisms of tissue repair, increasing susceptibility to tissue remodeling and altering responses to injury (8).
Indeed, epidemiologic associations between chronic exposure to air pollution and lung function have been demonstrated in several studies (9–12). One still unanswered question is whether exposure to air pollution at early stages of life predisposes children to the development of respiratory diseases in adulthood. There is evidence of such predisposition in the case of environmental tobacco smoke (13). In addition, some studies show that long-term exposure to outdoor air pollution causes reduction of lung growth in children, as assessed by pulmonary function tests (14–18).
In epidemiologic studies, the impact of long-term exposure to air pollution on lung function is probably the result of exposure to both gases and particles, making it difficult to ascribe a single agent as the cause of the observed changes. The use of animal models allows the role of selected pollutants on developmental parameters to be studied. For instance, alterations of the development of distal airways and pulmonary parenchyma have been elegantly demonstrated after prolonged exposure to ozone in rhesus monkeys (19).
Among the components of air pollution, particulate matter (PM) has been consistently associated with chronic respiratory symptoms and pulmonary function alterations in children (3, 10, 15, 17, 18). In addition, data provided by autopsy studies reveal that young adults living in areas with high ambient levels of inhalable particles have pathologic alterations of airways and distal pulmonary parenchyma (20–22). We reasoned that the role of PM in causing alterations in pulmonary development would be better evaluated in animal models, because confounding variables such as exposure assessment, co-morbidity, and individual variability can be controlled. Therefore, in this study we exposed mice to urban levels of PM dominated by traffic at selected phases of lung development: intrauterine, postnatal, and a combination of both. Some of the results of this study have been previously reported in the form of an abstract (23).
METHODS
An extended version of the methods is available in the online supplement. This study was approved by the Institutional Review Board.
Exposure Site Characterization
The exposure site was located 20 m from the roadside, 150 m from a busy traffic cross-road in downtown São Paulo, and 160 m from a monitoring station of the State of São Paulo Sanitation Agency (24). At this intersection, it is estimated that approximately 83,941 cars, 9,936 diesel vehicles, and 6,321 motorcycles circulate daily on the main street, and 25,590 cars, 5,299 diesel vehicles, and 808 motorcycles circulate on the lateral street of the crossing (25). There are no industries or significant biomass burning sources in the surroundings.
Air pollution at this site has been characterized as mainly vehicular. Previous characterization of PM2.5 (PM ≤ 2.5 μm) mass collected at the monitoring station and from the roof of the Medical School has shown that approximately 67% of the PM2.5 mass is derived from vehicular sources, with a black carbon/organic carbon ratio ranging between 40 and 70% throughout the day (26–28). We have further performed elemental analyses in PM2.5 filters collected at the exposure site between August 2005 and May 2007 confirming that vehicular emissions and crust resuspension are the major PM2.5 components at this site. The mean ± SD ratio black carbon/total carbon was 39 ± 22% during the course of our analysis. Therefore, historical and recent data show that vehicular sources are the major components of PM2.5 mass at this exposure site (see the online supplement for details).
Inhalation Chambers
Exposures were performed from August 2005 to April 2006, using two open-top chambers installed on the campus of the São Paulo University Medical School.
The exposure system employed in this study has been previously described (29–31). The two side-by-side exposure chambers consisted of cylindrical aluminum structures, measuring 2.0 m in diameter and 2.15 m in height and covered by a plastic ultraviolet film. Air entered the chamber at the base of the cylinder and was uniformly distributed throughout the chamber. The air was forced into the chamber and exited at the top, where there was a wide opening. It was a normobaric system; the pressure inside the chambers did not exceed 30 mm H2O. In the filtered system, three stages of filters were in line. The first (plain and bag filters) eliminated large particles and the second (model JFL-90) and third stages (High Efficiency Particulate Air, HEPA) trapped fine particles. Filters were purchased from Purafil (São Paulo, Brazil).
One of the chambers received ambient air at a flow rate of 20 m3/minute (nonfiltered chamber), whereas the other chamber received filtered air (filtered chamber) at the same flow rate. Inside the chambers, animals were kept at the same ambient conditions of temperature, humidity, and noise.
Exposure Assessment
The 24-hour concentration of PM2.5 was determined gravimetrically (32) using Harvard impactors (Air Diagnostics, Harrison, ME) at a flow rate of 10 L · m−1. Impactors were equipped with polycarbonate filters and results were expressed as μg/m3.
Hourly concentrations of CO (Gas Filter Correlation, 48 series; Thermo Scientific, Waltham, MA), PM10 (PM ≤ 10 μm) (FH62 I-n Beta Attenuation Monitor; Graseby Andersen, Smyrna, GA), NO2 (Chemiluminescence 42 Series; Thermo Scientific) and SO2 (Pulsed Fluorescence 43 Series; Thermo Scientific) were obtained from the monitoring station of the State of São Paulo Sanitation Agency (CETESB) (24) located at close proximity with the chambers. Temperature and relative humidity (inside chambers and outdoor) were measured daily; there were no differences in daily temperature and relative humidity between the two chambers (see online supplement for details).
Animals
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” published by the National Institutes of Health (NIH publication 86-23, revised 1985). A parental generation composed of 10-day-old BALB/c mice and their offspring were used in the study. Animals were fed ad libitum with water and commercial pelleted food for small rodents from Nuvital (Nuvilab CR-1; Colombo, Brazil).
Study Design
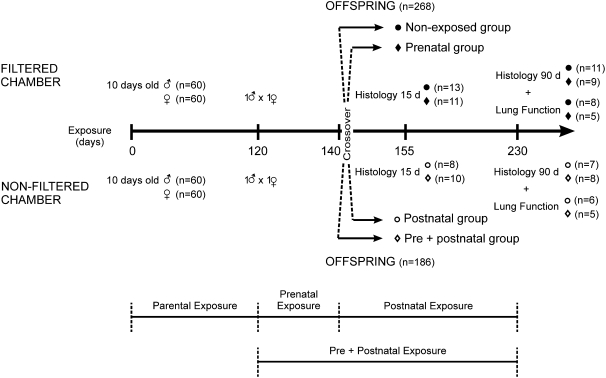
Male (n = 60) and female (n = 60) BALB/c mice were continuously exposed to São Paulo air pollution in a nonfiltered exposure chamber 24 hours/day for 4 months. The corresponding control group, with the same number of animals, was housed in a similar chamber with filters for the same period. After 120 days of exposure, animals were mated. The first generation was obtained from full-term pregnancies that developed in the two exposure chambers. Soon after birth, 30 females and offspring were transferred from the filtered chamber to the nonfiltered chamber (postnatal exposure); likewise, 30 females and offspring were transferred from the nonfiltered chamber to the filtered chamber (prenatal exposure). The remaining 60 females and offspring were kept in their original location, thus constituting two final experimental groups (nonexposed and pre+postnatal exposure). Figure 1 depicts the study flow chart.
Figure 1.
Study flow-chart. The number of animals used in each procedure is depicted.
Animals were killed with sodium pentobarbital (50 mg/kg body weight, intraperitoneally) at 15 or 90 days of age for lung histologic analysis. In the group killed at 90 days of age, pulmonary pressure–volume (P–V) curves were performed.
Histology
Lungs were fixed by intratracheal instillation of formalin (4% formaldehyde in 2% buffer) at a pressure of 20 cm H2O for 24 hours. Longitudinal lung sections were paraffin embedded and 5-μm-thick histologic sections were stained with hematoxylin and eosin for qualitative and morphometric analyses. Histologic slides were coded for blind analysis. Morphometric measurements were performed by the same observer. An integrating eyepiece with a coherent system made of a 100-point and 50 known length lines grid, coupled to a conventional light microscope, was used for the analysis. Lung surface density can be estimated by intersection counting, so that the probability of a test line of given length to intercept the alveolar septum is directly proportional to its surface density in the lung parenchyma. The surface-to-volume ratios were calculated using the equation Sv = 2 I/L, where I is the number of intersections of the alveolar septum with a test line and L is the cumulative length of the test line. L values were obtained using the equation L = Pd/2, where P is the number of points counted over the air spaces and d is the length of the test line in μm. Results are expressed as μm−1. The surface-to-volume ratio is considered to be a shape estimator of gas-exchanging parenchyma (33, 34).
P–V Curves
The lungs were placed in a 20-ml plethysmograph connected to a graded pipette, as previously described (33). Initially, lungs were subjected to three inflations up to 30 cm H2O, and then allowed to return to their resting volume. Lungs were filled with air in small increments of pressure up to 30 cm H2O. For measurements during deflation, lungs were inflated to 30 cm H2O and then deflated to 0 cm H2O. Thirty seconds were allowed to elapse at each step for equilibration before tracheal pressure was recorded. During the experiment, lungs were kept moist with saline.
Statistical Analysis
Data are presented as mean ± SD, unless otherwise specified. Comparison between numerical parameters was performed using Student's t test or ANOVA, and Chi-square when categorical. The comparison of the degree of alveolization among the four animal groups was performed using general linear models, considering surface-to-volume ratio as the dependent variable of age, exposure, as well as an interaction term for both predictive terms. Post hoc comparisons were done using Bonferroni's test. The P–V curves were analyzed by using two-way repeated measures ANOVA, and multiple comparisons were performed by using the Holm-Sidak method. Differences in curve areas among groups were evaluated by ANOVA followed by Tukey's post hoc test. The level of significance was set to 5%.
RESULTS
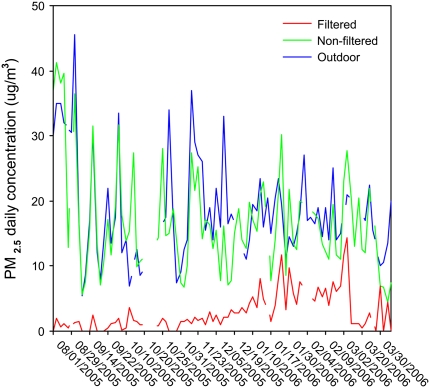
The mean ± SD value of PM2.5 was 2.9 ± 3.0 μg/m3 in the filtered chamber and 16.9 ± 8.3 μg/m3 in the nonfiltered chamber, P < 0.0001. Daily variations in PM2.5 inside both chambers and outdoors are depicted in Figure 2.
Figure 2.
Daily concentrations of PM2.5 (μg/m3) measured in filtered (red line) and nonfiltered chambers (green line) and outdoors (blue line) during the study period.
During the course of our study, the mean outdoor concentration of PM10 was 36.3 ± 15.8 μg/m3 (24-h mean), CO was 1.7 ± 0.7 ppm (8-h mean), NO2 was 89.4 ± 31.9 μg/m3 (24-h mean), and SO2 was 8.1 ± 4.8 μg/m3 (24-h mean). These values were determined by CETESB. Since gaseous pollutants were not retained by the filtering system, the concentrations of NO2, CO, and SO2 were assumed to be similar in both chambers.
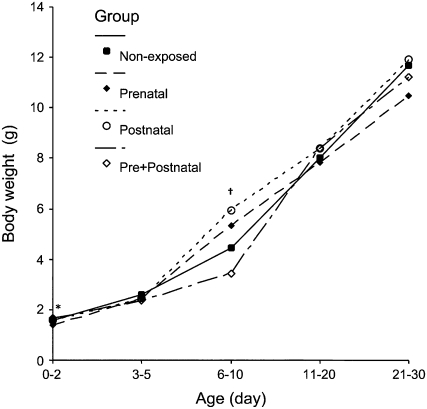
No mortality occurred in parental animals within the exposure chambers. At mating, parental weights (26.3 ± 2.8 g nonexposed and 25.0 ± 2.1 g exposed males, 22.5 ± 2.0 g nonexposed and 21.4 ± 4.0 g exposed females) were similar between chambers (P = 0.052 for males and P = 0.07 for females). After mating, 268 animals were born in the filtered chamber, whereas only 186 were born in the nonfiltered chamber. Interestingly, this difference was due to a lower percentage of pregnant mice (85% in the filtered chamber and 63% in the nonfiltered chamber, P = 0.007), since the number of offspring per female mouse was similar between the filtered chamber (4.4 ± 5.0) and the nonfiltered chamber (3.2 ± 3.3), P = 0.095. Figure 3 depicts the mean body weight of pups in time from birth to 30 days of age. The prenatal group presented a lower body weight at birth (0–2 d) when compared with pre+postnatal mice (P = 0.03). Six- to 10-day-old animals of the prenatal and postnatal groups presented significantly higher body weight when compared with nonexposed and pre+postnatal groups (P = 0.01). All groups had similar body weights at the weaning period (21–30 d).
Figure 3.
Body weight (g) of pups in the different study groups from birth (0–2 d) to weaning (21–30 d) periods. Values are expressed as mean. * Prenatal group had a statistically significant lower weight than did the pre+postnatal group at birth (P = 0.03). † Prenatal and postnatal groups were significantly different from nonexposed and pre+postnatal groups at 6 to 10 days of age (P = 0.01).
Random samples of offspring were chosen from both chambers for this study. Table 1 shows body weight (Bw, g), lung weight (Lw, mg), and the Lw/Bw ratio of examined offspring at 15 and 90 days of age. There were no significant differences in Lw/Bw ratios among the groups (P = 0.15 for 15 d of age and P = 0.13 for 90 d of age).
TABLE 1.
BODY WEIGHTS (g), LUNG WEIGHTS (mg), AND LUNG/BODY WEIGHT RATIO (mg/g) IN THE DIFFERENT STUDY GROUPS AT TIME OF KILLING
| Age (d) | Study Group | Body Weight (g) | Lung Weight (mg) | Lung/Body Weight Ratio (mg/g)* |
|---|---|---|---|---|
| 15 | Nonexposed (n = 13) | 7.8 ± 1.1 | 340.0 ± 82.0 | 43.2 ± 6.9 |
| 15 | Prenatal (n = 11) | 7.9 ± 0.6 | 352.7 ± 99.1 | 44.3 ± 12.6 |
| 15 | Postnatal (n = 8) | 8.6 ± 1.0 | 362.8 ± 77.5 | 42.7 ± 9.7 |
| 15 | Pre + Postnatal (n = 10) | 9.0 ± 1.0 | 383.0 ± 98.8 | 42.0 ± 3.0 |
| 90 | Nonexposed (n = 11) | 27.2 ± 1.6 | 436.0 ± 68.9 | 16.0 ± 2.5 |
| 90 | Prenatal (n = 9) | 20.3 ± 2.3 | 352.9 ± 40.6 | 17.5 ± 6.5 |
| 90 | Postnatal (n = 7) | 25.4 ± 0.6 | 343.7 ± 24.9 | 13.5 ± 2.6 |
| 90 | Pre + Postnatal (n = 8) | 27.4 ± 1.8 | 381.1 ± 21.4 | 13.9 ± 1.0 |
Data are presented as mean ± SD.
There was no statistical significance in the lung/body weight ratios among groups for 15 and 90 days.
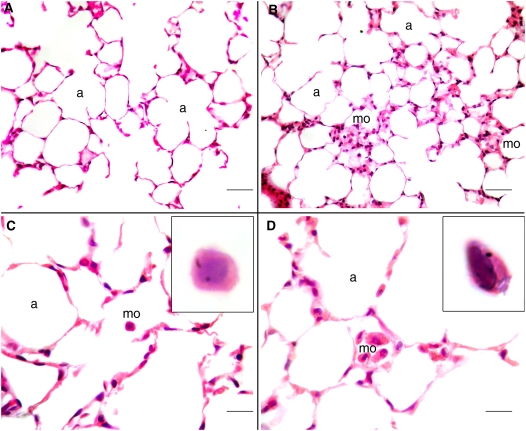
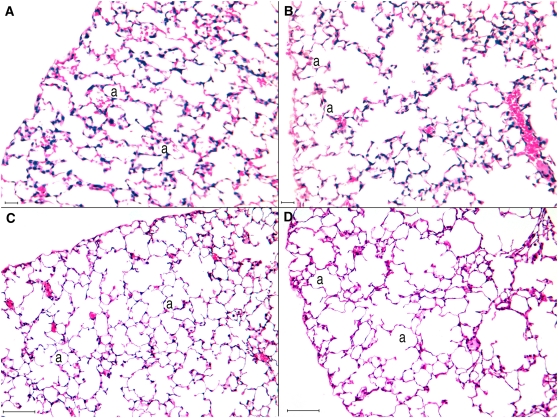
Qualitative analysis of lung tissue revealed that in animals exposed at 90 days of age, mild foci of macrophage accumulations occurred in the alveolar areas, with some macrophages containing minute dots of cytoplasmatic black carbon pigment (Figure 4).
Figure 4.
Histologic samples of distal lung parenchyma of 90-day-old old mice. A and C represent the alveolar parenchyma of nonexposed mice, with a normal histologic aspect. In the 90-day-old pre+postanally exposed mice sparse, mild foci of macrophages could be observed (B and D). In some macrophages of these animals, minute, black dots of carbon pigment could be observed (D, inset). a = alveolar spaces, mo = macrophages. Scale bar: A, B = 100 μm; C, D = 25 μm.
The results of surface-to-volume ratio (Sv) are presented in Table 2. Sv decreased from 15 to 90 days of age. Alveolization is largely complete by 15 days of age (35), therefore the decrease in Sv between 15 and 90 days of age indicates that air spaces enlarge more than create new alveolar septa (Figure 5). General linear modeling disclosed significant effects of age (P < 0.001) and group (P = 0.019), and nonsignificant effects for the term accounting for age × group interaction (P = 0.184). Post hoc comparison among the groups indicated that animals maintained in the filtered chamber (nonexposed) exhibited a higher Sv ratio compared with animals exposed to air pollution during both pregnancy and in the postnatal period (P = 0.036). Sv of animals exposed only during pregnancy or after birth was not different in respect to the nonexposed animals.
TABLE 2.
SURFACE TO VOLUME RATIO VALUES (μM−1) IN MICE OF 15 AND 90 d OF AGE IN THE DIFFERENT EXPOSURE PROTOCOLS
| Age (d) | Study Group | Surface-Volume Ratio (μm−1) |
|---|---|---|
| 15* | Nonexposed (n = 13) | 0.09 ± 0.01 |
| 15 | Prenatal (n = 11) | 0.10 ± 0.01 |
| 15 | Postnatal (n = 8) | 0.09 ± 0.01 |
| 15 | Pre + Postnatal (n = 10) | 0.08 ± 0.01† |
| 90 | Nonexposed (n = 11) | 0.06 ± 0.01 |
| 90 | Prenatal (n = 9) | 0.05 ± 0.01 |
| 90 | Postnatal (n = 7) | 0.05 ± 0.01 |
| 90 | Pre + Postnatal (n = 8) | 0.05 ± 0.01† |
Data are presented as mean ± SD. Statistical significance was achieved for age (*15 > 90, P < 0.001) and exposure to PM2.5 during pre + postnatal development († P = 0.036).
Figure 5.
Histologic samples of distal lung parenchyma. A represents alveolar parenchyma of 15-day-old nonexposed mice. In B, lung parenchyma of 15-day-old pre+postanally exposed mice. The alveolar spaces in B are enlarged when compared with those in A, as a result of incomplete alveolization. C represents distal alveolar parenchyma of 90-day-old nonexposed mice. The alveolar spaces are larger than in A and B. D shows the alveolar parenchyma of 90-day-old of pre+postanally exposed mice. The alveolar spaces are enlarged when compared with D. a = alveolar spaces. Hematoxylin and eosin staining. Scale bar: A, B = 20 μm; C, D = 100 μm.
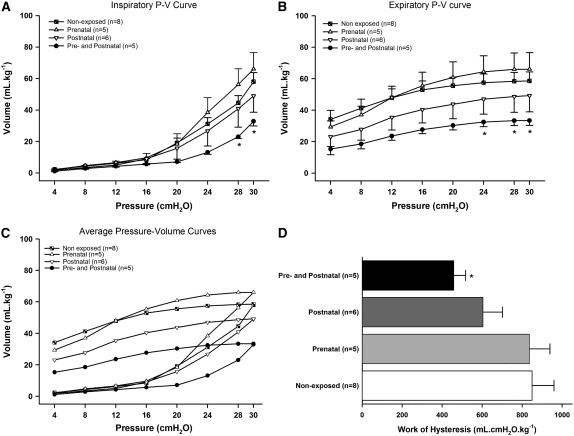
The graphic representation of inspiratory and expiratory limbs of the static P–V curves of the four experimental groups is depicted in Figure 6. Analysis of inspiratory curves showed that the pre+postnatal exposure group had a significant reduction of volume (mean ± SE) at 28 cm H2O (23.0 ± 2.6 ml · kg−1) and 30 cm H2O (32.9 ± 6.6 ml · kg−1) compared with nonexposed (44.5 ± 13.2 at 28 cm H2O, 58.0 ± 16.4 ml · kg−1 at 30 cm H2O, P < 0.001) and prenatally exposed animals (56.2 ± 22.3 at 28 cm H2O, 66.1 ± 23.2 ml at 30 cm H2O, P < 0.001) (Figure 6A). Analysis of expiratory curves showed that animals in the pre+postnatal groups had significant reduction of volume at 24 cm H2O (32.4 ± 6.6 ml · kg−1), 28 cm H2O (33.2 ± 7.0 ml · kg−1), and 30 cm H2O (33.4 ± 7.0 ml · kg−1) compared with prenatally exposed animals (64.3 ± 22.7 ml · kg−1 at 24 cm H2O, 66.0 ± 23.2 ml · kg−1 at 28 cm H2O, and 66.1 ± 23.2 ml · kg−1 at 30 cm H2O, P < 0.001) (Figure 6B). Lungs of animals born to exposed mothers (prenatal exposure) or exposed after term (postnatal exposure) did not show significant differences in the static pulmonary P–V curve compared with mice maintained in the filtered chamber. However, animals from the pre+postnatal exposure group showed a slight change in the shape of the P–V curve. There was a significant interaction between pressure and groups (P < 0.001) for both inspiratory and expiratory curves (Figure 6C). Mechanical work due to hysteresis was calculated and a trend to significance in decreased hysteresis was apparent in the pre+postnatal group compared with nonexposed and prenatally exposed mice (P = 0.054); a statistically significant difference was not observed among the remaining groups (Figure 6D).
Figure 6.
Pressure–volume (P–V) curves in the 90-day-old mice; data are presented as means ± SE. (A) Inspiratory curves showed that the pre+postnatal exposure group had a significant reduction of volume at 28 and 30 cm H2O compared with nonexposed and prenatally exposed animals. (B) Expiratory curves showed that animals of the pre+postnatal groups have significant reduction of volume at 24, 28, and 30 cm H2O compared with prenatally exposed animals. (C) Inspiratory and expiratory limbs of the static P–V curves of the four experimental groups. (D) In the mechanical work due to hysteresis there was a trend toward a difference between pre+postnatal group compared with nonexposed mice and prenatally exposed animals (*P = 0.054). The remaining groups did not show significant decrease in hysteresis.
DISCUSSION
In this study, we have shown that chronic exposure to urban levels of traffic-related particulate matter during the pre- and postnatal periods leads to decreased surface-to-volume ratios in 15-day-old and 90-day-old mice. These changes were accompanied by decreased lung volumes at different inspiratory and expiratory pressures in the young adult mice. To our knowledge, this is the first study in which the effects of chronic exposure to urban levels of traffic-related PM on mouse lung development have been assessed. Our data give definitive anatomic substrate to the previous epidemiologic studies that showed adverse effects of PM ambient exposure on the lung growth of children (9–18).
Of particular relevance to this study, the role of traffic as a major contributor to urban levels of PM2.5 and its effects on children's respiratory health has been highlighted in recent years (36, 37). Gauderman and coworkers (18) demonstrated in an 8-year longitudinal study that living within 500 m of motorways resulted in reduced lung function development in children.
São Paulo, a Latin American mega-city, has 17 million inhabitants and 7.4 million vehicles. In 2006, the emission of PM to the atmosphere of São Paulo was 60.5 thousand tons, and it is estimated that 40% of this amount was derived from traffic sources. Whereas PM10 emission was within acceptable ranges that year, PM2.5 emission exceeded the year-values of 15 μg/m3 in all monitoring stations (38). Our animals were exposed in chambers in close vicinity (< 100 m) to a road with high traffic density and without nearby industries. The exposure site was located near an environmental monitoring station, which performed continuous monitoring of gases and particles. Emissions at this site have been characterized as typically vehicular, as demonstrated by PM2.5 elemental analysis (26–28).
In mice, alveoli are absent at birth and the gas exchanging units are the primary saccules. Secondary crests appear within the first days of life to form the alveoli, and the process is believed to be largely complete by 14 days of age (35). In humans, alveolization begins in utero, by Week 36. Since it is estimated that 85% of alveoli are formed after birth, alveolization in humans is also considered a postnatal event. Controversy exists regarding the time needed to complete alveolization in humans. It is accepted to occur by 2 to 3 years of age, but development of full functionality does not occur until 6 years of age (39–41). Despite differences in postnatal alveolization, we believe that studies in mice can provide useful insights into the potential effects of urban air pollution. Adequate lung growth during the pre- and early postnatal periods is highly dependent on developmental, genetic, and environmental factors (39).
Our group and others have described epidemiologic associations of early fetal loss, prenatal deliveries, and low birth weight with São Paulo air pollution, suggesting an adverse effect of air pollution on intrauterine events (42–44). PM exposure during early pregnancy was associated with reduced fetal growth in a Czech study by Bobak (45). The adverse effects of intrauterine exposure to toxicants such as environmental tobacco smoke are well documented in humans and in experimental models, leading to fetus chronic hypoxia and several adverse effects on the lungs, such as increased airway hyperresponsiveness, loss of alveolar attachments, and persistent deficits in lung function (46–49). It was suggested that environmental tobacco smoke shares many characteristics with particulate air pollution from automotive sources, which is rich in particles and polycyclic aromatic hydrocarbons (41).
A significant portion of lung development in humans, especially the alveolization phase, occurs postnatally (40). It is likely that the sensitivity of fetal and neonatal cells to toxicants is completely different from adult cell sensitivity. Despite lower levels of xenobiotic activating enzymes and elevated detoxification mechanisms, neonatal lungs seem to have abnormal repair and differentiation responses and elevated susceptibility to lung target toxicants, representing a window of high susceptibility to lung damage (47). Further, in growing children, the immune system is also developing and recent emphasis has been given to the role of environmental factors on Th1–Th2 cell equilibrium (36).
In this study, we could not detect differences in surface–volume ratios or significant alterations in the P–V curves observed when animal exposure occurred only pre- or postnatally. This suggests that, at least in mice, a combination of exposure to ambient PM is necessary during both periods to alter lung development. Similarly, Joad and colleagues, when studying the effects of environmental tobacco smoke on lung function in rats, could only find alterations in lung function when exposure occurred in both pre- and postnatal periods. The reasons for such findings are not clear, but it is possible that prenatal exposure to air pollution produces in utero priming, followed by maintenance with postnatal exposure (48). Indeed, experimental and human evidence exists supporting the role of in utero exposures to postnatal outcomes. Singh and coworkers demonstrated that mice exposed prenatally but not postnatally to cigarette smoke exhibited increased airway hyperresponsiveness after intratracheal of Aspergillus fumigatus extract (50). In children, in utero exposures to tobacco smoke and early-onset asthma were associated with persistent deficits in lung function (51).
We have not analyzed animals at later ages, so that we cannot exclude the possibility that the observed effects are transitory. Mauderly and colleagues (52) studied the effects of inhaled NO2 and diesel exhaust in developing rats (prenatal until 6 mo of age) and reported that the young animals did not develop more severe alterations than adults animals exposed for the same period, suggesting that young rats were not a susceptible population to automotive emissions. However, it is possible that even transitory effects on lung development may have an adverse impact in susceptible individuals, like children with asthma.
Our study has some important limitations. We have not studied specific components of PM to identify the causative constituents in the development of the observed effects. It is known that the components of PM vary markedly from region to region and from source to source, which may greatly affect toxicity. However, exposure to multiple toxicants may result in injuries not predicted by analyzing exposures to toxicants individually. In addition, it seems that many components of PM generate reactive oxygen species during their interactions with bioactive molecules, and despite the initial difference in mediators, similar secondary mechanisms leading to cardiopulmonary impairment are generated (53). Because of the “real world exposure” approach employed in this study, we were limited in the number of characterizations performed (such as some gases or organic elements) and in the filtering efficiency within the chambers. Although we cannot exclude the possibility that gases and some other ambient factors such as noise and temperature could have influenced our results, we believe that animals in both chambers were equally affected by these variables. We have not identified mechanistic pathways by which alterations leading to impaired alveolization could occur. Assessing the mechanisms associated with PM and impaired fetal growth and lung development is complex; the timing of exposure and the different toxic components of PM may variably affect the fetus either after transfer across the placenta or indirectly by impairing maternal health and the uterine environment (36). We believe that our findings give pathologic support to epidemiologic data and may contribute to the generation of more studies in this largely unexplored field.
In summary, our findings that mice develop significant alterations of alveolar structure and elastic properties reinforce the hypothesis that urban levels of pollution may adversely impact lung growth. In addition, our results suggest that at ambient levels, intrauterine and postnatal exposures have synergic adverse effects on lung growth.
Supplementary Material
Acknowledgments
The authors thank the technical staff of the Experimental Air Pollution Laboratory for animal care during the exposure period of this study.
Funded by: Fundação de Amparo a Pesquisa do Estado de São Paulo (grant#03/10772-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação Faculdade de Medicina, and the Brazilian Ministry of Health. D.I.K. is financially supported by NIH grant # R01 HL068865.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.200803-436OC on July 2, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Farhat SC, Paulo RL, Shimoda TM, Conceicao GM, Lin CA, Braga AL, Warth MP, Saldiva PH. Effect of air pollution on pediatric respiratory emergency room visits and hospital admissions. Braz J Med Biol Res 2005;38:227–235. [DOI] [PubMed] [Google Scholar]
- 2.Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol 2005;115:689–699. [DOI] [PubMed] [Google Scholar]
- 3.Annesi-Maesano I, Moreau D, Caillaud D, Lavaud F, Le Moullec Y, Taytard A, Pauli G, Charpin D. Residential proximity fine particles related to allergic sensitisation and asthma in primary school children. Respir Med 2007;101:1721–1729. [DOI] [PubMed] [Google Scholar]
- 4.Bedeschi E, Campari C, Candela S, Collini G, Caranci N, Frasca G, Galassi C, Francesca G, Vigotti MA. Urban air pollution and respiratory emergency visits at pediatric unit, Reggio Emilia, Italy. J Toxicol Environ Health A 2007;70:261–265. [DOI] [PubMed] [Google Scholar]
- 5.Magas OK, Gunter JT, Regens JL. Ambient air pollution and daily pediatric hospitalizations for asthma. Environ Sci Pollut Res Int 2007;14:19–23. [DOI] [PubMed] [Google Scholar]
- 6.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Gehring U, Koletzko S, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med 2007;64:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol 2006;164:505–517. [DOI] [PubMed] [Google Scholar]
- 8.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther 2007;114:129–145. [DOI] [PubMed] [Google Scholar]
- 9.Calderon-Garciduenas L, Mora-Tiscareno A, Fordham LA, Chung CJ, Valencia-Salazar G, Flores-Gomez S, Solt AC, Gomez-del Campo A, Jardon-Torres R, Henriquez-Roldan C, et al. Lung radiology and pulmonary function of children chronically exposed to air pollution. Environ Health Perspect 2006;114:1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu LJ, Kaufman JD, Koenig JQ. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest 2006;129:1614–1622. [DOI] [PubMed] [Google Scholar]
- 11.Kasamatsu J, Shima M, Yamazaki S, Tamura K, Sun G. Effects of winter air pollution on pulmonary function of school children in Shenyang, China. Int J Hyg Environ Health 2006;209:435–444. [DOI] [PubMed] [Google Scholar]
- 12.Moshammer H, Hutter HP, Hauck H, Neuberger M. Low levels of air pollution induces changes of lung function in a panel of schoolchildren. Eur Respir J 2006;27:1138–1143. [DOI] [PubMed] [Google Scholar]
- 13.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax 2004;59:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 2004;351:1057–1067. [DOI] [PubMed] [Google Scholar]
- 15.Horak F Jr, Studnicka M, Gartner C, Spengler JD, Tauber E, Urbanek R, Veiter A, Frischer T. Particulate matter and lung function growth in children: a 3-yr follow-up study in Austrian schoolchildren. Eur Respir J 2002;19:838–845. [DOI] [PubMed] [Google Scholar]
- 16.Jedrychowski W, Flak E, Mroz E. The adverse effect of low levels of ambient air pollutants on lung function growth in preadolescent children. Environ Health Perspect 1999;107:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, McDonnell W, Loomis D, Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med 2007;176:377–384. [DOI] [PubMed] [Google Scholar]
- 18.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, Lurmann F, Avol E, Kunzli N, Jerrett M, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 2007;369:571–577. [DOI] [PubMed] [Google Scholar]
- 19.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 2006;291:L644–L650. [DOI] [PubMed] [Google Scholar]
- 20.Sherwin RP, Richters V, Everson RB, Richters A. Chronic glandular bronchitis in young individuals residing in a metropolitan area. Virchows Arch 1998;433:341–348. [DOI] [PubMed] [Google Scholar]
- 21.Churg A, Brauer M, del Carmen Avila-Casado M, Fortoul TI, Wright JL. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect 2003;111:714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souza MB, Saldiva PH, Pope CA III, Capelozzi VL. Respiratory changes due to long-term exposure to urban levels of air pollution: a histopathologic study in humans. Chest 1998;113:1312–1318. [DOI] [PubMed] [Google Scholar]
- 23.Mauad T, Bueno HMS, Rivero DHRF, Oliveira RC, Lichtenfels AJFC, André PA, Kasahara DI, Saldiva PHN. Effects of pre- and post-natal chronic exposure to ambient levels of particulate matter air pollution in mice lung development. E2784–17th European Respiratory Society Annual Congress 2007; Stockholm, Sweden, September 15–19.
- 24.Companhia de Tecnologia de Saneamento Ambiental (CETESB) [internet]. Available from: http://www.cetesb.sp.gov.br (Accessed 18 August, 2008).
- 25.Caracterização das Estações da Rede Automática de Monitoramento da Qualidade do Ar na RMSP-Estação Cerqueira César (CETESB) [internet]. 2005. Série Relatórios/Secretaria de Estado do Meio Ambiente, ISSN 0103-4103. Available from: http://www.cetesb.sp.gov.br (Accessed 18 August, 2008).
- 26.Modelo Receptor-Estudo de Caracterização de Aerossóis na Região Metropolitana de São Paulo-Cerqueira César [internet]. 2002. Available from: http://www.cetesb.sp.gov.br/Ar/relatorios.asp (Accessed 18 August, 2008).
- 27.Ynoue RY, Andrade MF. Characteristics of Urban Aerosols. Size-resolved mass balance of aerosol particles over the São Paulo metropolitan area of Brazil. Aerosol Sci Technol 2004;38:52–62. [Google Scholar]
- 28.Castanho ADA, Artaxo P. Wintertime and summertime Sao Paulo aerosol source apportionment study. Atmos Environ 2001;35:4889–4902. [Google Scholar]
- 29.Mohallem SV, de Araujo Lobo DJ, Pesquero CR, Assuncao JV, de Andre PA, Saldiva PH, Dolhnikoff M. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ Res 2005;98:196–202. [DOI] [PubMed] [Google Scholar]
- 30.Pires-Neto RC, Lichtenfels AJ, Soares SR, Macchione M, Saldiva PHN, Dolhnikoff M. Effects of Sao Paulo air pollution on the upper airways of mice. Environ Res 2006;101:356–361. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenfels AJ, Gomes JB, Pieri PC, El Khouri Miraglia SG, Hallak J, Saldiva PH. Increased levels of air pollution and a decrease in the human and mouse male-to-female ratio in Sao Paulo, Brazil. Fertil Steril 2007;87:230–232. [DOI] [PubMed] [Google Scholar]
- 32.Hinds WC. Aerosol technology: properties, behavior and measurement of airborne particles, 2nd ed. New York: Wiley-Interscience; 1998.
- 33.Silva MF, Zin WA, Saldiva PH. Airspace configuration at different transpulmonary pressures in normal and paraquat-induced lung injury in rats. Am J Respir Crit Care Med 1998;158:1230–1234. [DOI] [PubMed] [Google Scholar]
- 34.Weibel ER, Hsia CC, Ochs MJ. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 2007;102:459–467. [DOI] [PubMed] [Google Scholar]
- 35.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 1977;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkerton KE, Joad JP. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol 2006;33:269–272. [DOI] [PubMed] [Google Scholar]
- 37.Sandstrom T, Brunekreef B. Traffic-related pollution and lung development in children. Lancet 2007;369:535–537. [DOI] [PubMed] [Google Scholar]
- 38.Companhia de Tecnologia de Saneamento Ambiental (CETESB). Relatório de qualidade do ar no estado de São Paulo 2006/CETESB. São Paulo: CETESB, 2007. Série Relatórios/Secretaria de Estado do Meio Ambiente, ISSN 0103-4103. Available from: http://www.cetesb.sp.gov.br
- 39.Kotecha S. Lung growth for beginners. Paediatr Respir Rev 2000;1:308–313. [DOI] [PubMed] [Google Scholar]
- 40.Burri PH. Structural aspects of prenatal and postnatal development and growth of the lung. In: Mcdonald JA, editor. Lung growth and development: lung biology in health and disease. New York: Marcel Dekker, Inc.; 1997. pp.1–32.
- 41.Schwartz J. Air pollution and children's health. Pediatrics 2004;113:1037–1043. [PubMed] [Google Scholar]
- 42.Lin CA, Pereira LA, Nishioka DC, Conceicao GM, Braga AL, Saldiva PH. Air pollution and neonatal deaths in Sao Paulo, Brazil. Braz J Med Biol Res 2004;37:765–770. [DOI] [PubMed] [Google Scholar]
- 43.Pereira LA, Loomis D, Conceicao GM, Braga AL, Arcas RM, Kishi HS, Singer JM, Bohm GM, Saldiva PH. Association between air pollution and intrauterine mortality in Sao Paulo, Brazil. Environ Health Perspect 1998;106:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouveia N, Bremner SA, Novaes HM. Association between ambient air pollution and birth weight in Sao Paulo, Brazil. J Epidemiol Community Health 2004;58:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bobak M. Outdoor pollution, low birth weight, and prematurity. Environ Health Perspect 2000;108:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joad JP. Smoking and pediatric respiratory health. Clin Chest Med 2000;21:37–46. [DOI] [PubMed] [Google Scholar]
- 47.Finkelstein JN, Johnston CJ. Enhanced sensitivity of the postnatal lung to environmental insults and oxidant stress. Pediatrics 2004;113:1092–1096. [PubMed] [Google Scholar]
- 48.Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol Appl Pharmacol 1995;132:63–71. [DOI] [PubMed] [Google Scholar]
- 49.Elliot JG, Carroll NG, James AL, Robinson PJ. Airway alveolar attachment points and exposure to cigarette smoke in utero. Am J Respir Crit Care Med 2003;167:45–49. [DOI] [PubMed] [Google Scholar]
- 50.Singh SP, Barrett EG, Kalra R, Razani-Boroujerdi S, Langley RJ, Kurup V, Tesfaigzi Y, Sopori ML. Prenatal cigarette smoke decreases lung cAMP and increases airway hyperresponsiveness. Am J Respir Crit Care Med 2003;168:342–347. [DOI] [PubMed] [Google Scholar]
- 51.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med 2003;167:917–924. [DOI] [PubMed] [Google Scholar]
- 52.Mauderly JL, Bice DE, Carpenter RL, Gillett NA, Henderson RF, Pickrell JA, Wolff RK. Effects of inhaled nitrogen dioxide and diesel exhaust on developing lung. Res Rep Health Eff Inst 1987;8:3–37. [PubMed] [Google Scholar]
- 53.Kodavanti UP, Watkinson W. Bioavailability of particle-associated air pollutants and relationship to cardiopulmonary injury. In: Foster M, Costa, DL, editors. Air pollutants and the respiratory tract. Lung biology in health and disease, 2nd ed. Boca Raton, FL: Taylor and Francis Group; 2005. pp. 75–133.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.